Key Points
IFN-γ impairs maintenance of HSCs by directly reducing their proliferative capacity and impairing their restoration upon viral infection.
IFN-γ induces SOCS1 expression in HSCs, which inhibits TPO-induced STAT5 phosphorylation, thereby deregulating key cell-cycle genes.
Abstract
Balancing the processes of hematopoietic stem cell (HSC) differentiation and self-renewal is critical for maintaining a lifelong supply of blood cells. The bone marrow (BM) produces a stable output of newly generated cells, but immunologic stress conditions inducing leukopenia increase the demand for peripheral blood cell supply. Here we demonstrate that the proinflammatory cytokine interferon-γ (IFN-γ) impairs maintenance of HSCs by directly reducing their proliferative capacity and that IFN-γ impairs restoration of HSC numbers upon viral infection. We show that IFN-γ reduces thrombopoietin (TPO)-mediated phosphorylation of signal transducer and activator of transcription (STAT) 5, an important positive regulator of HSC self-renewal. IFN-γ also induced expression of suppressor of cytokine signaling (SOCS) 1 in HSCs, and we demonstrate that SOCS1 expression is sufficient to inhibit TPO-induced STAT5 phosphorylation. Furthermore, IFN-γ deregulates expression of STAT5-mediated cell-cycle genes cyclin D1 and p57. These findings suggest that IFN-γ is a negative modulator of HSC self-renewal by modifying cytokine responses and expression of genes involved in HSC proliferation. We postulate that the occurrence of BM failure in chronic inflammatory conditions, such as aplastic anemia, HIV, and graft-versus-host disease, is related to a sustained impairment of HSC self-renewal caused by chronic IFN-γ signaling in these disorders.
Introduction
Since most mature blood cells have a limited life span, the hematopoietic system in the bone marrow (BM) continuously generates new cells in order to maintain sufficient numbers in the periphery. This process of hematopoiesis is hierarchically organized, with the most potent cells, the hematopoietic stem cells (HSCs), on top. These can give rise to new HSCs through self-renewal, but they can also generate all blood cell lineages via step-wise differentiation to downstream progenitors and fully mature cells. A large fraction of HSCs remain quiescent (nonproliferating) during homeostasis, and thus do not contribute to hematopoiesis. A combination of cell-intrinsic (transcription factors) and external (cytokines, membrane-bound molecules) regulatory mechanisms control the balance among quiescence, self-renewal, and differentiation, which is critical in preserving HSCs and generating sufficient numbers of differentiated mature blood cells.
Whereas this balance is rather constant during the steady-state situation, hematopoietic stress conditions, like infection and inflammation, dramatically alter this equilibrium and change the hematopoietic output from the BM (reviewed in Takizawa et al1 ). Hematopoietic stem and progenitor cells can directly sense the presence of pathogens,2 but they can also be influenced by proinflammatory cytokines, such as interleukin (IL) 1,3 tumor necrosis factor α,4,5 granulocyte colony-stimulating factor,6 and interferon-α.7 We have recently shown that interferon-γ (IFN-γ), upon production by activated T cells in the BM, also has a profound impact on the hematopoietic system, as it suppresses the formation of several hematopoietic lineages, such as B cells,8 erythrocytes,9 and eosinophilic10 and neutrophilic granulocytes.11 BM failure in multiple chronic inflammatory diseases has been associated with elevated IFN-γ levels,12-15 and the beneficial effect of immune-suppressive drugs on hematopoietic function in BM-suppressed patients might result from a reduction in IFN-γ–producing lymphocytes.16 HSCs can also be affected by IFN-γ, but there are contradictory reports on the functional consequences of IFN-γ on the self-renewal and maintenance of HSCs.17-20 Moreover, the underlying molecular mechanism by which IFN-γ would affect HSCs remains obscure. Hence, we examined the functional consequences of IFN-γ on HSCs and we found that IFN-γ inhibits, in a direct manner, the proliferation of HSCs and their recovery after viral infection. We also provide a molecular mechanism by which IFN-γ impairs HSC proliferation. We believe that these findings can explain the detrimental effect of IFN-γ on hematopoiesis in chronic inflammatory diseases.
Materials and methods
Mice
Wild-type (WT) C57BL/6 (CD45.2), C57BL/6.SJL (CD45.1), litters of WT C57BL/6 and C57BL/6.SJL (CD45.1.2), and IFN-γ−/− C57BL/6 (CD45.2) mice were used. IFN-γR1−/− C57BL/6 (CD45.2) (stock number 3288) mice were obtained from The Jackson Laboratory. All animals were housed at the animal research institute of the Academic Medical Center under specific-pathogen–free conditions. Animal experiments were approved by the Animal Ethics Committee and performed in accordance with institutional and national guidelines. For lymphocytic choriomeningitis virus (LCMV) infection, mice were injected intraperitoneally with 1 × 105 plaque-forming units of LCMV clone Armstrong in 200 μL phosphate-buffered saline (PBS).
Transplantations
For transplantation of cultured HSCs, CD45.1 and CD45.1.2 cultured cells were pooled, thoroughly washed in PBS, mixed with 2 × 105 CD45.2 whole BM cells, and injected intravenously into CD45.2 mice lethally irradiated with a split dose of 10 Gy. For WT:IFN-γR1−/− chimeras, 2 × 106 WT CD45.1.2 whole BM cells were mixed with 2 × 106 IFN-γR1−/− CD45.2 and injected into irradiated WT CD45.1 recipients. Recipient mice received 1.75 g/L neomycin in sterile water for the first 3 weeks after transplantation. Chimeric mice were infected with LCMV 2 months after transplantation.
Quantitative real-time PCR
RNA was extracted using Trizol (Invitrogen) and complementary DNA was made with random hexamers and Superscript II reverse transcriptase (Roche). Quantitative real-time polymerase chain reaction (PCR) was performed in duplicate with Express SYBR GreenER reagents (Invitrogen) on the StepOnePlus RT-PCR system (Applied Biosystems), and data were normalized using 18S as a reference gene. Primer sequences are listed in supplemental Table 1 on the Blood Web site.
Cell cultures
For 7-day cultures, 150 HSCs (Lin−c-Kit+Sca-1+CD48−CD150+) were sorted directly in 96-well plates cultured in X-VIVO 15 medium (Lonza) supplemented with 5 ng/mL thrombopoietin (TPO), stem cell factor (SCF), IL-3, IL-6, and Flt3-L. IFN-γ (20 ng/mL) was added when indicated. Cells were cultured at 37°C in a humidified incubator at 5% CO2. For signal transducer and activator of transcription (STAT) 5 analysis and RNA collection, HSCs (Lin−c-Kit+Sca-1+CD48−CD150+) were cultured for 24 hours with SCF or SCF and TPO in the presence or absence of IFN-γ. All cytokines were obtained from Peprotech. For 5-bromo-29-deoxyuridine (BrdU) incorporation, HSCs (Lin−c-Kit+Sca-1+CD34−), short-term HSCs and multipotent progenitors (Lin−c-Kit+Sca-1+CD34+) and common myeloid progenitors (CMPs) (Lin−c-Kit+Sca-1−CD34+CD16/32low) were purified and cultured for 18 hours with SCF or SCF and IFN-γ for 18 hours. A total of 10 μM BrdU (Sigma) was added for 18 hours (HSCs) or for the last 3 hours of culture (CD34+LKS and CMP). For carboxyfluorescein succinimidyl ester (CFSE) labeling, purified HSCs (Lin−c-Kit+Sca-1+CD48−CD150+) were washed in PBS, labeled with 0.5 μM CFSE (Invitrogen) in PBS for 12 minutes at 37°C, washed, and subsequently cultured.
Flow cytometry
FACSCanto II and FACSAria were used for flow cytometric analysis and cell sorting. For cell sorting, whole BM was stained with the biotinylated lineage markers CD4 (GK1.5), CD8α (53-6.7), B220 (RA3-6B2), CD11b (M1/70), Gr1 (RB6-8C5), Ter119 (Ly-76), and IL-7Rα (B12-1) and enriched for progenitors by negative depletion of labeled lineage cells using streptavidin microbeads and MACS LS-Columns (Miltenyi Biotec). Cells were stained with CD34 (RAM34), Sca-1 (D7), fluorochrome-conjugated streptavidin, CD48 (HM-48-1), CD150 (TCF15-12F12.2, Biolegend), CD16/32 (93), and c-Kit. HSCs in all experiments were purified as Lin−c-Kit+Sca-1+CD48−CD150+. For purification of HSCs from LCMV-infected chimeric mice, cells were stained with additional antibodies against CD45.1 (A20) and CD45.2 (104) and purified as CD45.1+ or CD45.2+ Lin−c-Kit+CD48−CD150+. For the BrdU incorporation assay, cells were purified as Lin−c-Kit+Sca-1+CD34− (HSCs), Lin−c-Kit+Sca-1+CD34+ (short-term HSCs and multipotent progenitors), and Lin−c-Kit+Sca-1−CD34+CD16/32low (CMPs). For immunophenotypic characterization of HSCs in culture or in mice, cells were stained as above, with additional antibodies against CD45.1 (A20) and CD45.2 (104) when required, and identified as Lin−c-Kit+CD48−CD150+ in all experiments. Dead cells were excluded by stringent gating on single cells and by using propidium iodide. For staining of phosphorylated STAT1 (pSTAT1) and phosphorylated STAT5 (pSTAT5), cultured cells were directly fixed for 10 minutes at 37°C by adding an equal volume of BD Cytofix/Cytoperm buffer (BD Biosciences), chilled on ice for 1 minute, washed, and fixed with −20°C 90% methanol for a least 1 hour. Cells were washed and incubated with pSTAT1 (K51-856/STAT1 [pS727]) or pSTAT5 antibody (47/STAT5a [pY694], both from BD Biosciences) at room temperature for 45 minutes. For detection of incorporated BrdU, cells were treated as described elsewhere.21 BrdU and DNA content was visualized using anti-BrdU (PRB-1) and propidium iodide. All antibodies were obtained from eBioscience, unless indicated otherwise.
Retroviral transduction of BM cells
Pinco retroviral vectors encoding murine suppressor of cytokine signaling (SOCS) 1 or an empty control in an internal ribosomal entry site green fluorescent protein backbone22 were transfected into the Plat-E packaging cell line using Turbofect (Fermentas) according to the manufacturer’s instructions. Supernatants were collected 48 and 72 hours posttransfection and coated on Retronectin-precoated (Takara) plates. Lineage-depleted BM cells were cultured overnight in serum-free StemSpan medium (Stem Cell Technologies) supplemented with 10 ng/mL of murine SCF and human fibroblast growth factor 1 and 20 ng/mL of murine TPO and murine insulin growth factor 2 and subsequently cultured on virus-coated plates for 48 hours, followed by TPO stimulation with or without IFN-γ and intracellular staining for pSTAT5, as described above.
Statistics
Mean values plus or minus standard deviation (SD) or standard error of the mean (SEM) are shown. Statistical analysis was performed using a 2-tailed Student t test with GraphPad Prism software and significance is indicated by *P < .05; **P < .01; or ***P < .001.
Results
IFN-γ reduces HSC maintenance in vitro
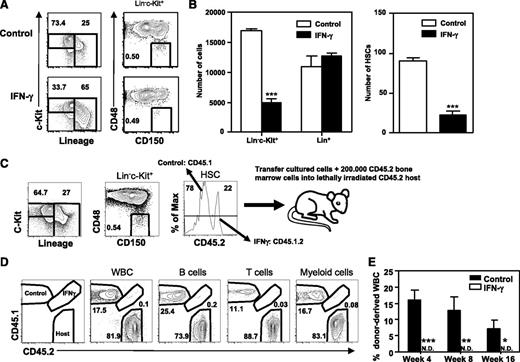
To address the effect of IFN-γ on HSC function, we cultured highly purified HSCs (Lin−c-Kit+Sca-1+CD48−CD150+) with cytokines supporting both self-renewal and differentiation of HSCs with or without IFN-γ. IFN-γ increased the percentage but not the absolute number of differentiated cells (Lin+), whereas it decreased both the percentage and absolute number of HSCs (Lin−c-Kit+CD48−CD150+) and progenitor cells (Lin−c-Kit+; Figure 1A-B). To assess the functional capacity of these remaining phenotypical HSCs, control and IFN-γ–cultured cells were mixed and transplanted into lethally irradiated recipient mice, using the congenic marker CD45 to distinguish IFN-γ–treated (CD45.1.2) and control donor cells (CD45.1) from host cells (CD45.2; Figure 1C). These experiments showed long-term, multilineage reconstitution of control cells, but a complete failure of IFN-γ–treated cells (Figure 1D), thus demonstrating that in vitro treatment with IFN-γ reduces HSC numbers both phenotypically and functionally.
IFN-γ reduces HSC maintenance in vitro. HSCs (Lin−c-Kit+Sca-1+CD48−CD150+) were purified and cultured for 7 days with SCF/TPO/IL-3/IL-6/Flt3-L with or without IFN-γ. Representative plots showing the analysis for progenitors (Lin−c-Kit+), differentiated cells (Lin+), and HSCs (Lin−c-Kit+CD48−CD150+) (A) and absolute numbers of these cells (B). Data represent 3 independent experiments with 4 to 5 wells per condition. (C) HSCs from CD45.1 and CD45.1.2 mice were cultured (CD45.1 HSCs without IFN-γ, CD45.1.2 HSCs with IFN-γ), pooled and analyzed, and injected into CD45.2 recipient mice. (D) Representative plots showing donor contribution to total white blood cells and B, T, and myeloid cell lineages in peripheral blood of lethally irradiated CD45.2 recipient mice at 4 weeks after transplantation. (E) Donor contribution to total white blood cells at indicated weeks (n = 8). The experiment was repeated with CD45.1.2 HSCs cultured without IFN-γ and CD45.1 HSCs with IFN-γ with similar results. Mean values ± SEM are shown. *P < .05; **P < .01; ***P < .001. N.D., not detectable; WBC, white blood cells.
IFN-γ reduces HSC maintenance in vitro. HSCs (Lin−c-Kit+Sca-1+CD48−CD150+) were purified and cultured for 7 days with SCF/TPO/IL-3/IL-6/Flt3-L with or without IFN-γ. Representative plots showing the analysis for progenitors (Lin−c-Kit+), differentiated cells (Lin+), and HSCs (Lin−c-Kit+CD48−CD150+) (A) and absolute numbers of these cells (B). Data represent 3 independent experiments with 4 to 5 wells per condition. (C) HSCs from CD45.1 and CD45.1.2 mice were cultured (CD45.1 HSCs without IFN-γ, CD45.1.2 HSCs with IFN-γ), pooled and analyzed, and injected into CD45.2 recipient mice. (D) Representative plots showing donor contribution to total white blood cells and B, T, and myeloid cell lineages in peripheral blood of lethally irradiated CD45.2 recipient mice at 4 weeks after transplantation. (E) Donor contribution to total white blood cells at indicated weeks (n = 8). The experiment was repeated with CD45.1.2 HSCs cultured without IFN-γ and CD45.1 HSCs with IFN-γ with similar results. Mean values ± SEM are shown. *P < .05; **P < .01; ***P < .001. N.D., not detectable; WBC, white blood cells.
IFN-γ impairs HSC proliferation
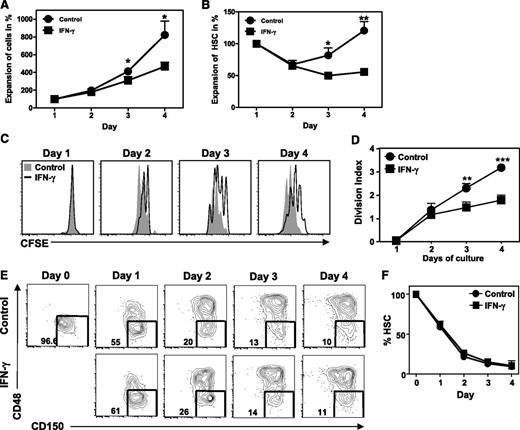
To analyze at what level IFN-γ affects HSC maintenance in these cultures, purified HSCs were labeled with CFSE, cultured for 4 days with IFN-γ, and analyzed daily. IFN-γ impaired the expansion of total cells in culture (Figure 2A) as well as HSC numbers (Figure 2B), which could not be attributed to increased cell death (supplemental Figure 1A-B). Based on CFSE dilution, we conclude that the reduction in HSC numbers was due to an inhibitory effect of IFN-γ on HSC proliferation (Figure 2C-D). Reduction in HSC proliferation was not due to an increased commitment to differentiate, since IFN-γ did not change the percentage of self-renewing CD48−CD150+ HSCs and more committed CD48+CD150+ progenitors, only their absolute number (Figure 2E-F). As HSCs are predominantly quiescent in naive mice, we tested whether IFN-γ influences this nonproliferative state. However, overnight culture with IFN-γ did not change the expression of the proliferation marker Ki67 (supplemental Figure 2A-B), which is absent in quiescent HSCs, or the incorporation of BrdU in HSCs and downstream progenitors (supplemental Figure 2C-D). This demonstrates that IFN-γ does not directly affect HSC quiescence or cell-cycle entry, which corresponds with the observation that significant differences in HSC proliferation could only be observed after 3 days of culture (Figure 2A-D). We therefore conclude that IFN-γ does not affect recruitment of quiescent HSCs into proliferation, but rather inhibits their subsequent self-renewal divisions.
IFN-γ impairs proliferation of HSCs. Purified HSCs (Lin−c-Kit+CD48−CD150+) were labeled with CFSE and cultured for 4 days with SCF/TPO/IL-3/IL-6/Flt3-L with or without IFN-γ. (A) Expansion of total cells in culture relative to day 1. (B) Expansion of HSCs (Lin−c-Kit+CD48−CD150+) relative to day 1. Histograms (C) and division index (defined as the average number of divisions that a cell that was present in the starting population has undergone) (D) of CFSE-labeled HSCs cultured with or without IFN-γ. (E) Representative plots showing percentage of HSC (Lin−c-Kit+CD48−CD150+) in cultures and (F) quantification of these data. Histograms and plots are representative of 3 independent experiments. Graphs represent data pooled from 3 independent experiments with duplicate cultures. Mean values ± SEM are shown. *P < .05; **P < .01; ***P < .001.
IFN-γ impairs proliferation of HSCs. Purified HSCs (Lin−c-Kit+CD48−CD150+) were labeled with CFSE and cultured for 4 days with SCF/TPO/IL-3/IL-6/Flt3-L with or without IFN-γ. (A) Expansion of total cells in culture relative to day 1. (B) Expansion of HSCs (Lin−c-Kit+CD48−CD150+) relative to day 1. Histograms (C) and division index (defined as the average number of divisions that a cell that was present in the starting population has undergone) (D) of CFSE-labeled HSCs cultured with or without IFN-γ. (E) Representative plots showing percentage of HSC (Lin−c-Kit+CD48−CD150+) in cultures and (F) quantification of these data. Histograms and plots are representative of 3 independent experiments. Graphs represent data pooled from 3 independent experiments with duplicate cultures. Mean values ± SEM are shown. *P < .05; **P < .01; ***P < .001.
IFN-γ inhibits HSC recovery upon viral infection
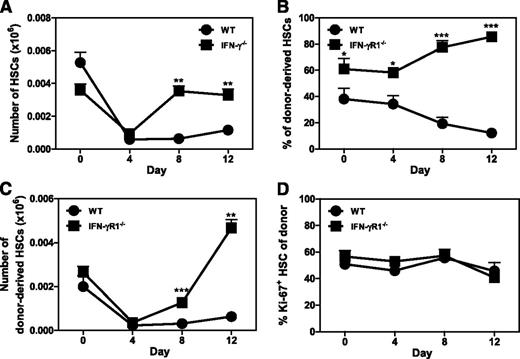
To examine whether IFN-γ has the same effect on HSC function in vivo, we infected WT and IFN-γ−/− mice with the Armstrong strain of LCMV. LCMV infection reduces BM output and induces leukopenia, which is completely dependent on production of type I interferons early after infection.23 LCMV thereby poses significant pressure on the hematopoietic system to restore blood cell homeostasis. As expected, HSC (Lin−c-Kit+CD48−CD150+) numbers were equally reduced in WT and IFN-γ−/− mice at day 4 of infection. However, HSC numbers were restored to normal levels in IFN-γ−/− mice at day 8, while only a slight recovery of WT HSCs was observed at day 12 (Figure 3A). Impaired recovery of WT HSCs around day 8 paralleled the occurrence of IFN-γ–producing LCMV-specific CD8 T cells.11,24 To exclude indirect effects of IFN-γ on HSCs, we generated mixed chimeric mice with BM from both WT and IFN-γR1−/− mice. Two months after transplantation, donor chimerism of HSCs from IFN-γR1−/− mice was higher that of WT mice (Figure 3B, day 0), suggesting that homeostatic levels of IFN-γ already influence HSC self-renewal. Upon LCMV infection, HSC numbers of both donors dropped equally early after infection, but IFN-γR1−/− HSCs recovered much better than their WT counterparts within the same host (Figure 3C). Importantly, there was no difference in HSC quiescence (Figure 3D), indicating that the impaired recovery of WT HSCs numbers was due to an IFN-γ–dependent reduction in the proliferative capacity of HSCs rather than a decrease in the fraction of cycling HSCs. We therefore conclude that IFN-γ directly reduces HSC proliferation, both in vitro and in vivo.
IFN-γ inhibits HSC recovery after LCMV infection. (A) WT and IFN-γ−/− mice were infected with LCMV and the number of HSCs (Lin−c-Kit+CD48−CD150+) was measured by flow cytometry at indicated days (n = 3-5). Data represent 3 independent experiments. Mixed BM-chimeric mice were generated with WT (CD45.1) and IFN-γR1−/− (CD45.2) BM (1:1 ratio), infected with LCMV, and donor HSC chimerism (B), donor HSC numbers (C), and proliferation of donor HSCs (D) were measured at indicated days (n = 3-5). Data are representative of 2 independent experiments. Mean values ± SEM are shown. *P < .05; **P < .01; ***P < .001.
IFN-γ inhibits HSC recovery after LCMV infection. (A) WT and IFN-γ−/− mice were infected with LCMV and the number of HSCs (Lin−c-Kit+CD48−CD150+) was measured by flow cytometry at indicated days (n = 3-5). Data represent 3 independent experiments. Mixed BM-chimeric mice were generated with WT (CD45.1) and IFN-γR1−/− (CD45.2) BM (1:1 ratio), infected with LCMV, and donor HSC chimerism (B), donor HSC numbers (C), and proliferation of donor HSCs (D) were measured at indicated days (n = 3-5). Data are representative of 2 independent experiments. Mean values ± SEM are shown. *P < .05; **P < .01; ***P < .001.
IFN-γ impairs TPO signaling
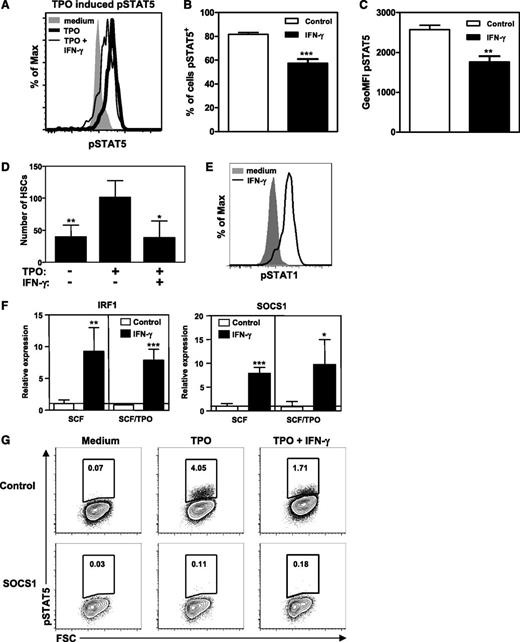
To investigate the molecular mechanism by which IFN-γ regulates HSC proliferation, we examined both the extrinsic and intrinsic pathways that mediate this process. The cytokine TPO is important for HSC self-renewal, as its receptor, c-mpl, can induce STAT5 phosphorylation and thereby regulate transcription of self-renewal genes.25-27 Competitive repopulation capacity of STAT5−/− or c-mpl−/− HSCs28,29 is severely impaired, while constitutively active STAT5 enhances the self-renewal and repopulation activity of HSCs.25 We found that IFN-γ reduced TPO-mediated phosphorylation of STAT5 in purified HSCs (Figure 4A-C) and fully annihilated TPO-driven HSC expansion (Figure 4D). IFN-γ induced phosphorylation of STAT1 in purified LKS cells (Figure 4E) and IRF-1 expression in HSCs, thus confirming direct activation of IFN-γR signaling, but also induced expression of SOCS1 (Figure 4F), a negative regulator of IFN-γR signaling through its ability to inhibit STAT1 phosphorylation.30 However, SOCS1 can also inhibit STAT5 phosphorylation,30 which can thereby explain the perturbed TPO signaling in HSCs by IFN-γ. To test if expression of SOCS1 is sufficient for the inhibition of TPO-mediated STAT5 phosphorylation, lineage-depleted BM cells were retrovirally transduced with a vector encoding SOCS1 or empty vector as a control. This revealed a complete abrogation of TPO-mediated STAT5 phosphorylation in SOCS1-expressing cells, while cells transduced with control vector showed normal TPO-mediated STAT5 phosphorylation that could be inhibited by IFN-γ (Figure 4G).
IFN-γ reduces TPO-mediated STAT5 phosphorylation. (A) Histogram showing phosphorylated STAT5 staining of purified HSCs (Lin−c-Kit+CD48−CD150+) cultured for 24 hours with SCF (shaded histogram), SCF and TPO (bold histogram), or SCF, TPO, and IFN-γ (thin histogram). Percentage of cells stained positive for pSTAT5 (B) and geometric mean fluorescence intensity of pSTAT5 (C). Data represent triplicate cultures and are representative of 2 independent experiments. (D) Purified HSCs were cultured as in Figure 1 with or without IFN-γ and/or TPO and HSC numbers were measured. Data are representative of 2 experiments with 4 wells per condition. (E) Histogram showing pSTAT1 staining of purified LKS cells stimulated for 45 minutes with SCF (shaded histogram) or SCF and IFN-γ (bold histogram). Histograms are representative of 4 independent experiments. (F) Messenger RNA expression levels of IRF-1 and SOCS1 in HSCs cultured for 24 hours with SCF or SCF and TPO with or without IFN-γ. Expression levels are relative to the expression in SCF cultured HSCs. Quantitative PCRs were performed in duplicate and data are pooled from 2 independent experiments with 1 to 3 cultures per condition. (G) Plots showing pSTAT5 staining of lineage-depleted BM cells after retroviral transduction with a vector encoding SOCS1 or with an empty vector as a control. Cells were cultured for 48 hours followed by TPO stimulation with or without IFN-γ and intracellular staining for pSTAT5. pSTAT5 staining of transduced (green fluorescent protein positive) cells is shown. Plots are representative of 3 independent experiments. Mean values ± SD are shown. *P < .05; **P < .01; ***P < .001. GeoMFI, geometric mean fluorescence intensity.
IFN-γ reduces TPO-mediated STAT5 phosphorylation. (A) Histogram showing phosphorylated STAT5 staining of purified HSCs (Lin−c-Kit+CD48−CD150+) cultured for 24 hours with SCF (shaded histogram), SCF and TPO (bold histogram), or SCF, TPO, and IFN-γ (thin histogram). Percentage of cells stained positive for pSTAT5 (B) and geometric mean fluorescence intensity of pSTAT5 (C). Data represent triplicate cultures and are representative of 2 independent experiments. (D) Purified HSCs were cultured as in Figure 1 with or without IFN-γ and/or TPO and HSC numbers were measured. Data are representative of 2 experiments with 4 wells per condition. (E) Histogram showing pSTAT1 staining of purified LKS cells stimulated for 45 minutes with SCF (shaded histogram) or SCF and IFN-γ (bold histogram). Histograms are representative of 4 independent experiments. (F) Messenger RNA expression levels of IRF-1 and SOCS1 in HSCs cultured for 24 hours with SCF or SCF and TPO with or without IFN-γ. Expression levels are relative to the expression in SCF cultured HSCs. Quantitative PCRs were performed in duplicate and data are pooled from 2 independent experiments with 1 to 3 cultures per condition. (G) Plots showing pSTAT5 staining of lineage-depleted BM cells after retroviral transduction with a vector encoding SOCS1 or with an empty vector as a control. Cells were cultured for 48 hours followed by TPO stimulation with or without IFN-γ and intracellular staining for pSTAT5. pSTAT5 staining of transduced (green fluorescent protein positive) cells is shown. Plots are representative of 3 independent experiments. Mean values ± SD are shown. *P < .05; **P < .01; ***P < .001. GeoMFI, geometric mean fluorescence intensity.
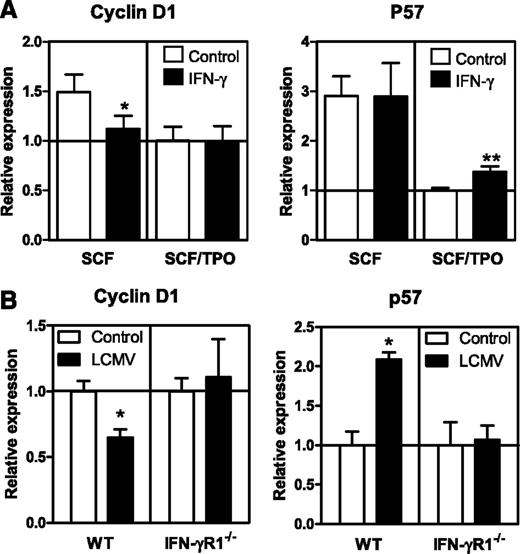
IFN-γ regulates expression of self-renewal genes
Next, we investigated the impact of IFN-γ on molecular mediators of HSC proliferation and found that IFN-γ significantly reduced the expression of cyclin D1 and inhibited TPO-mediated downregulation of the cell-cycle inhibitor p57 (Figure 5A). Cyclin D1 and p57 are both important mediators of HSC self-renewal, and their expression is associated with TPO signaling and STAT5 expression.26,27 These findings are also relevant for the in vivo situation, as cyclin D1 and p57 were significantly downregulated and upregulated, respectively, in WT HSCs but not in IFN-γR1−/− HSCs from mixed BM-chimeric mice upon LCMV infection (Figure 5B). The combined decrease in STAT5 phosphorylation and changes in expression of these key cell-cycle genes provide a molecular explanation of how IFN-γ negatively affects the proliferative capacity of HSCs both in vitro and in vivo.
IFN-γ affects expression of genes involved in cell cycle regulation. (A) Messenger RNA expression levels of cyclin D1 and p57 in HSCs cultured for 24 hours with SCF or SCF and TPO with or without IFN-γ. Expression levels are relative to the expression in SCF/TPO cultured HSCs. Quantitative PCRs were performed in duplicate and data are pooled from 2 independent experiments with 1 to 3 cultures per condition. (B) Messenger RNA expression levels of indicated genes in WT and IFN-γR1−/− HSCs from chimeric mice 8 days after LCMV infection. Expression levels are relative to the expression in WT and IFN-γR1−/− HSCs from naive WT: IFN-γR1−/− chimeric mice. Quantitative PCRs were performed in duplicate of 2 or 3 pooled samples of purified HSCs. Mean values ± SD are shown. *P < .05; **P < .01.
IFN-γ affects expression of genes involved in cell cycle regulation. (A) Messenger RNA expression levels of cyclin D1 and p57 in HSCs cultured for 24 hours with SCF or SCF and TPO with or without IFN-γ. Expression levels are relative to the expression in SCF/TPO cultured HSCs. Quantitative PCRs were performed in duplicate and data are pooled from 2 independent experiments with 1 to 3 cultures per condition. (B) Messenger RNA expression levels of indicated genes in WT and IFN-γR1−/− HSCs from chimeric mice 8 days after LCMV infection. Expression levels are relative to the expression in WT and IFN-γR1−/− HSCs from naive WT: IFN-γR1−/− chimeric mice. Quantitative PCRs were performed in duplicate of 2 or 3 pooled samples of purified HSCs. Mean values ± SD are shown. *P < .05; **P < .01.
Discussion
Our conclusion that IFN-γ directly inhibits HSC proliferation supports previous studies with human HSCs demonstrating that IFN-γ inhibits HSC self-renewal.18-20 However, our findings differ from a model recently reported in Nature concluding that IFN-γ rather induces proliferation of quiescent murine HSCs and causes a concomitant decrease in myeloid progenitors.17 We postulate that these conflicting findings can be explained in part by indirect effects of IFN-γ on other cell types, which we have carefully excluded by treating HSCs with IFN-γ after rather than before cell sorting and by using WT:IFN-γR1−/− mixed chimeric mice for in vivo studies. Another possible explanation is related to the use of Sca-1 for identification of HSCs and progenitor cells. Sca-1 is an interferon-responsive molecule that is highly upregulated by IFN-γ on all hematopoietic progenitors upon treatment in vitro (supplemental Figure 3A) or infection in vivo (supplemental Figure 3B). As a consequence, myeloid progenitor cells (normally Sca-1−), of which a fraction express CD150, become Sca-1+ and thereby substantially contaminate the HSC pool and reduce the myeloid progenitor fraction (supplemental Figure 3C). We ruled out this contamination by not using Sca-1 when identifying progenitor cells that had been exposed to interferons and by excluding CD48+ myeloid progenitors. As shown previously,31 Sca-1 is not required when HSCs are identified as Lin−c-Kit+CD48−CD150+ cells, both in vivo (supplemental Figure 3D) and in vitro (supplemental Figure 3E). Since myeloid progenitor cells are actively cycling cells and thus not quiescent, any contamination of the HSC fraction with these cells due to Sca-1 upregulation will lead to a reduction in the number of quiescent HSCs. However, when carefully avoiding this contamination, we found no evidence that IFN-γ had any direct influence on the quiescent state of HSCs (supplemental Figure 3). Importantly, Sca-1 upregulation most likely also explains other studies that have reported an infection- and/or interferon-induced increase in HSC numbers.7,32,33
Although we found no direct evidence that IFN-γ induced differentiation of HSCs, differentiation of more downstream progenitors was enhanced by IFN-γ (supplemental Figure 4). This supports our previous finding that production of IFN-γ in the BM upon viral infection is beneficial, as it strongly enhances the formation of monocytes.11 Moreover, we do not exclude that IFN-γ acts as an inducer of HSC differentiation in vivo, since different BM niches regulate the diverse fates of HSCs, like quiescence, self-renewal, and differentiation.34 Thus, it could be that IFN-γ impairs HSC self-renewal in order to relocalize these cells to a microenvironment that supports differentiation. In addition, IFN-γ–induced differentiation of downstream progenitors might indirectly induce differentiation of HSCs to compensate for the declining numbers of downstream progenitors. Alternatively, sustained production of IFN-γ might reduce HSC self-renewal in order to prevent exhaustion and loss of HSC activity resulting from excessive HSC proliferation during hematopoietic stress. In both cases, chronic exposure to IFN-γ results in declining HSC numbers and impaired hematopoietic output. Based on our findings, we postulate that this is due to perturbed TPO signaling, since IFN-γ induces the expression of SOCS1 in HSCs, which is sufficient to inhibit TPO-induced activation of STAT5 (Figure 4). Interestingly, IFN-γ also perturbs granulocyte colony-stimulating factor–mediated phosphorylation of STAT3 in a SOCS3-dependent manner and thereby actively inhibits neutrophil differentiation.11 Similarly, IFN-γ also induces the expression of SOCS1 in developing B cells and it thereby inhibits their responsiveness to IL-7.35 It is thus important to further investigate this emerging concept that SOCS-mediated inhibition of STAT-driven cytokine receptors is part of the mechanism underlying the negative impact that interferons have on hematopoiesis.
Although temporary inhibition of HSC proliferation during acute infection will not immediately influence peripheral blood cell numbers, prolongation of this process will threaten the maintenance and/or restoration of blood cell homeostasis. A better understanding of the underlying mechanism is also clinically important, because increased IFN-γ production has been associated with BM failure in patients with chronic inflammation, such as aplastic anemia, Fanconi anemia, and HIV.12-15 In fact, IFN-γ neutralization improves the in vitro capacity of hematopoietic progenitors from patients with aplastic anemia.36 Furthermore, IFN-γ also plays an important role in the pathogenesis of graft-versus-host disease37 and disease progression of chronic myeloid leukemia.38 Our study suggests that the negative impact of IFN-γ on HSC proliferation contributes to the impaired hematopoietic function in multiple human diseases, and we speculate that repression of IFN-γ signaling could alleviate the hematopoietic suppression in patients with chronic inflammation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Claudia Brandão, Klaas van Gisbergen, Sten Libregts, Katarina Ochodnicka-Mackovicova, and Astrid Eichler for technical assistance and Prof Dr Rene van Lier, Dr Marieke von Lindern, Dr Esther Nolte-‘t Hoen, and Dr Monika Wolkers for critical reading of the manuscript.
This work is supported by grants from the German Cancer Aid (Deutsche Krebshilfe 108699 and 108688) (C.H.B.), the German Research Foundation (Deutsche Forschungsgemeinschaft SFB834) (C.H.B.), The Netherlands Organization of Scientific Research (VIDI grant 91776310) (M.A.N.), and the Landsteiner Foundation for Blood Transfusion Research (LSBR fellowship #1014) (M.A.N.).
Authorship
Contribution: A.M.d.B. designed and performed experiments, analyzed the data, and wrote the paper; Ö.D. designed and performed experiments and analyzed the data; B.H. performed sorting experiments; C.H.B. designed and supervised experiments and analyzed the data; and M.A.N. designed and supervised experiments and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Martijn A. Nolte, Department of Hematopoiesis, Sanquin Research and Landsteiner Laboratory AMC/UvA, Plesmanlaan 125, 1066 CX Amsterdam, The Netherlands; e-mail: m.nolte@sanquin.nl.