In this issue of Blood, Shindo et al show differences in signal transduction between T cells across the stages of memory differentiation, which could be exploited clinically in a novel approach for preventing acute graft-versus-host disease (GVHD).1
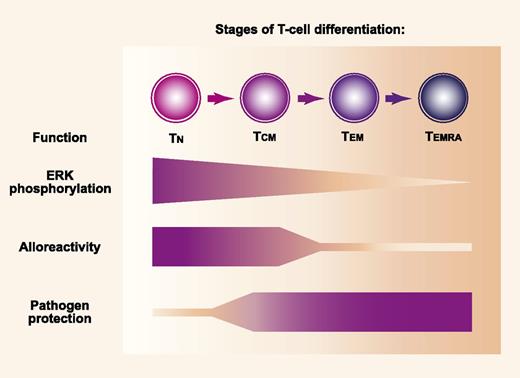
MEK-mediated phosphorylation of ERK and the repertoire of antigen specificities differ according to the stage of memory T-cell differentiation. MEK-mediated phosphorylation of ERK decreases progressively across stages of memory differentiation after activation of TN, TCM, TEM, and TEMRA cells that express CD45RA (TEMRA). Alloantigens are recognized more effectively by the TN cell population than by memory populations, while protection against pathogens is mediated more effectively by memory populations than by the TN cell population. Professional illustration by Paulette Dennis.
MEK-mediated phosphorylation of ERK and the repertoire of antigen specificities differ according to the stage of memory T-cell differentiation. MEK-mediated phosphorylation of ERK decreases progressively across stages of memory differentiation after activation of TN, TCM, TEM, and TEMRA cells that express CD45RA (TEMRA). Alloantigens are recognized more effectively by the TN cell population than by memory populations, while protection against pathogens is mediated more effectively by memory populations than by the TN cell population. Professional illustration by Paulette Dennis.
The thymus exports newly developed T cells in a “naive” stage characterized by expression of both CD45RA and CD27. After stimulation of naive T (TN) cells, asymmetric cell division differentiates effector and memory T-cell fates. Memory T cells can be divided into at least 3 distinct subsets.2 The CD45RA−CD27+ “central” memory (TCM) population migrates preferentially through lymphoid tissue and is long-lived, whereas the CD45RA−CD27− “effector” memory (TEM) population migrates preferentially in nonlymphoid tissues and is short-lived. A CD45RA+CD27− “late effector” memory (TEMRA) population of CD8 cells has been identified in humans and is closely related to the TEM population.
The TN cell population contains a wide repertoire of receptors specific for alloantigens and pathogen-associated antigens (see figure). Because the immune system is challenged far more often by pathogens than by alloantigens, the repertoire of memory populations is enriched for receptors that recognize pathogen-specific antigens and contains relatively few cells that recognize alloantigens. As compared with TN cells, TEM cells have a reduced ability to cause GVHD in mice. Data are mixed for TCM cells, with some studies showing that they cause no GVHD and others showing that they cause less severe GVHD as compared with TN cells.3 Recent data suggest that the reduced capacity of TEM cells to cause GVHD relates both to T-cell receptor repertoire-dependent and -independent properties.4,5
T-cell receptor signaling activates rat sarcoma, which triggers a mitogen-activated protein kinase relay involving sequential phosphorylation of RAF, mitogen-activated protein kinase kinase (MEK), and extracellular receptor kinase (ERK). In this signal cascade, activated RAF phosphorylates MEK, and activated MEK phosphorylates ERK. Phosphorylated ERK then enters the nucleus and contributes to the activation program of T cells through interactions with transcription factors. Shindo et al1 used MEK inhibitors to determine the extent to which ERK phosphorylation is required for activation of T cells at each stage of memory differentiation. This question was motivated by previous studies showing that inhibition of ERK phosphorylation blocked proliferation of murine T cells stimulated with alloantigen in mixed lymphocyte cultures.6
Shindo et al1 report 4 main findings. (1) ERK was phosphorylated in progressively lower proportions of activated T cells across the spectrum of memory stages. (2) Inhibition of ERK phosphorylation caused progressively less suppression of cytokine production by activated T cells across the spectrum of memory stages. (3) ERK phosphorylation was required for proliferation of human T cells after stimulation with alloantigen but not for cytokine expression after simulation with cytomegalovirus or Epstein-Barr virus–specific peptides. (4) Inhibition of ERK phosphorylation delayed the onset of acute GVHD in mice.
Memory populations are generally characterized by selection of cells with receptors having high affinity for antigen, increased expression of adhesion molecules, and distinctive cytokine production and response profiles that accelerate protective immune responses after a repeated pathogen exposure.7 The reduced dependence of memory cells on ERK phosphorylation may help to explain why they are activated more easily than naive cells. The biochemical mechanisms that give rise to these differences and the signaling pathways that enable activation of memory cells in the presence of MEK inhibitors remain to be elucidated.8
From a translational perspective, the findings of Shindo et al1 suggest that MEK inhibitors could be used to control activation of alloantigen-specific TN cells that cause GVHD, while sparing pathogen-specific memory cells needed for initial immune reconstitution after allogeneic hematopoietic cell transplantation. The results suggest that a simple pharmacologic approach could replace the far more cumbersome physical methods of separating donor naive and memory cell populations currently being explored in clinical trials to prevent GVHD. As pointed out by the authors, however, administration of a MEK inhibitor for the first 7 days after transplantation only delayed the onset of GVHD and was not sufficient to prevent GVHD. Whether a higher dose or prolonged administration of the inhibitor could prevent GVHD remains to be determined. Alternatively, improved efficacy might be gained by combining a calcineurin inhibitor with a MEK inhibitor, as suggested by in vitro results.
At least 3 major questions would remain even if MEK inhibition could prevent GVHD. The premise that MEK inhibition would spare memory T cells needed for immune reconstitution could be questioned, since previous studies have demonstrated that murine CD4 memory cells express high levels of phosphorylated ERK on day 14 after hematopoietic cell transplantation.6 Moreover, long-lived TCM cells that depend on ERK phosphorylation could have an important role in immune reconstitution after hematopoietic cell transplantation.9 Additional studies will also be needed to determine whether MEK inhibition impairs the ability of alloreactive donor T cells to prevent graft rejection10 or the ability of donor T cells to eliminate malignant T cells in the recipient after transplantation.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal