Key Points
Microtubule tip complex proteins control EC lumen formation through tubulin acetylation to support the developing apical membrane surface.
Microtubule assembly and tubulin modifications interface with proteolytic and kinase cascades to control EC lumen formation in 3D matrices.
Abstract
Vascular tube morphogenesis requires the establishment of endothelial cell (EC) apical–basal polarity in three-dimensional (3D) extracellular matrices. To date, there is little understanding of how EC polarity is controlled during these highly dynamic and rapid morphogenic events. We show that the microtubule tip complex proteins, end binding 1 (EB1), p150Glued, and Clasp1, control human EC tube formation by (1) inducing microtubule assembly and asymmetric cytoskeletal polarization, whereby acetylated and detyrosinated tubulins distribute in a subapical membrane location and filamentous actin distributes basally; (2) increasing tubulin posttranslational modifications, including required acetylation events; and (3) regulating an EC lumen signaling cascade that involves membrane type 1 matrix metallopatrinase (MT1-MMP)–dependent proteolysis as well as Pak, Raf, and Erk kinases. Another regulator of this process is the microtubule stabilizing protein, tau, which binds p150Glued and similarly affects EC lumen formation by controlling the levels of acetylated and detyrosinated tubulins. Increased expression of the tubulin deacetylases, sirtuin 2, and histone deacetylase 6 (HDAC6), blocks EC tube formation and cytoskeletal polarization, while siRNA suppression of these deacetylases stimulates these events. Overall, this work reveals a fundamental role for microtubule tip complex proteins in coordinating microtubule assembly, posttranslational modifications including acetylation, and apical–basal cytoskeletal polarization to control the developing apical membrane surface during blood vessel tubulogenesis in 3D matrix environments.
Introduction
The microtubule cytoskeleton represents a major building block for cell shape and provides a structural and functional framework for the control of cell polarity.1-5 Microtubule minus ends anchor at the centrosome, while plus ends extend to the cell periphery.3,6-10 Microtubules interface with both actin and intermediate filaments to control cell structure and function. They also play a key role in providing a scaffold for signaling molecules.3,11-16 They are highly dynamic structures that continuously remodel and undergo different types of posttranslational modifications, including acetylation, detyrosination, phosphorylation, tyrosination, and glutamylation, all of which are important for the diversity of microtubule functions.2,17-20 Posttranslationally modified tubulins have been implicated in disease states,21,22 intracellular trafficking events,20,23,24 and the control of interactions with other binding partners. In the case of acetylated tubulin, the level of tubulin acetylation is controlled by the reversible enzyme activity of acetyltransferases and the tubulin deacetylases, histone deacetylase 6 (HDAC6) and sirtuin 2 (SIRT2).25-28 Tubulin acetylation appears to be essential for the maturation of primary cilia,2,29 an important polarized structure in select cell types that controls signal transduction.
Microtubules are regulated by tubulin-binding plus-end proteins that directly bind to growing microtubule tips in a manner that is dependent on posttranslational modifications.2,3,5-10 Microtubule plus-end proteins are of special interest because they regulate microtubule dynamics and thus determine the organization and stability of microtubules. They control cell polarity, vesicle trafficking, secretory functions, and centrosome positioning and can localize to both the growing distal tip of microtubules as well as the centrosome at MT minus ends.3,7,30,31 These proteins include end binding 1 (EB1) (a protein with separate microtubule and actin-binding sites),32,33 adenomatous polyposis coli (APC), p150Glued, CLIP-170 (cytoplasmic linker proteins) and CLIP-115, and CLIP-associating proteins (CLASPs).3,4,6,16,34
Vascular tube morphogenesis in three-dimensional (3D) extracellular matrices is a very dynamic process that leads to endothelial cell (EC) lumen formation and multicellular tube assembly, with creation of apical and basal plasma membrane domains.35-39 Considerable progress has been made in elucidating molecular signaling requirements; however, many fundamental questions remain, particularly those concerning how apical–basal polarity is established and maintained during these events. Here, we address this latter question by focusing on a key regulator of this EC polarity mechanism—the microtubule cytoskeleton. Previously, we reported that microtubules play a pivotal role in maintaining and supporting vessel structure and stability and are largely responsible for the shape of tube structures in 3D matrices.40 Microtubule-depolymerizing drugs were found to rapidly collapse endothelial cell tube networks, while actin-depolymerizing drugs did not.40 In the work presented here, we identify key microtubule tip complex proteins, including EB1, p150Glued, and Clasp1, that are required for EC tubulogenesis because of their ability to control an EC lumen signaling cascade. They further regulate the formation of posttranslationally modified tubulins, including acetylated and detyrosinated forms that distribute in a subapical pattern to support the developing apical EC luminal membrane during this process. Our novel data demonstrate a fundamental role for tubulin assembly and posttranslational modifications, which are necessary for EC tubes to form and stabilize during vascular morphogenesis in 3D extracellular matrices.
Methods
Cell culture
Human umbilical vein ECs (Lonza) were cultured as described.38 EC tube morphogenesis assays in 3D collagen gels were performed as described using 3.75 mg/mL of type I collagen.38,39 Cultures were fixed with 3% glutaraldehyde and stained with 0.1% toluidine blue prior to photography, visualization, and quantitation of tube areas.38 For western blot analysis, 3D collagen gels were lysed using sodium dodecyl sulfate sample buffer, and immunoblots were performed.
Confocal microscopy
ECs in collagen I matrices were imaged on a confocal microscope (Leica TC5 SP5) connected to a multiphoton system (Leica, Buffalo Grove, IL) using excitation wavelengths of 488 nm and 633 nm sequentially. The resolution images were captured using the 63× water immersion objective (NA 1.2). A through-focus image set was collected for each cell with a z-step interval of 0.5 μm. The 3D scans progressed from the upper surface of the specimen downward into the tissue and were collected with a z-step interval of 0.5 μm. Data reconstruction into 3D images was performed using IMARIS 3D software (Bitplane).
Results
EB1, p150Glued, and Clasp1 are required for EC lumen and tube formation in 3D collagen matrices
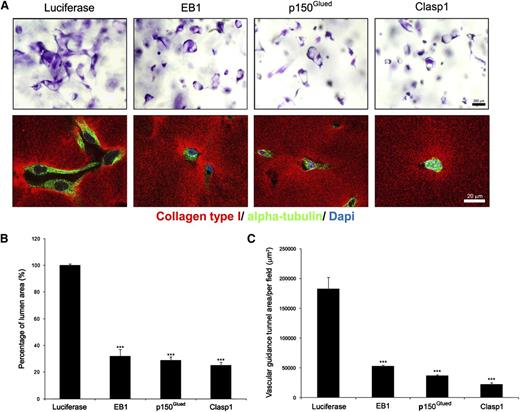
Previous work from our laboratory revealed a role for the microtubule cytoskeleton in EC tube stabilization, since disruption of microtubules led to rapid tube collapse and EC apoptosis in 3D matrices.40 To address a potential role for microtubules in EC tube formation, we focused on known microtubule tip complex proteins that control microtubule assembly as well as vesicular trafficking events relevant to morphogenesis. An siRNA screen evaluated the role of EB1, p150Glued, Clasp1, APC, and CLIP-170 because of their ability to control such microtubule-dependent events. Marked blockade of EC lumen formation was observed following siRNA suppression of EB1, p150Glued, and Clasp1 compared with the control luciferase siRNA (Figure 1A-B; Figure 2A; supplemental Figure 1; supplemental Videos 1–4), while lesser but significant effects were observed following siRNA suppression of APC but not CLIP-170. Real-time videos of these events over 24 hours of culture reveals marked inhibition of tube formation and EC motility following siRNA suppression of EB1, p150Glued, and Clasp1 compared with control siRNA (supplemental Videos 1–4). A consequence of EC tube formation is the creation of vascular guidance tunnels,41 which are physical spaces generated in the collagen matrices that result from MT1-MMP–dependent proteolytic events (supplemental Video 5, supplemental Figure 2). siRNA suppression of EB1, p150Glued, and Clasp1 inhibits EC lumen formation and also markedly blocks vascular guidance tunnel formation, which is revealed by immunostaining of the collagen matrix and confocal microscopy (Figure 1A, lower panels; Figure 1C). Time course experiments were performed to determine whether these proteins are differentially expressed during EC tube formation. Both EB1 and p150Glued are co-induced in a similar time course (6–24 hours), which directly correlates with the induction of the EC lumen formation process (Figure 2A-B). Clasp1 appears to be induced from 24 to 72 hours compared to control proteins such as actin and tubulin isoforms.
The microtubule tip complex proteins, EB1, p150Glued, and Clasp1, are required for EC tubular morphogenesis in 3D collagen matrices. (A) ECs were treated with control (luciferase), p150Glued, Clasp1, or EB1 siRNAs and suspended within 3D collagen matrices for 24 hours. Fixed cultures were stained with toluidine blue and photographed (upper panel). Bar equals 200 μm. Cultures were double stained with anticollagen antibodies to examine vascular guidance tunnel spaces that are created as a result of the tube formation (left lower panel) and anti-α tubulin antibodies to visualize the EC cytoskeleton under the indicated conditions (lower panel). Representative confocal microscopy images of control, EB1-, p150Glued-, or Clasp1 siRNA–treated cells in 3D collagen matrices are shown (lower panel). Bar equals 20 μm. (B) Cultures from (A) were quantitated for EC lumen (B) and vascular guidance tunnel (C) formation. Data are shown as mean EC lumenal area ± standard deviation (n = 6) measured using Metamorph software. Statistical significance ***P < .0005.
The microtubule tip complex proteins, EB1, p150Glued, and Clasp1, are required for EC tubular morphogenesis in 3D collagen matrices. (A) ECs were treated with control (luciferase), p150Glued, Clasp1, or EB1 siRNAs and suspended within 3D collagen matrices for 24 hours. Fixed cultures were stained with toluidine blue and photographed (upper panel). Bar equals 200 μm. Cultures were double stained with anticollagen antibodies to examine vascular guidance tunnel spaces that are created as a result of the tube formation (left lower panel) and anti-α tubulin antibodies to visualize the EC cytoskeleton under the indicated conditions (lower panel). Representative confocal microscopy images of control, EB1-, p150Glued-, or Clasp1 siRNA–treated cells in 3D collagen matrices are shown (lower panel). Bar equals 20 μm. (B) Cultures from (A) were quantitated for EC lumen (B) and vascular guidance tunnel (C) formation. Data are shown as mean EC lumenal area ± standard deviation (n = 6) measured using Metamorph software. Statistical significance ***P < .0005.
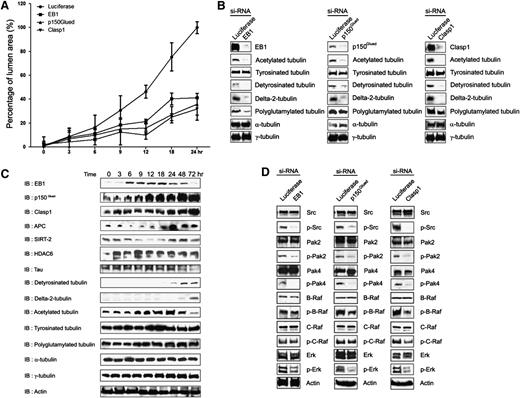
EB1, p150Glued, and Clasp1 control vascular tube morphogenesis by affecting a lumen signaling kinase cascade and tubulin posttranslational modifications, including induced acetylation and detyrosination events. (A) Time course experiment to monitor the influence of siRNA-treated control, EB1, p150Glued, and Clasp1 ECs during lumen and tube formation after the indicated times of culture. EC luminal areas were traced and analyzed using Metamorph software. Data are shown as mean EC luminal area ± standard deviation (n = 3). (B) EB1, p150Glued, and Clasp1 control EC morphogenesis in 3D collagen matrices through posttranslationally modified tubulins. EC lysates were harvested at 24 hours for immunoblot analysis and probed with indicated antibodies. Depletion of EB1, p150Glued, and Clasp1 by siRNA interference markedly decreases posttranslationally modified tubulin expression (compared with total α -tubulin) that accompanies blockade of EC lumen and tube formation in 3D collagen matrices. (C) ECs in 3D collagen matrices were lysed at the indicated time points for immunoblot analysis to analyze the expression or presence of EB1, p150Glued, Clasp1, APC, SIRT2, HDAC6, polyglutamylated tubulin, detyrosinated-tubulin, delta-2-tubulin, acetylated tubulin, tyrosinated tubulin, α-tubulin, and γ-tubulin during EC tube formation over time. An actin immunoblot was used as a loading control. (D) Depletion of EB1, p150Glued, or Clasp1 by siRNA interference impairs an EC lumen kinase-signaling cascade in 3D collagen matrices. Phospho-Pak2, -Pak4, -B-Raf, and -Erk are dramatically inhibited during EC lumen and tube formation in EB1, p150Glued, or Clasp1 knockdown cells. Total kinase levels as well as actin levels were determined. These blots were from a representative experiment that was repeated twice. The specificity of siRNAs and their ability to knockdown the protein expression was confirmed by immunoblot analysis.
EB1, p150Glued, and Clasp1 control vascular tube morphogenesis by affecting a lumen signaling kinase cascade and tubulin posttranslational modifications, including induced acetylation and detyrosination events. (A) Time course experiment to monitor the influence of siRNA-treated control, EB1, p150Glued, and Clasp1 ECs during lumen and tube formation after the indicated times of culture. EC luminal areas were traced and analyzed using Metamorph software. Data are shown as mean EC luminal area ± standard deviation (n = 3). (B) EB1, p150Glued, and Clasp1 control EC morphogenesis in 3D collagen matrices through posttranslationally modified tubulins. EC lysates were harvested at 24 hours for immunoblot analysis and probed with indicated antibodies. Depletion of EB1, p150Glued, and Clasp1 by siRNA interference markedly decreases posttranslationally modified tubulin expression (compared with total α -tubulin) that accompanies blockade of EC lumen and tube formation in 3D collagen matrices. (C) ECs in 3D collagen matrices were lysed at the indicated time points for immunoblot analysis to analyze the expression or presence of EB1, p150Glued, Clasp1, APC, SIRT2, HDAC6, polyglutamylated tubulin, detyrosinated-tubulin, delta-2-tubulin, acetylated tubulin, tyrosinated tubulin, α-tubulin, and γ-tubulin during EC tube formation over time. An actin immunoblot was used as a loading control. (D) Depletion of EB1, p150Glued, or Clasp1 by siRNA interference impairs an EC lumen kinase-signaling cascade in 3D collagen matrices. Phospho-Pak2, -Pak4, -B-Raf, and -Erk are dramatically inhibited during EC lumen and tube formation in EB1, p150Glued, or Clasp1 knockdown cells. Total kinase levels as well as actin levels were determined. These blots were from a representative experiment that was repeated twice. The specificity of siRNAs and their ability to knockdown the protein expression was confirmed by immunoblot analysis.
Differential induction of tubulin posttranslational modifications including acetylated and detyrosinated tubulins during EC lumen and tube formation in 3D collagen matrices
We also assessed whether posttranslational modifications of tubulin occur during these events. Interestingly, there are strong increases in tubulin acetylation between 9 and 24 hours of culture, which temporally correlates with increases in EC lumen formation (Figure 2B). It is interesting that this increase correlates with a corresponding decrease in the expression of SIRT-2, a tubulin deacetylase (Figure 2B). There are also increases observed in detyrosinated tubulin (Glu-tubulin) and delta2-tubulin, which occurred later in the time course (> 24 hour). Of these modifications, the appearance of acetylated tubulin appeared to most directly correlate with the development of EC lumens and tube networks (Figure 2A-B). We found that siRNA suppression of EB1, p150Glued, or Clasp1 affects these tubulin modifications and demonstrate that there are marked decreases in acetylated and detyrosinated tubulin as well as delta2 tubulin (compared with controls) that correlated with a lack of EC lumen formation (Figure 2C). This data suggests that tubulin modifications, which are induced during EC tubulogenesis, may play a role in tube formation or stabilization events and that these modifications are controlled by microtubule tip complex proteins.
EB1, p150Glued, and Clasp1 control a kinase-signaling cascade that controls the EC tube formation process
Our laboratory recently identified a critical kinase cascade downstream of Cdc42- and Rac1-dependent signaling that is necessary for ECs to form lumens in 3D matrices.42 To determine whether EB1, p150Glued, and Clasp1 affect this signaling cascade, ECs were treated with siRNAs to each of these molecules, and lysates were obtained after 24 hours of culture to assess kinase activation during EC lumen formation (Figure 2D). Strong reductions in phosphorylation of Src, Pak-2, Pak-4, and Erk 1/2 were observed in all cases compared with luciferase siRNA–treated cells (and also compared with total kinase levels; Figure 2D). B-Raf phosphorylation was strongly reduced in p150Glued- and Clasp1 siRNA–treated cells, while it was only modestly reduced with EB1 knockdown. A modest reduction in C-Raf phosphorylation was also observed. These data indicate that EB1, p150Glued, and Clasp1 control EC lumen formation by affecting a key kinase-signaling cascade that is known to be required for these events.42
EB1 and its C-terminal p150Glued and Clasp1 binding domain control EC lumen and tube formation
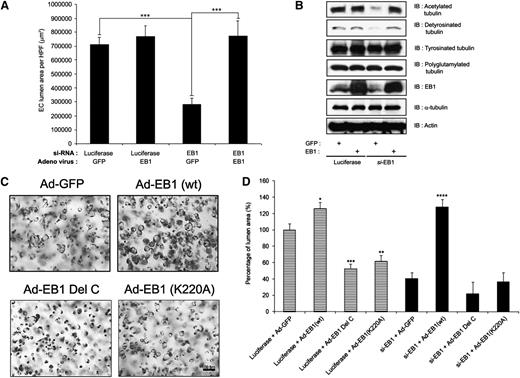
We further examined the functional role of EB1 during lumen formation by generating adenoviral vectors that express wild-type and mutant versions of EB1. We generated a C-terminal deletion of EB1 (EB1 Del C residues 235-268) that eliminates its known binding sites for both p150Glued and Clasp1 as well as a point mutation (EB1 K220A) construct that disrupts its binding to APC.16,33 Increased expression of wild-type EB1 enhances EC lumen formation compared with control and, furthermore, EB1 siRNA–treated ECs (which fail to form lumens) could be rescued to form lumens and tubes by reexpression of wild-type EB1, but not by control GFP expression (Figure 3A). Lysates of these cultures reveal a loss of both acetylated and detyrosinated tubulin in EB1 siRNA–treated ECs, which correlates with inhibition of lumen formation (Figure 3A), and a rescue of the expression of these modified tubulins in conjunction with the rescue of EC lumen formation by reexpression of EB1 (Figure 3A-B). In addition, expression of EB1 Del C or EB1 K220A significantly reduced EC lumen formation (compared with control GFP and wild-type EB1), suggesting that both constructs have dominant negative mutant activities (Figure 3C-D). In addition, neither EB1 mutant was able to rescue the lumen formation defect secondary to EB1 siRNA suppression compared with wild-type EB1 (Figure 3D). These data confirm our conclusions above, demonstrating an important role for EB1, p150Glued, and Clasp1 during EC tubulogenesis, and also show a critical role for an EB1 C-terminal domain that is known to interact with both p150Glued and Clasp1.
Reexpression of wild-type EB1 restores vascular tube formation in EB1 siRNA–treated ECs, but a C-terminal mutant of EB1 without affinity for p150Glued or Clasp1 fails to rescue this phenotype. (A) EB1 siRNA–treated cells were infected with adenoviral vectors carrying GFP or EB1 and allowed to undergo EC tube morphogenesis for 24 hours. Reexpression of EB1 (but not control GFP expression) successfully restores vascular tube formation in these siRNA-treated ECs. Data are presented as lumen area ± standard deviation (SD; n = 6), which was measured by Metamorph software. Statistical significance ***P < .0005. (B) Cell lysates were prepared from the samples in (A) and immunoblots were performed with the indicated antibodies. siRNA suppression of EB1 decreased both acetylated and detyrosinated tubulin, whereas reexpression of EB1 in these cells fully rescued the expression of acetylated and detyrosinated tubulins, which accompanied the restoration of vascular lumen formation. (C) Endothelial cells were induced to express GFP, EB1 (wt), EB1 (K220A), or EB1 δ C using recombinant adenoviral vectors and suspended within collagen matrices for 24 hours in 3D collagen matrices. Cultures were fixed with glutaraldehyde, stained with toluidine blue, and photographed. Scale bar is 100 μm. (D) EB1 or control siRNA-treated cells were induced to express control GFP, EB1 (wt), EB1 (K220A), or EB1 δ C, suspended within collagen matrices and allowed to undergo EC tube morphogenesis for 24 hours. Fixed and stained cultures were quantitated for lumen formation. Data are presented as lumen area ± SD (n = 3). Statistical significance *P < .05, **P < .005, ***P < .0005.
Reexpression of wild-type EB1 restores vascular tube formation in EB1 siRNA–treated ECs, but a C-terminal mutant of EB1 without affinity for p150Glued or Clasp1 fails to rescue this phenotype. (A) EB1 siRNA–treated cells were infected with adenoviral vectors carrying GFP or EB1 and allowed to undergo EC tube morphogenesis for 24 hours. Reexpression of EB1 (but not control GFP expression) successfully restores vascular tube formation in these siRNA-treated ECs. Data are presented as lumen area ± standard deviation (SD; n = 6), which was measured by Metamorph software. Statistical significance ***P < .0005. (B) Cell lysates were prepared from the samples in (A) and immunoblots were performed with the indicated antibodies. siRNA suppression of EB1 decreased both acetylated and detyrosinated tubulin, whereas reexpression of EB1 in these cells fully rescued the expression of acetylated and detyrosinated tubulins, which accompanied the restoration of vascular lumen formation. (C) Endothelial cells were induced to express GFP, EB1 (wt), EB1 (K220A), or EB1 δ C using recombinant adenoviral vectors and suspended within collagen matrices for 24 hours in 3D collagen matrices. Cultures were fixed with glutaraldehyde, stained with toluidine blue, and photographed. Scale bar is 100 μm. (D) EB1 or control siRNA-treated cells were induced to express control GFP, EB1 (wt), EB1 (K220A), or EB1 δ C, suspended within collagen matrices and allowed to undergo EC tube morphogenesis for 24 hours. Fixed and stained cultures were quantitated for lumen formation. Data are presented as lumen area ± SD (n = 3). Statistical significance *P < .05, **P < .005, ***P < .0005.
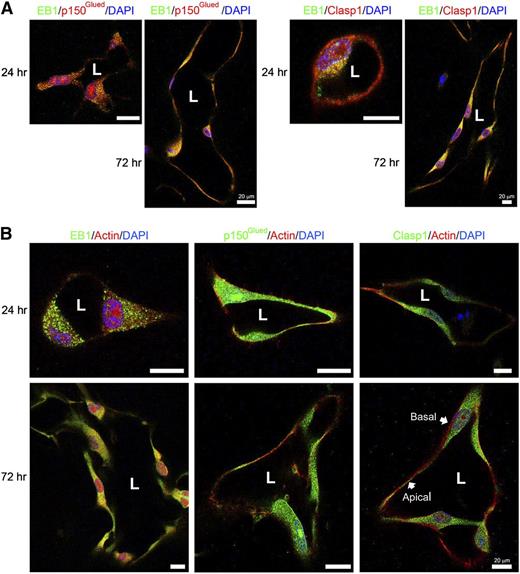
Finally, we examined the ability of EB1, p150Glued, and Clasp1 to colocalize during EC lumen formation in 3D collagen matrices by immunofluorescence analysis and confocal microscopy (Figure 4). We observed both overlapping and nonoverlapping staining for EB1 and p150Glued as well as EB1 and Clasp1. EB1 staining is very punctate, while p150Glued and Clasp1 staining is more continuous (Figure 4B). We first compared each protein with its relationship to filamentous actin (F-actin) at 2 time points of EC lumen and tube formation (Figure 4B). With time, there appeared to be some overlapping staining of EB1 with actin, but this was much less apparent for p150Glued and Clasp1. Of great interest is the appearance of both p150Glued and Clasp1 in a subapical band of staining while F-actin is heavily localized in a basal fashion (Figure 4B). These experiments reveal a strong degree of overlap in staining for these tip complex proteins, which are predominantly distributed in a subapical domain and appear positioned to support the developing apical EC luminal membrane during tubulogenesis in 3D matrices.
Overlapping staining of EB1 with p150Glued or Clasp1 during EC tubulogenesis and presence of these microtubule tip complex proteins in a subapical band supporting the apical luminal surface while F-actin is localized along the basal surface. (A) Confocal microscope images derived from optical sections of vascular tube structures in 3D matrices. EB1 was co-immunostained with p150Glued or Clasp1. EB1 (green) is colocalized with p150Glued or Clasp1 (red) in EC tubes within 3D matrices. Scale bar is 20 μm. (B) ECs were cultured for 24 hours and 72 hours in 3D collagen matrices and fixed and stained for EB1, p150Glued, or Clasp1 and F-actin. EB1, p150Glued, or Clasp1 distributed in a subapical domain, while F-actin was distributed basally. Stained samples were analyzed by confocal microscopy and representative images are shown. Scale bar is 20 μm.
Overlapping staining of EB1 with p150Glued or Clasp1 during EC tubulogenesis and presence of these microtubule tip complex proteins in a subapical band supporting the apical luminal surface while F-actin is localized along the basal surface. (A) Confocal microscope images derived from optical sections of vascular tube structures in 3D matrices. EB1 was co-immunostained with p150Glued or Clasp1. EB1 (green) is colocalized with p150Glued or Clasp1 (red) in EC tubes within 3D matrices. Scale bar is 20 μm. (B) ECs were cultured for 24 hours and 72 hours in 3D collagen matrices and fixed and stained for EB1, p150Glued, or Clasp1 and F-actin. EB1, p150Glued, or Clasp1 distributed in a subapical domain, while F-actin was distributed basally. Stained samples were analyzed by confocal microscopy and representative images are shown. Scale bar is 20 μm.
The microtubule stabilizing protein, tau, controls EC lumen and tube formation in 3D collagen matrices
A known binding partner for p150Glued is the key microtubule stabilizing protein, tau, which has been strongly implicated in the pathogenic development of neurodegenerative diseases as well as vascular endothelial growth factor (VEGF)-induced pathologic angiogenesis through its susceptibility to cleavage by calpain.43 To address a potential role for tau during EC lumen and tube formation, we suppressed the expression of tau using siRNA and, alternatively, we increased its expression using a recombinant adenoviral vector (supplemental Figure 3A-B). siRNA suppression of tau markedly inhibited EC lumen formation (with reductions in acetylated and detyrosinated tubulin compared with controls), while increased expression stimulated this process (with increases in acetylated and detyrosinated tubulin; supplemental Figure 3A-B). Thus, microtubule tip complex proteins as well as an interacting partner, tau, together control the EC tubulogenic process in 3D matrices.
Tubulin acetylation controls EC lumen formation and regulation of these events by the tubulin deacetylases, SIRT2 and HDAC6
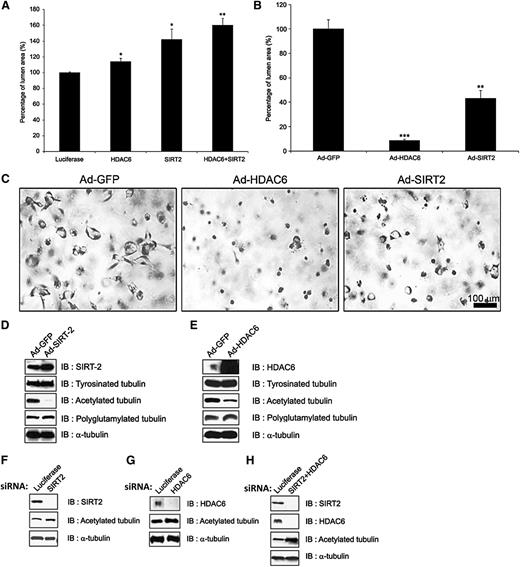
As shown in Figure 2B, a temporal increase in tubulin acetylation directly correlates with the time course and extent of lumen formation (Figure 2A). This also coincides with increases in EB1 and p150Glued expression; siRNA suppression of these molecules markedly inhibits tubulin acetylation, which correlates with their ability to control EC lumen formation (Figure 2B-C). To more directly assess the contribution of tubulin acetylation to this process, we manipulated the expression of 2 known tubulin deacetylases that are expressed by ECs, namely, SIRT2 and HDAC6 (Figure 5A-B). siRNA suppression of both deacetylases individually led to increased EC lumen formation, while knockdown of both molecules together resulted in greater increases in lumen formation (Figure 5A). In contrast, increased expression of SIRT2 or HDAC6 led to blockade of EC lumen formation, suggesting that tubulin acetylation levels directly correlate with the ability of ECs to form lumens in 3D collagen matrices (Figure 5B-C). Importantly, increased expression of either deacetylase strongly reduces tubulin acetylation (Figure 5D-E), while siRNA suppression of SIRT2, HDAC6, and particularly their combination leads to increased acetylation (Figure 5F-H). These data, coupled with our demonstration that EB1, p150Glued, and Clasp1 control lumen formation as well as tubulin acetylation in 3D matrices, strongly suggest that tubulin acetylation directly modulates EC lumen and tube formation. Finally, EC-lined tubes at 72 hours of culture were collapsed following colchicine treatment, as we reported previously, and this leads to a rapid and marked loss of tubulin acetylation and lesser but detectable loss of detyrosinated tubulin (compared with control tubulin and actin) as the tubes collapse (supplemental Figure 4). Thus, the presence of acetylated tubulin appears to correlate with both the formation of EC tubes and with the ability of EC-lined tubes to be maintained in 3D matrices. Finally, siRNA suppression of tubulin tyrosine ligase, an enzyme that regulates C-terminal tyrosination of tubulin, leads to increased lumen formation as well as levels of detyrosinated tubulin, suggesting that tyrosination of tubulin inhibits this morphogenic event (supplemental Figure 3C).
The tubulin deacetylases, HDAC6 and SIRT2, negatively regulate EC lumen and tube formation in 3D matrices. (A) ECs were treated with siRNAs directed to HDAC6 and SIRT2 and their combination, which leads to increased EC lumen formation and increased tubulin. Data are shown as mean EC lumenal area ± standard deviation (SD; n = 6). Statistical significance *P < .05, **P < .005. (B,C) ECs were induced to express HDAC6 vs SIRT2 (vs control GFP) using recombinant adenoviruses, leading to markedly reduced lumen formation after 24 hours of culture. This was assessed by photography (C) and marked loss of acetylated tubulin (D-E) in culture lysates, which correlates with a lack of lumen formation (B-C). Western blots were performed to assess protein knockdown and the levels of acetylated tubulin in the indicated siRNA-treated EC cultures undergoing lumen formation (F-H). Lysates were made after 24 hours of culture. Data are shown as mean EC lumenal area ± SD (n = 6). Statistical significance *P < .05, **P < .005.
The tubulin deacetylases, HDAC6 and SIRT2, negatively regulate EC lumen and tube formation in 3D matrices. (A) ECs were treated with siRNAs directed to HDAC6 and SIRT2 and their combination, which leads to increased EC lumen formation and increased tubulin. Data are shown as mean EC lumenal area ± standard deviation (SD; n = 6). Statistical significance *P < .05, **P < .005. (B,C) ECs were induced to express HDAC6 vs SIRT2 (vs control GFP) using recombinant adenoviruses, leading to markedly reduced lumen formation after 24 hours of culture. This was assessed by photography (C) and marked loss of acetylated tubulin (D-E) in culture lysates, which correlates with a lack of lumen formation (B-C). Western blots were performed to assess protein knockdown and the levels of acetylated tubulin in the indicated siRNA-treated EC cultures undergoing lumen formation (F-H). Lysates were made after 24 hours of culture. Data are shown as mean EC lumenal area ± SD (n = 6). Statistical significance *P < .05, **P < .005.
EC lumen and tube formation in 3D collagen matrices leads to asymmetric polarization of the tubulin cytoskeleton subapically to support the developing apical luminal membrane
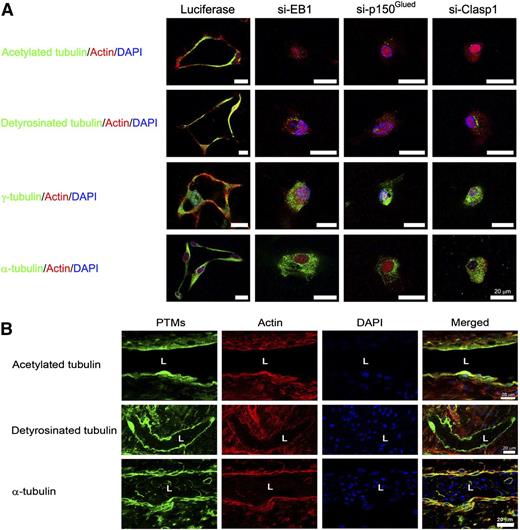
A major unanswered question in vascular biology has been how apical–basal polarity is controlled during vascular tube formation in 3D extracellular matrices. A key question is how the forming apical membrane (which is disconnected from extracellular matrix and exposed to apical luminal fluid) is physically supported during the dynamic tubulogenic process. Because our data show that microtubule assembly and posttranslational modifications such as acetylation and detyrosination are critical for these events (in an EB1-, p150Glued-, and Clasp1-dependent manner), we addressed how these modified tubulins are distributed within ECs during lumen and tube network formation. Immunostaining for EB1, p150Glued, and Clasp1 revealed that there was a subapical band of staining for these microtubule regulators (Figure 4). This raised the possibility that they are participating directly in cytoskeletal support for the EC apical membrane surface during the tubulogenic process (Figures 6 and 7). Disruption of their expression leads to inhibition of EC lumen formation and, thus, a corresponding lack of development of an apical membrane (Figure 6A). In addition, we demonstrate that the developing apical surface during EC lumen formation possesses an underlying band of posttranslationally modified tubulins, including acetylated and detyrosinated tubulin whose presence is markedly dependent on EB1, p150Glued, and Clasp1 expression (Figure 6A, supplemental Figure 5). Confocal microscopy reveals a cytoskeletal asymmetry that develops during these events, with acetylated tubulin presented in a subapical band and F-actin strongly expressed in a basal manner (Figures 6 and 7A-B; supplemental Figures 5 and 6; supplemental Videos 6 and 7). Three-dimensional reconstruction of such images reveals a marked apical–basal polarity of apically distributed acetylated tubulin and basally distributed F-actin (Figure 7B, supplemental Video 7). Similar distributions of detyrosinated tubulin and γ-tubulin are observed in a subapical position, which is also dependent on EB1, p150Glued, and Clasp1 expression (Figure 6A, supplemental Figure 5). Staining of blood vessels during quail vascular development reveals a similar blood vessel apical distribution of acetylated and detyrosinated tubulin as well as basally expressed F-actin (Figure 6B). Thus, our data reveal a fundamental role for the microtubule tip complex proteins, EB1, p150Glued, and Clasp1, in controlling EC lumen formation by inducing asymmetrical subapical polarization of acetylated and detyrosinated tubulins to form and maintain EC apical plasma membranes during the tube assembly process in 3D matrices.
EB1, p150Glued, and Clasp1 control accumulation of acetylated and detyrosinated tubulins in a subapical distribution during EC tubulogenesis in 3D collagen matrices. (A) ECs were treated with control (luciferase), p150Glued, Clasp1, or EB1 siRNAs and suspended within 3D collagen matrices for 24 hours. After fixation, cultures were immunostained with antibodies to the indicated tubulins and modified tubulins. Cultures were costained with AlexaFluorX-conjugated phalloidin to label F-actin and Dapi to label nuclei. Bar equals 20 μm. (B) Day 5 embryonic quail chorioallantoic membranes were stained with the indicated antibodies to label tubulins, with phalloidin to label F-actin, and Dapi to label nuclei within endothelial cells. A subapical distribution of modified tubulins is noted in the developing embryonic quail vasculature along with a basal distribution for F-actin; this recapitulates what we observe using our in vitro EC tubulogenesis model (A). L indicates lumen space. Bar equals 20 μm.
EB1, p150Glued, and Clasp1 control accumulation of acetylated and detyrosinated tubulins in a subapical distribution during EC tubulogenesis in 3D collagen matrices. (A) ECs were treated with control (luciferase), p150Glued, Clasp1, or EB1 siRNAs and suspended within 3D collagen matrices for 24 hours. After fixation, cultures were immunostained with antibodies to the indicated tubulins and modified tubulins. Cultures were costained with AlexaFluorX-conjugated phalloidin to label F-actin and Dapi to label nuclei. Bar equals 20 μm. (B) Day 5 embryonic quail chorioallantoic membranes were stained with the indicated antibodies to label tubulins, with phalloidin to label F-actin, and Dapi to label nuclei within endothelial cells. A subapical distribution of modified tubulins is noted in the developing embryonic quail vasculature along with a basal distribution for F-actin; this recapitulates what we observe using our in vitro EC tubulogenesis model (A). L indicates lumen space. Bar equals 20 μm.
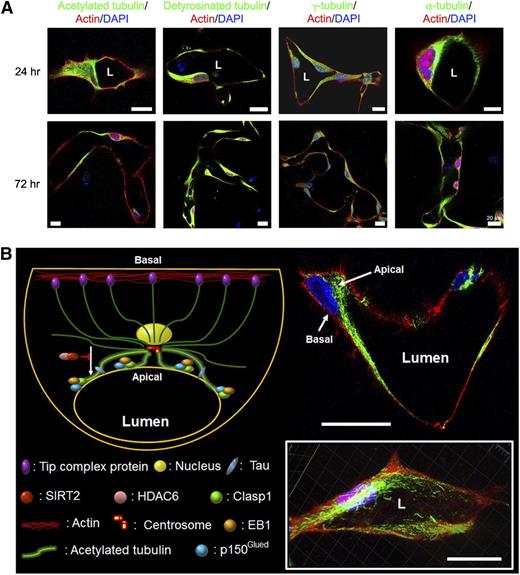
Posttranslationally modified tubulins, including acetylated and detyrosinated tubulins, accumulate in a subapical membrane location during EC tubulogenesis in 3D extracellular matrices, while F-actin is predominantly observed in a basal distribution. (A) ECs were cultured in 3D collagen matrices for 24 or 72 hours and then fixed for immunostaining for the indicated molecules. L indicates lumen space. Bar equals 20 μm. (B) The 72-hour cultures were stained for acetylated tubulin (green) and F-actin (red); a representative image is shown in the upper right. A 3D reconstruction of these images is shown in the lower right. L indicates lumen space. Bars equal 20 μm. The schematic diagram illustrates major findings in this work including a fundamental role for microtubule assembly and posttranslational modifications in EC lumen and tube formation by supporting the development and maintenance of the apical membrane surface and, thus, controlling cytoskeletal apical–basal polarization of EC-lined tubes.
Posttranslationally modified tubulins, including acetylated and detyrosinated tubulins, accumulate in a subapical membrane location during EC tubulogenesis in 3D extracellular matrices, while F-actin is predominantly observed in a basal distribution. (A) ECs were cultured in 3D collagen matrices for 24 or 72 hours and then fixed for immunostaining for the indicated molecules. L indicates lumen space. Bar equals 20 μm. (B) The 72-hour cultures were stained for acetylated tubulin (green) and F-actin (red); a representative image is shown in the upper right. A 3D reconstruction of these images is shown in the lower right. L indicates lumen space. Bars equal 20 μm. The schematic diagram illustrates major findings in this work including a fundamental role for microtubule assembly and posttranslational modifications in EC lumen and tube formation by supporting the development and maintenance of the apical membrane surface and, thus, controlling cytoskeletal apical–basal polarization of EC-lined tubes.
Discussion
Considerable progress has made in recent years in the elucidation of the molecular mechanisms underlying how ECs form lumens and tubes within 3D matrix environments.35-37 However, many key questions remain, including how EC tubes are stabilized and polarized during the tubulogenic process. A previous study from our laboratory demonstrated a fundamental role for microtubules in governing the shape and stability of EC-lined tubes. We demonstrated that microtubule depolymerization, but not actin disassembly, led to rapid collapse of tubes in 3D matrices.40 This latter tube collapse response required RhoA activation, a GTPase-mediated event that we have shown, in a variety of studies, actively inhibits lumen and tube formation.35,40 In the current study, we advance our understanding of how microtubule regulatory proteins control this critical vascular tubulogenic process. Our work demonstrates a major role for the microtubule tip complex proteins, EB1, p150Glued, and Clasp1 as well as their associated regulators, including the tubulin deacetylases, SIRT2 and HDAC6, in controlling EC lumen and tube formation in 3D extracellular matrices.
Previous work demonstrates critical roles for microtubules in neuronal morphogenesis and axonal stability. Interestingly, disruptions in microtubule function underlie the pathogenesis of various neurodegenerative disorders including Alzheimer and Parkinson diseases.44,45 Many studies have demonstrated functional overlaps in molecules and signaling pathways that control morphogenesis and stabilization of the vascular and nervous systems.46 The novel work presented here demonstrates a similar fundamental role for microtubule assembly and tubulin posttranslational modifications that occur downstream of EB1-, p150Glued-, and Clasp1-dependent signaling, which allows for EC lumen formation, cytoskeletal apical–basal polarity, and physical support for the developing apical membrane during this tubulogenic process. It is clear that these microtubule tip complex proteins are key regulators of the EC lumen formation mechanism because they play a major role in controlling a kinase cascade as well as an MT1-MMP–dependent cell surface proteolytic mechanism that are required for EC lumen formation35,36 (Figures 1 and 2). In addition, they control tubulin posttranslational modifications such as acetylation and detyrosination, which directly correlate with their ability to regulate lumen and tube formation (Figures 2, 5, and 6). Furthermore, our data show that both acetylated and detyrosinated tubulins accumulate in a subapical membrane band that is positioned to support the apical domain of these tubes in vitro and in vivo, while F-actin is predominantly distributed in a basal fashion during these events (Figures 6 and 7; supplemental Figures 5 and 6; supplemental Videos 6 and 7). Also, p150Glued and Clasp1 are distributed in a colocalizing manner in this subapical position, along with posttranslationally modified α tubulin and γ tubulin with additional overlapping staining of EB1 (Figure 4).
Our data provide important insight into mechanisms that underlie the development of EC polarity during tubulogenesis. Although known polarity regulators such as Cdc42, Par6, and Par3 have been shown to be critical during EC lumen and tube formation in 3D matrices,35,36,47 mechanistic understanding of how this leads to apical vs basal specialization in ECs during these events has been unknown. One key point is that EC lumen polarity appears to be quite distinct from epithelial lumen polarity where actin-based apical constriction and strong accumulation of apical membrane F-actin occur during tubulogenesis.48 In contrast, we show here that during EC tubulogenesis, F-actin accumulates along the basal membrane surface, with much less accumulation in an apical distribution (see schematic diagram in Figure 7B). Importantly, our data show that a major cytoskeletal system, the microtubule cytoskeleton, underlies and critically supports the apical plasma membrane to allow EC tubes to form and be stabilized (Figure 7B). Microtubule cytoskeletal disruption following the addition of colchicine (supplemental Figure 4) or vinblastine (but not cytochalasin B) results in rapid collapse of tube structures in 3D matrices, suggesting that the shape and structure of EC-lined tubes is primarily tubulin based.40 Furthermore, colchicine also caused a rapid loss of acetylated tubulin, which accompanies the disappearance of apical membrane polarity during EC tube collapse in 3D matrices (and concomitant loss of basal F-actin localization; supplemental Figure 4). Previously, we reported that microtubule depolymerization led to EC tube collapse and RhoA activation, a GTPase that needs to be suppressed during EC lumen formation and tube stabilization.40 Many genes appear to contribute to this inactivated RhoA state, including Cdc42, Rac1, Rasip1, and the cerebral cavernous malformation (CCM) proteins, CCM1, CCM2, and CCM3. Interestingly, all of these molecules control EC lumen and tube formation.35-37 Also, Pak-4, a key kinase downstream of Cdc42 activation that controls EC lumen formation,47 is also known to control microtubule assembly49 and to contribute to RhoA inactivation through blockade of GEF-H1, a microtubule-binding Rho guanine exchange factor.50 siRNA suppression of EB1, p150Glued, and Clasp1 leads to marked reductions in Pak4 activation (Figure 2B), suggesting that these molecules contribute to EC tube formation and stability through this mechanism. Clearly, more work is necessary to understand how these different interacting partners affect the EC lumen and tube formation process through control of microtubule structure and function (and thereby influence Rho GTPase activity such as Cdc42 and Rac1 activation and RhoA inactivation).
Expression of a C-terminal deletion mutant of EB1, which eliminates its known binding domain for p150Glued and Clasp133 in ECs, leads to inhibition of lumen formation (Figure 3). In addition, expression of a mutant EB1 that fails to interact with another tip complex protein, APC, also blocks lumen formation. EB1 siRNA–treated ECs fail to form lumens in 3D matrices, but their lumen formation ability could be restored through expression of wild-type EB1, but not these mutant EB1 constructs (Figure 3). Thus, EB1 and its interacting partners, p150Glued and Clasp1, are necessary for EC lumen and tube formation. We also demonstrated that these molecules control critical tubulin posttranslational modifications including acetylation and detyrosination, which are strongly induced during the lumen formation process. siRNA suppression of EB1, p150Glued, and Clasp1 markedly reduces these modifications, and this correlates with a lack of EC lumen and tube formation (Figures 2 and 6). Interestingly, the tubulin acetylases, SIRT2 and HDAC6, regulate tubulin acetylation during these events and also control the lumen formation process (Figure 5). Increased expression of both acetylases blocks lumen formation, while combined siRNA suppression of these acetylases increases lumen formation (Figure 5). Also, SIRT2 and HDAC6 are known to interact with each other in order to regulate their affinity for tubulin. Furthermore, HDAC6 is a known binding partner for EB1. Thus, it appears that during EC lumen formation, EB1, p150Glued, and Clasp1 lead to suppression of SIRT2 and HDAC6 deacetylase activities in order to facilitate increased tubulin acetylation and thereby stimulate the lumen and tube formation process. Another contributing molecule to this process is tau, a known microtubule stabilizing protein, that also influences EC lumen formation and tubulin acetylation and detyrosination (supplemental Figure 3A-B).
Overall, this novel work highlights a critical functional role for microtubules and their regulators as major controllers of the dynamic process of vascular tube morphogenesis in 3D extracellular matrices (Figure 7B). They control tube formation and tube stability by affecting key signal transduction events downstream of Rho GTPases and by controlling apical–basal cytoskeletal polarity to stabilize the apical membrane, a necessary step in shaping biologic tube structures.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from the National Institutes of Health (HL59373 and HL87308 to G.E.D.).
Authorship
Contribution: D.J.K. performed experiments; D.J.K., L.M.-L., and G.E.D. designed experiments, analyzed data, made the figures, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: George E. Davis, Margaret Proctor Mulligan Professor of Medical Research, Department of Medical Pharmacology and Physiology School of Medicine, University of Missouri-Columbia, MA415 Medical Sciences Building, One Hospital Dr, Columbia, MO 65212; e-mail: davisgeo@health.missouri.edu.