Key Points
Risk stratification treatment of t(8;21) acute myeloid leukemia may decrease relapse and improve long-term survival.
Allo-HSCT benefited high-risk patients, but impaired the survival of low-risk patients.
We aimed to improve the outcome of t(8;21) acute myeloid leukemia (AML) in the first complete remission (CR1) by applying risk-directed therapy based on minimal residual disease (MRD) determined by RUNX1/RUNX1T1 transcript levels. Risk-directed therapy included recommending allogeneic hematopoietic stem cell transplantation (allo-HSCT) for high-risk patients and chemotherapy/autologous-HSCT (auto-HSCT) for low-risk patients. Among 116 eligible patients, MRD status after the second consolidation rather than induction or first consolidation could discriminate high-risk relapse patients (P = .001). Allo-HSCT could reduce relapse and improve survival compared with chemotherapy for high-risk patients (cumulative incidence of relapse [CIR]: 22.1% vs 78.9%, P < .0001; disease-free survival [DFS]: 61.7% vs 19.6%, P = .001), whereas chemotherapy/auto-HSCT achieved a low relapse rate (5.3%) and high DFS (94.7%) for low-risk patients. Multivariate analysis revealed that MRD status and treatment choice were independent prognostic factors for relapse, DFS, and OS. We concluded that MRD status after the second consolidation may be the best timing for treatment choice. MRD-directed risk stratification treatment may improve the outcome of t(8;21) AML in CR1. This trial was registered at http://www.chictr.org as #ChiCTR-OCH-12002406.
Introduction
Although acute myeloid leukemia (AML) with t(8;21) generally has a favorable prognosis, relapse occurs in ∼40% of cases, and long-term survival is <50%.1,,,,,,,-9 Patients with a KIT mutation exhibit an even higher relapse rate (up to 70%) and poor survival rates.10,-12 Once patients experience a relapse, their outcomes are extremely poor even after receiving allogeneic hematopoietic stem cell transplantation (allo-HSCT).4,-6 Therefore, rapid identification of high-risk relapse patients and preemptively treating them with more aggressive therapy, such as HSCT, may decrease relapse and improve survival.
Cytogenetics is the most powerful predictor of prognosis in AML, and integrating additional genetic data may further improve prediction.13,-15 However, minimal residual disease (MRD) monitoring during treatment remains an efficient tool to estimate an individual leukemia’s susceptibility to chemotherapy and enhance the delivery of risk-directed therapies.16,17 More recently, a trial of a prospective MRD-directed risk stratification strategy successfully led to improvement in the outcomes of high-risk pediatric AML patients.18
AML with t(8;21) harbors a specific fusion gene, RUNX1/RUNX1T1, that provides an ideal target for MRD monitoring. Several small, retrospective cohort studies have suggested that monitoring MRD using real-time quantitative reverse transcriptase-polymerase chain reaction (RQ-PCR) may identify patients at high risk for relapse.19,,,,-24 Two studies found that a reduction in MRD (RUNX1/RUNX1T1 transcript levels) of <3 logs compared with pretreatment levels was a significant predictor of relapse after 2 courses of consolidation or within 3 to 4 months after achieving complete remission (CR).19,21 Because HSCT exhibits the strongest anti-leukemia effects, we hypothesize that HSCT can decrease the relapse rate and improve the outcomes of patients with t(8;21) AML who are classified as high risk according to their MRD status. Therefore, we performed a prospective multicenter cohort study to determine whether risk-directed treatment according to MRD status could decrease relapse rates and improve long-term survival of t(8;21) AML patients. We also investigated whether allo-HSCT could improve the outcomes of high-risk and KIT-mutated patients with t(8;21) AML.
Methods
Patients
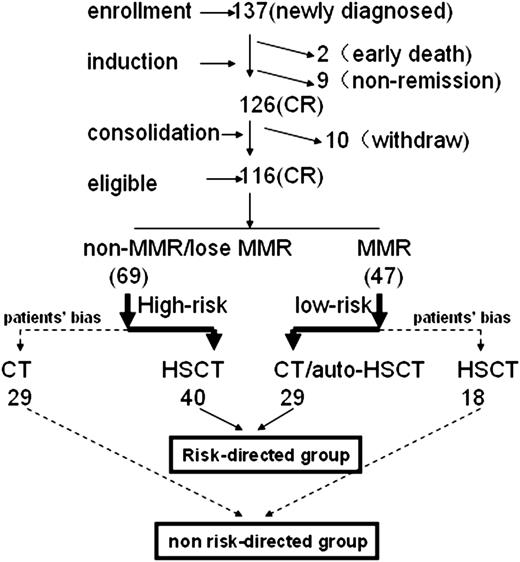
One hundred thirty-seven newly diagnosed AML patients with t(8;21) were enrolled at 3 centers between June 2005 and December 2011. The following inclusion criteria were applied: (1) 14 to 60 years old; (2) newly diagnosed AML with t(8;21) and/or RUNX1/RUNX1T1 transcripts and had achieved CR after 2 induction cycles; and (3) no contraindications to consolidation chemotherapy or HSCT. Figures 1 and 2 present the treatment scheme and study protocol. This protocol was approved by the institutional review boards at each center. Written informed consent was obtained from all patients or guardians before entry into the study in accordance with the Declaration of Helsinki.
Trial design and patient accrual flowchart. Eighty-two and 34 patients achieved CR after 1 and 2 induction cycles. Sixty-three of 116 patients received anthracycline during consolidation cycles 1 and 2, including 45 mg/m2 daunorubicin or 8 mg/m2 mitoxantrone for 3 days. CT, chemotherapy.
Trial design and patient accrual flowchart. Eighty-two and 34 patients achieved CR after 1 and 2 induction cycles. Sixty-three of 116 patients received anthracycline during consolidation cycles 1 and 2, including 45 mg/m2 daunorubicin or 8 mg/m2 mitoxantrone for 3 days. CT, chemotherapy.
MRD monitoring and KIT mutation screening
Bone marrow samples were collected from patients at the time of diagnosis, after induction therapy, after every consolidation chemotherapy, and then at 3-month intervals for 1 year. In total, 978 bone marrow samples (median, 8 samples per patient) were collected. MRD was monitored using RQ-PCR to quantify the level of RUNX1/RUNX1T1 transcripts. Total RNA was extracted with TRIzol reagent (Invitrogen) according to the manufacturer’s instructions. PCR reaction conditions have been previously published.25 Each RQ-PCR reaction and analysis were performed on an AB 7500 platform. Primers and probes for the RUNX1/RUNX1T1 gene and the control gene ABL have been previously described.25 c-KIT mutations in exons 17 and 8 were screened using the direct sequencing method. We did not include the c-KIT mutation screening in the initial protocol. After the prognostic value of the c-KIT mutation was confirmed in 2006, we only added to detect the c-KIT mutation without modifying the treatment protocol according to KIT status. In total, the pretreatment bone marrow samples for 84 patients were successfully sequenced, whereas the KIT mutational status of 32 patients was undetermined because of unavailable or poor-quality samples.
Diagnosis and treatment response assessment
The diagnosis of AML was performed as previously described.26 CR was defined as <5% bone marrow blasts, the absence of blasts with Auer rods, the absence of extramedullary disease, an absolute neutrophil count >1.0 × 109/L, and a platelet count >100 × 109/L with no red cell transfusions. Relapse was defined as the recurrence of ≥5% bone marrow blasts and the reappearance of blasts in the blood or the development of extramedullary disease infiltrates at any site.
Definitions of MMR and risk groups
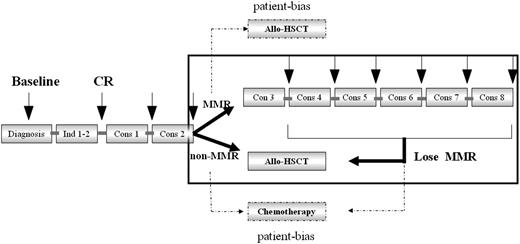
Major molecular remission (MMR) was defined as a >3-log reduction in RUNX1/RUNX1T1 transcripts (<0.4%) compared with the pretreatment baseline of 388% in our center, as previously described.25 Loss of MMR was defined as RUNX1/RUNX1T1 transcript levels >0.4% in MMR patients. The high-risk group was defined as those patients not achieving MMR after the second consolidation therapy19,21 or those exhibiting the loss of MMR within 6 months of achieving MMR. The low-risk group was defined as patients who achieved MMR after the second consolidation therapy and maintained MMR for 6 months thereafter.
Treatment protocols
Induction chemotherapy was composed of 1 to 2 cycles of induction with an anthracycline (either daunorubicin at 45 mg/m2 or idarubicin at 8-10 mg/m2 for 3 days) in combination with cytarabine at 100 mg/m2 for 7 days. The first and second consolidation therapies included intermediate-dose cytarabine (IDAC; 1-2 g/m2 every 12 hours for 3 days) with or without an anthracycline (daunorubicin at 45 mg/m2 or mitoxantrone at 8 mg/m2 for 3 days). High-risk patients defined as above were recommended for allo-HSCT, and low-risk patients were recommended to continue 6 cycles of chemotherapy, including IDAC (1-2 g/m2 every 12 hours for 3 days) for 2 cycles and then 45 mg/m2 daunorubicin or 8 to 10 mg/m2 idarubicin for 3 days plus 100 mg/m2 cytarabine for 7 days; 2 mg/m2 homoharringtonine for 7 days plus 100 mg/m2 cytarabine for 7 days; 8 mg/m2 mitoxantrone for 3 days plus 100 mg/m2 cytarabine for 7 days; and 20 mg/d aclamycin for 7 days plus 100 mg/m2 cytarabine for 7 days. Autologous-HSCT (auto-HSCT) was permitted in low-risk patients after 4 courses of consolidation. Patients who lost MMR within 6 months of the second consolidation were redefined as high-risk patients and recommended for allo-HSCT (Figures 1 and 2). Allo-HSCT required an HLA-matched sibling donor (MSD), a matched unrelated donor (MUD), or a haploidentical related donor (HRD) HSCT. For MSD and HRD HSCT patients, granulocyte-colony-stimulating factor–mobilized bone marrow and peripheral blood stem cells were used as a graft source. For MUD HSCT patients, only granulocyte-colony-stimulating factor–mobilized peripheral blood stem cells were used as a graft source. The HSCT conditioning regimen and graft-versus-host disease prophylaxis were previously described.27
Patients who could not comply with the risk-directed treatment recommendations described above due to patient bias, which meant that low-risk patients preferred to undergo allo-HSCT and high-risk patients preferred to undergo chemotherapy as postremission treatment, were categorized into the non–risk-directed therapy group (Figure 2).
End points and statistical methods
The primary end points studied were relapse and disease-free survival (DFS). The secondary end point was overall survival (OS). DFS was measured from the date when CR was achieved. Events for DFS included death in first CR (CR1) or relapse. The event for OS was death (regardless of the cause), and patients were queried at the date of last follow-up to determine whether they were still alive. At a significant level of 5%, the study included 106 patients, providing an overall power of 80% for a 2-sided test to detect a difference of 25% between the 2 groups, assuming that 1 group had a relapse rate of 45%.
Survival functions were estimated using the Kaplan-Meier method and compared using the log-rank test. To exclude bias that may arise from including patients who relapsed or died too early to receive allo-HSCT in CR1 in high-risk patients, landmark analysis was used when comparing the outcomes of patients receiving allo-HSCT with those receiving chemotherapy/auto-HSCT.28 In our study, the median time from CR achievement to allo-HSCT was 4 months. Thus, we defined 4 months after achieving CR as a landmark for this analysis.
Cox regression model was also used to identify prognostic variables for the patients, which included c-KIT status (mutation or no mutation), treatment choice (risk directed or not risk directed), white blood cell (WBC) count at diagnosis (<20 or ≥20 × 109/L), CD56 (positive or negative), additional chromosome abnormalities (yes or no), and the number of courses required to achieve CR1 (1 or >1 course). The independence of categorical parameters was calculated using either a χ2 or Fisher’s exact test. The distribution of the continuous variables was calculated using a Mann-Whitney U test. Any probability mentioned in this study is the probability at 5 years.
Results
Patient characteristics
A total of 137 newly diagnosed patients with t(8;21) were enrolled in this study (Figure 1). Twenty-one of these patients were excluded from the final analysis due to death during induction (n = 2), failure to achieve CR after 2 courses of induction chemotherapy (n = 9), or withdrawal before the second consolidation treatment (n = 10; Figure 2). Eight-two patients and 34 patients achieved CR after 1 and 2 cycles of induction, respectively. Therefore, we analyzed 116 patients composed of 69 patients who received risk-directed treatment and 47 patients (18 low risk and 29 high risk) who received non–risk-directed treatment due to patient bias. Potential reasons for the 18 low-risk patients to be withdrawn from the trial were loss of Y or X chromesome (n = 10), KIT-mutation (n = 5), extramedullary disease (n = 2), and patient’s persistence (n = 1). The potential reasons for the 29 high-risk patients to be withdrawn from the trial might result from complex factors such as fear of transplantation-related mortality, donors available, and consulting with other doctors about treatment options. There was no significant difference in the presenting features between the risk-directed and non–risk-directed groups (Table 1).
Characteristics of t(8;21) AML
| Characteristic . | Risk directed . | Not risk directed . | |||||
|---|---|---|---|---|---|---|---|
| Low risk—chemo (n = 29) . | High risk—HSCT (n = 40) . | Total . | Low risk—HSCT (n = 18) . | High risk—Chemo (n = 29) . | Total . | P . | |
| Age (years) | 28 (15-56) | 37 (15-54) | 33 (15-56) | 35 (16-54) | 37 (15-60) | 36 (15-60) | NS |
| Gender (male/female) | 16/16 | 26/14 | 39/30 | 12/6 | 16/13 | 28/19 | NS |
| WBC (109/L) | 5.4 (1.4-79) | 9.0 (1.9-59) | 8.1 (1.4-79) | 6.0 (1.2-30) | 8.0 (2.5-80.5) | 7.2 (1.2-80.5) | NS |
| HGB (g/L) | 83 (34-136) | 81 (49-133) | 81(45-136) | 95 (41-123) | 74 (34-135) | 78 (34-135) | NS |
| PLT (109/L) | 37 (5-286) | 27 (4-108) | 32.5 (4-286) | 33 (2-106) | 35 (7-225) | 34 (2-225) | NS |
| Blasts in BM (%) | 52 (26-89) | 51 (20-86) | 52(20-89) | 56 (21-82) | 39 (24-92) | 46 (21-92) | NS |
| RUNX1-RUNX1T1 (%) | 506 (353-993) | 411(97-1082) | 439 (35-1082) | 422 (98-848) | 358(52-1163) | 380 (43-1163) | NS |
| KIT mutation (%) | 17.6(3/17) | 54.8(17/31) | 54.8(20/48) | 50(5/10) | 34.6(9/26) | 38.9(14/36) | NS |
| Comorbidity score (0/1-2/3)* | 23/17/0 | 18/11/0 | 41/28/0 | 16/13/0 | 11/7/0 | 27/20/0 | NS |
| Characteristic . | Risk directed . | Not risk directed . | |||||
|---|---|---|---|---|---|---|---|
| Low risk—chemo (n = 29) . | High risk—HSCT (n = 40) . | Total . | Low risk—HSCT (n = 18) . | High risk—Chemo (n = 29) . | Total . | P . | |
| Age (years) | 28 (15-56) | 37 (15-54) | 33 (15-56) | 35 (16-54) | 37 (15-60) | 36 (15-60) | NS |
| Gender (male/female) | 16/16 | 26/14 | 39/30 | 12/6 | 16/13 | 28/19 | NS |
| WBC (109/L) | 5.4 (1.4-79) | 9.0 (1.9-59) | 8.1 (1.4-79) | 6.0 (1.2-30) | 8.0 (2.5-80.5) | 7.2 (1.2-80.5) | NS |
| HGB (g/L) | 83 (34-136) | 81 (49-133) | 81(45-136) | 95 (41-123) | 74 (34-135) | 78 (34-135) | NS |
| PLT (109/L) | 37 (5-286) | 27 (4-108) | 32.5 (4-286) | 33 (2-106) | 35 (7-225) | 34 (2-225) | NS |
| Blasts in BM (%) | 52 (26-89) | 51 (20-86) | 52(20-89) | 56 (21-82) | 39 (24-92) | 46 (21-92) | NS |
| RUNX1-RUNX1T1 (%) | 506 (353-993) | 411(97-1082) | 439 (35-1082) | 422 (98-848) | 358(52-1163) | 380 (43-1163) | NS |
| KIT mutation (%) | 17.6(3/17) | 54.8(17/31) | 54.8(20/48) | 50(5/10) | 34.6(9/26) | 38.9(14/36) | NS |
| Comorbidity score (0/1-2/3)* | 23/17/0 | 18/11/0 | 41/28/0 | 16/13/0 | 11/7/0 | 27/20/0 | NS |
There are no differences in the characteristics between the low risk—chemotherapy group and the low risk—HSCT group and between the high risk—chemotherapy group and the high risk—HSCT group. HGB, hemoglobin; PLT, platelet; BM, bone marrow; NS, not significant.
According to the Charlson Comorbidity Index.
Fifty-eight patients received chemotherapy-based consolidation therapy (including 17 auto-HSCT), and 58 patients received allo-HSCT in CR1 (MSD, 30 patients; MUD, 5 patients; HRD, 23 patients). The similar outcomes of auto-HSCT and chemotherapy as consolidation therapy were confirmed (data were not shown). There were no significant differences in transplantation outcomes between the 23 patients who received HRD HSCT and the 35 patients who received MSD/MUD HSCT (supplemental Appendix on the Blood website).
Patient outcomes
The median follow-up time was 36 months (6-83 months) in surviving patients. Of the 116 patients analyzed, 29 died (18 of relapse; 11 of treatment-related mortality) during treatment and 87 survived; 33 patients experienced a relapse. For the entire study population, the cumulative incidence of relapse (CIR) was 32.8%. The DFS and OS rates were 58.8% and 69.3%, respectively.
Impact of MRD on relapse at different checkpoints
MRD status (non-MMR vs MMR) at the end of induction could not discriminate high-risk relapse patients (P = .87 for CIR) and had a trend to discriminate agaist high-risk relapse patients (P = .06 for CIR) at the end of first consolidation. However, it could significantly discriminate high-risk relapse patients at the second consolidation (CIR, 46.9% vs 22.9%; P = .001).
Relapse
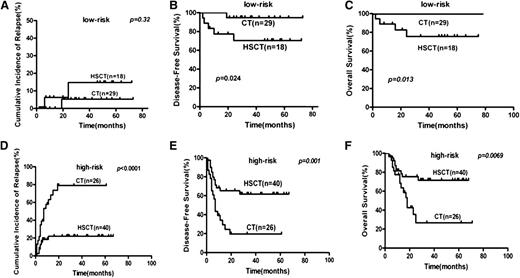
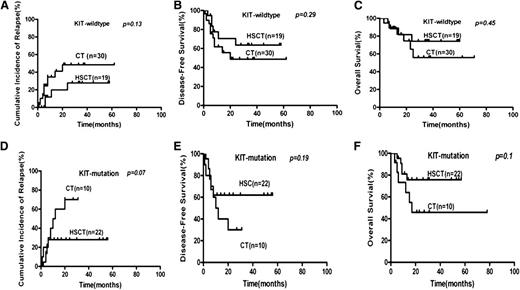
By subgroup analysis, allo-HSCT lowered the relapse rate compared with chemotherapy for high-risk patients using the landmark analysis (CIR, 22.1% vs 78.9%, P < .0001; Figure 3), even for high-risk patients without achieving MMR after second consolidation (CIR, 20.6% vs 100%; P < .0001; n = 47). Allo-HSCT did not lower the relapse rate compared with chemotherapy for low-risk patients (14.7% vs 5.3%; P = .33). Allo-HSCT had a trend to lower the relapse of KIT-mutated patients (CIR, 28% vs 70%; P = .07; Figure 4). When we took all patients that received traditional high-dose cytarabine-based consolidation (according to National Comprehensive Cancer Network [NCCN] guidelines) as a subgroup (n = 58), risk-directed therapy also decreased the relapse rate (CIR, 15.0% vs 54.6%; P = .0066). To avoid the influence of KIT mutation, we also analyzed the data in KIT wild-type patients (n = 50) and found that risk-directed therapy also may be superior to the traditional approach (CIR, 11.4% vs 50.1%; P = .003).
Subgroup analysis of risk stratification treatments and outcomes of t(8;21) AML. (A,D) Relapse. (B,E) DFS. (C,F) OS. HSCT, hematopoietic stem cell transplantation; CT, chemotherapy.
Subgroup analysis of risk stratification treatments and outcomes of t(8;21) AML. (A,D) Relapse. (B,E) DFS. (C,F) OS. HSCT, hematopoietic stem cell transplantation; CT, chemotherapy.
Outcome by KIT gene mutations and treatments of t (8;21) AML patients. The comparison of (A,D) relapse, (B,E) DFS, and (C,F) OS between patients receiving hematopoietic stem cell transplantation (HSCT) and chemotherapy (CT).
Outcome by KIT gene mutations and treatments of t (8;21) AML patients. The comparison of (A,D) relapse, (B,E) DFS, and (C,F) OS between patients receiving hematopoietic stem cell transplantation (HSCT) and chemotherapy (CT).
The 5-year CIR of risk-directed therapy was 15.0%. The CIR of high-risk patients receiving allo-HSCT was 22.1%, and low-risk patients receiving chemotherapy/auto-HSCT was 5.3%.
Based on multivariate analysis, MRD status (high risk vs low risk), treatment strategy (risk directed vs not risk directed), and KIT status (mutation vs no mutation) were all independent risk factors for relapse (Table 2). The hazard ratio for relapse was 8.85 (95% confidence interval [CI], 2.05-38.13; P = .003) for high-risk patients, 0.26 (95% CI, 0.12-0.61; P = .002) for risk-directed treatment, and 2.12 (95% CI, 1.01-4.48; P = .049) for mutant KIT patients. Other variables such as age (<35 vs ≥35 years), high WBC count at diagnosis (>20 × 109/L vs ≤20 × 109/L), CD56 expression (positive vs negative), additional chromosome abnormalities (yes vs no), and the number of courses to achieve CR (1 vs >1) did not influence the risk of relapse.
Multivariable analysis of prognostic factors
| Covariate . | CIR P . | DFS P . | OS P . |
|---|---|---|---|
| MRD (high vs low risk) | .0030 | .0020 | .020 |
| Treatment (risk directed vs not risk directed) | .026 | .036 | .037 |
| KIT (mutation vs wild type) | .049 | NS | NS |
| BM blasts (≥50% vs <50%) | NS | NS | NS |
| WBC (≥20 vs <20) | NS | NS | NS |
| CD56 (positive vs negative) | NS | NS | NS |
| Age (<35 vs ≥35 years old) | NS | NS | NS |
| CR (1 vs >1 course) | NS | NS | NS |
| Cytogenetic (with vs without additional changes) | NS | NS | NS |
| Covariate . | CIR P . | DFS P . | OS P . |
|---|---|---|---|
| MRD (high vs low risk) | .0030 | .0020 | .020 |
| Treatment (risk directed vs not risk directed) | .026 | .036 | .037 |
| KIT (mutation vs wild type) | .049 | NS | NS |
| BM blasts (≥50% vs <50%) | NS | NS | NS |
| WBC (≥20 vs <20) | NS | NS | NS |
| CD56 (positive vs negative) | NS | NS | NS |
| Age (<35 vs ≥35 years old) | NS | NS | NS |
| CR (1 vs >1 course) | NS | NS | NS |
| Cytogenetic (with vs without additional changes) | NS | NS | NS |
BM, bone marrow.
Disease-free survival
Landmark analysis was used when comparing the DFS of patients receiving allo-HSCT with those receiving chemotherapy/auto-HSCT, which defined 4 months after achieving CR as a landmark.By subgroup analysis, allo-HSCT could improve DFS rate when compared with chemotherapy for high-risk patients (61.7% vs 19.6%, P = .001; Figure 3) and had a potential to improve DFS for KIT-mutated patients (62.0% vs 30.0%, P = .19; Figure 4) using the landmark analysis. Allo-HSCT also improved DFS rate when compared with chemotherapy for high-risk patients without achieving MMR after second consolidation (DFS, 60.5% vs 0%, P < .0001, n = 47). DFS decreased in low-risk patients when compared with allo-HSCT recipients (70.3% vs 94.7%, P = .024; Figure 4). When we took all patients that received traditional high-dose cytarabine-based consolidation (according to NCCN guidelines) as a subgroup to study (n = 58), risk-directed therapy (n = 69) also improved DFS (74.7% vs 53.1%, P = .01). To avoid the influence of KIT mutation, we also analyzed the data in KIT-wild type patients (n = 50) and found that risk-directed therapy might be superior to traditional approach (DFS 80.7% vs 46.7%, P = .013).
The 5 year DFS of risk-directed therapy was 74.7%. The DFS of high-risk patients receiving allo-HSCT was 61.7%, and low-risk patients receiving chemotherapy/auto-HSCT was 94.7%.
Multivariate analysis demonstrated that MRD status and treatment strategy were independent risk factors for DFS (Table 2). The hazard ratio for DFS was 9.32 (95% CI 2.21–39.3; P = .002) for high-risk patients and 0.36 (95% CI 0.17–0.75, P = .007) for risk-directed treatment. Other variables, such as KIT mutational status, age, high WBC count, CD56 expression, additional chromosome abnormalities and the number of courses to achieve CR, did not influence the risk of DFS.
Overall survival
By subgroup analysis, allo-HSCT improved OS compared with chemotherapy for high-risk patients (71.6% vs 26.7%; P = .007) but decreased OS for low-risk patients (75.7% vs 100%; P = .013; Figure 3) using the landmark analysis. Allo-HSCT also improved OS compared with chemotherapy for high-risk patients without achieving MMR after second consolidation (OS, 69.95% vs 0%; P < .0002; n = 47). Allo-HSCT tended to improve OS compared with chemotherapy for KIT-mutated patients, but this trend did not achieve statistical significance (75.7% vs 45.8%; P = .1; Figure 4).
The 5-year OS of risk-directed therapy was 82.7%. The OS of high-risk patients receiving allo-HSCT was 71.6% and of low-risk patients receiving chemotherapy/auto-HSCT was 100%.
When we took all patients that received traditional high-dose cytarabine-based consolidation (according to NCCN guidelines) as a subgroup to study (n = 58), risk-directed therapy (n = 69) tended to have improved OS (82.7% vs 65.1%; P = .07). To avoid the influence of the KIT mutation, we also analyzed the data in KIT wild-type patients (n = 50) and found that risk-directed therapy might be potentially superior to the traditional approach (OS, 85.7% vs 55.9%; P = .17).
Multivariate analysis demonstrated that MRD status and treatment strategy were independent risk factors for DFS (Table 2). The hazard ratio for DFS was 10.53 (95% CI, 1.41-78.83; P = .022) for high-risk patients and 0.37 (95% CI, 0.15-0.93, P = .035) for risk-directed treatment. Other variables, such as KIT mutation, age, high WBC count, CD56 expression, additional chromosome abnormalities, and the number of courses to achieve CR, did not influence the risk of OS.
Discussion
In this multicenter prospective cohort study, we demonstrated that allo-HSCT could reduce relapse and improve survival compared with chemotherapy/auto-HSCT for high-risk patients, and chemotherapy/auto-HSCT achieved a low relapse rate and high survival for low-risk patients. MRD-directed risk stratification treatment may improve the outcome of patients with AML with t(8;21) in CR1.
Combining pretreatment and posttreatment parameters into an optimal, risk-directed treatment algorithm likely contributed to our improved results. Our novel risk stratification system has significant power to identify patients at high risk for relapse. As demonstrated by our results, among patients who received chemotherapy as postremission therapy, high-risk patients had a very high CIR (78.9%) compared with low-risk patients (5.3%). Multivariate analysis further demonstrated that high-risk patient status is an independent prognostic factor for relapse. Our results agree with those of 2 earlier studies in patients with t(8;21).19,21 These studies found that a reduction in the MRD of <3 logs compared with pretreatment levels was a significant predictor of relapse after 2 courses of consolidation or within 3 to 4 months after achieving CR.19,21
Identifying the best checkpoints for MRD examination is very important for applying MRD-directed risk stratification treatment. We found MRD status after second consolidation may be the best timing for MRD examination in our treatment protocol. The reasons why we chose the key checkpoint for MRD assessment after 2 courses of IDAC-based consolidation was according to 2 previous reports,19,21 which suggested that MRD levels at this time may reflect the sensitivity of leukemia cells to high-dose cytarabine. However, we did not find the predictive value of MRD at earlier checkpoints (after induction and first consolidation) as reported by Liu Yin et al,29 which might result from our relative low intense of induction (eg, daunorubicine dose, 45 mg/m2) and consolidation compared with the United Kingdom Medical Research Council AML-15 protocol. Not only the MRD level but also the kinetics of MRD is reliable for the prediction of imminent relapse. Recently published results of the United Kingdom Medical Research Council AML-15 trial showed that not only the >3 log reduction of RUNX1/RUNX1T1 transcript levels at the time of achieving CR but also rising MRD levels on serial monitoring accurately predicted hematological relapse.29 Therefore, combination monitoring of MRD levels and MRD kinetics translates into a timely, accurate prediction for relapse.
In our study, we could not only predict relapse accurately, but we could also identify who would benefit from allo-HSCT. We demonstrated that allo-HSCT could significantly lower the relapse rates of high-risk patients, translating into improved DFS and OS. Recently, Boissel et al30 reported primary results in high-risk patients (<3 log reduction before second consolidation), demonstrating that 10 of 18 patients who did not receive allo-HSCT experienced relapse compared with only 1 of 9 patients receiving allo-HSCT. The results of Boissel et al were in agreement with our study. Moreover, we found for the first time that allo-HSCT impaired the outcome of low-risk patients. The reason for the negative effect of allo-HSCT in low-risk patients may be its inability to lower the risk of relapse combined with an increase in treatment-related mortality. Our results support the perspectives reported in the NCCN guidelines that t(8;21) AML is a favorable subgroup of AML and that high-dose cytarabine-based chemotherapy/auto-HSCT is recommended as postremission therapy for low-risk patients. We also found that allo-HSCT tended to lower the relapse rates of KIT-mutated patients and improved their survival. Not reaching statistical difference may result from relatively limited numbers of KIT-mutated patients in our study (supplemental Appendix) and needs to be confirmed by future studies with large samples. Our results suggest recommending allo-HSCT to high-risk, but not low-risk, patients to avoid under- or overtreatment of t(8;21) AML, which might be a complement to current NCCN guidelines.
The primary aim of risk-directed treatment is to improve the survival of the entire patient population. Our results suggested that risk-directed treatment might improve survival (5-year DFS and OS of 74.7% and 82.7%, respectively). This improved survival advantage in the risk-directed treatment group is better than those previous reported without risk-directed treatment, including the French AML Intergroup (5-year DFS and OS of 52% and 59%, respectively; n = 154),3 the German AML Intergroup (3-year DFS and OS of 60% and 65%, respectively; n = 191),4 the Cancer and Leukemia Group B Study (5-year OS of 46%; n = 139),5 the Southwest Oncology Group/Eastern Cooperative Oncology Group/MD. Anderson Study (5-year DFS and OS of 45% and 45%, respectively; n = 174),6 and the European Group for Blood and Marrow Transplantation Study (5-year DFS: 60% for allo-HSCT and 66% for auto-HSCT; n = 166).8
However, our study has some limitations. Forty percent (47/116) of the patients went off the trial. This made it difficult to compare the outcome of risk-directed treatment and non–risk-directed treatment directly, and a randomization controlled trial is needed in the future. Similarly, we compared the efficacy of allo-HSCT and chemotherapy in a nonrandomized condition, which also was a source of bias. Although best compared in a randomized trial, no such study is in progress or is being planned. The low incidence (∼10%) of t(8;21) AML within AML makes a randomized trial enrolling >100 patients very difficult. Therefore, our study enrolling 137 patients is likely the best comparison of allo-HSCT and chemotherapy.
In summary, our results suggested that MRD-directed risk stratification treatment may improve the outcome of t(8;21) AML patients in CR1. Treatment of t(8;21) by risk stratification according to MRD status might be incorporated in clinical decision making.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Key Program of National Natural Science Foundation of China (grant 81230013), Key Clinical Program of the Ministry of Health (China, 2010), and the Beijing Municipal Science and Technology Commission (Z111107067311070).
Authorship
Contribution: H.-H.Z., X.-J.H., X.-H.Z., and Y.-Z.Q. designed the study; D.-H.L., H.J., H.C., Q.J., L.-P.X., J.L., W.H., L.B., Y.W., Y.-H.C., J.-Z.W., F.-R.W., Y.-Y.L., J.-Y.C., L.-R.W., and Y.-R.L. collected and assembled the data; K.-Y.L. and B.J. analyzed and interpreted the data; H.-H.Z. and X.-J.H. wrote the manuscript; and all authors gave final approval for the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Xiao-Jun Huang, Peking University People’s Hospital, Peking University Institute of Hematology, 11 Xizhimen South St, Beijing 10044, China; e-mail: xjhrm@medmail.com.cn.
References
Author notes
H.-H.Z., X.-H.Z., and Y.-Z.Q. contributed equally to this work.