Key Points
RAS/MEK/ERK signaling is memory stage-dependent in human T cells, conferring susceptibility to alloreactive T-cell selective inhibition.
MEK inhibitors selectively inhibit alloreactive but not herpesvirus-specific human T cells and inhibit murine GVHD.
Abstract
Immunosuppressive strategies currently used in hematopoietic stem cell transplantation reliably decrease graft-versus-host disease (GVHD) rates, but also impair pathogen-specific immunity. Experimental transplant studies indicate that GVHD-initiating alloreactive T cells reside primarily in naive and central memory T-cell compartments. In contrast, virus-specific T cells comprise a more differentiated memory population. After finding that the rat sarcoma/mitogen-activated protein kinase kinase/extracellular receptor kinase (RAS/MEK/ERK) pathway is preferentially activated in naive and central memory human T cells, we hypothesized that MEK inhibitors would preferentially inhibit alloreactive T cells, while sparing more differentiated virus-specific T cells. Confirming our hypothesis, we found that MEK inhibitors including selumetinib preferentially inhibited cytokine production and alloreactivity mediated by naive and central memory human CD4+ and CD8+ T cells while sparing more differentiated T cells specific for the human herpesviruses cytomegalovirus and Epstein-Barr virus. We then demonstrated that short-term posttransplant administration of selumetinib in a major histocompatibility complex major- and minor-mismatched murine model significantly delayed the onset of GVHD-associated mortality without compromising myeloid engraftment, demonstrating the in vivo potential of MEK inhibitors in the setting of hematopoietic stem cell transplantation. These findings demonstrate that targeting memory-dependent differences in T-cell signaling is a potent and selective approach to inhibition of alloreactivity.
Introduction
Allogeneic stem cell transplantation (SCT) is the preferred treatment of many high-risk and/or relapsed hematologic malignancies. Unfortunately, graft-versus-host disease (GVHD) remains a frequent and often life-threatening complication.1,2 GVHD arises following the activation of alloreactive donor T cells that recognize host antigens.3,4 Calcineurin inhibitors (eg, cyclosporine and tacrolimus) have remained the mainstay of GVHD prevention strategies for decades, but suppress T cells indiscriminately, thereby increasing the risk of opportunistic infections, including herpesvirus reactivation. Similarly, corticosteroids, the first line of therapy for GVHD, dramatically increase the risk of serious infections, which remain the leading cause of death following GVHD.5,6 The development of selective immunosuppressive strategies that effectively inhibit alloreactivity, while sparing pathogen-specific immunity, remains an important and elusive goal.
The T-cell repertoire consists of naive T cells that have not yet encountered antigen, and progressively differentiated central memory and effector memory T-cell subsets, each characterized by distinct patterns of surface marker expression, homing, and effector functions.7 Combinations of surface markers (eg, CD45 isoforms, CCR7, CD27, CD62L) may discriminate memory compartments, given the lack of distinct molecular signatures that define and distinguish human T-cell subsets.8
In murine GVHD, increasing evidence suggests that naive and central memory T-cell subsets are more potent at inducing GVHD than effector memory cells.9-13 Initially, it was demonstrated that naive T cells, but not memory cells, were essential for GVHD induction.11 Subsequent studies confirmed that effector memory cells, in contrast to naive T cells, were poorly capable of mediating GVHD. Relative to naive and more differentiated effector memory T cells, central memory cells are intermediate in their ability to induce GVHD.12-14 Thus, the potential to induce GVHD diminishes with maturation, with little to no contribution by the most differentiated (effector memory) cells in GVHD initiation. In contrast to the relative immaturity of the most critical GVHD-initiating cells, we have shown that human CMV-specific T cells are usually highly differentiated.15 Consequently, we reasoned that selective inhibition of alloreactive T cells might be achieved by targeting a pathway that is differentially activated in naive and progressively differentiated memory cells.
Triggering of a T-cell receptor by its cognate antigen results in nearly immediate activation of downstream signaling cascades, including the rat sarcoma/mitogen-activated protein kinase kinase/extracellular receptor kinase (RAS/MEK/ERK) pathway.16 Single-cell analysis of ERK1/2 phosphorylation in murine T cells suggested that ex vivo MEK inhibition inhibited alloreactivity, suggesting the potential to ameliorate GVHD.17 MEK1/2 inhibitors are being tested for efficacy in multiple cancers dependent on RAS/MEK/ERK signaling, with little apparent hematologic toxicity reported in over 60 ongoing human clinical trials.18,19 Recently, extremely promising results have been evident in multiple cancer trials, either using MEK inhibition alone or with other targeted inhibitors.20-22 In this report, we demonstrate that MEK inhibitors selectively suppress human alloreactivity in a memory stage-dependent manner, and inhibit experimental GVHD.
Materials and methods
Drugs
Tacrolimus (FK506; Sigma-Aldrich), U0126 (Cell Signaling Technology), and selumetinib (AZD6244/selumetinib; Selleck Chemicals) were reconstituted in dimethylsulfoxide (DMSO), and stored at −20°C before adding to culture media.
Human T-cell isolation and sorting
Peripheral blood mononuclear cells (PBMCs) were obtained from healthy donor buffy coat specimens obtained following written informed consent in accordance with the Declaration of Helsinki. PBMCs were isolated by density gradient sedimentation using Ficoll-Hypaque Plus (GE Healthcare). CD4+ or CD8+ T cells were enriched by RosetteSep and EasySep (StemCell Technologies). PBMCs were stained with anti–CD4- or CD8-V450 (BD Biosciences), anti–CD45RA-Qdot605 (Life Technologies), and anti–CD27-allophycocyanin (BD Pharmingen) antibodies and sorted to >99% purity using a FACSAria cytometer (BD).
Assessment of ERK1/2 phosphorylation using phosphoflow cytometry
PBMCs were stimulated with phorbol-12-myristate-13-acetate (PMA):ionomycin (1 ng/mL:1 µM; Sigma-Aldrich) for 5 minutes in RPMI 1640 (Life Technologies) supplemented with 10% fetal bovine serum (Atlanta Biologicals), and immediately fixed and permeabilized with Fix & Perm A/B buffers (Life Technologies). Intracellular phosphorylated ERK1/2 (pERK1/2) and surface staining were analyzed using a LSR-II cytometer (BD) with FlowJo software (TreeStar) using Mac computers (Apple). Intracellular pERK1/2 was stained with rabbit anti-pERK1/2 (Cell Signaling Technology) and donkey anti-rabbit immunoglobulin G (IgG) conjugated with Alexa 647 (Life Technologies). pERK1/2 was assessed at different maturation stages (naive, central memory, effector memory, and late effector memory “EMCD45RA+” T cells).
Western blotting
CD8 T-cell subset lysates were prepared (50 mM Tris-HCl, 150 mM NaCl, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, 1% NP-40; Thermo Scientific) supplemented with protease inhibitors (Sigma-Aldrich) and centrifuged (13 000 rpm, 15 minutes at 4°C). Proteins were quantitated by modified Bradford assay (Bio-Rad). Protein (25 µg) was resolved on a 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (PAGE) gel and transferred to polyvinylidene difluoride membranes (Bio-Rad), blocked, rinsed, and incubated with rabbit anti-human RAS (Cell Signaling Technology), mouse anti–human cRAF (BD Transduction Laboratories), rabbit anti-human MEK1 (Epitomics) and MEK2 (Cell Signaling Technology), mouse anti-human ERK1/2 (Cell Signaling Technology), or mouse anti-human β-actin antibodies (Sigma-Aldrich) overnight. Signals were detected with horseradish peroxidase–conjugated anti-mouse IgG or anti-rabbit IgG (Santa Cruz Biotechnology) using an ECL system (PerkinElmer).
Mixed lymphocyte reactions
Monocytes from healthy donors were matured and differentiated to dendritic cells (DCs) with granulocyte macrophage–colony-stimulating factor (1000 IU/mL; Bayer), interleukin-4 (IL-4) (500 IU/mL; R&D Systems), IL-1β (10 ng/mL; R&D Systems), tumor necrosis factor α (TNFα) (10 ng/mL; R&D Systems), IL-6 (15 ng/mL; R&D Systems), and prostaglandin E2 (1 µg/mL; Sigma-Aldrich) for 7 days. Allogeneic PBMCs or sorted naive T cells were labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE) (Life Technologies) and cultured with 25 Gy-irradiated DCs for 7 days. Proliferation was assessed by CFSE dilution and functional differentiation by CD45RA and CD27 staining.
Cytokine flow cytometry
PBMCs were stimulated with 10 µg/mL staphylococcal enterotoxin B (SEB) (Sigma-Aldrich) or pentadecapeptide pools spanning cytomegalovirus (CMV) pp65 and Epstein-Barr virus (EBV) EBNA1 antigens (JPT) as previously described.23-25 After 1 hour, 10 µg/mL brefeldin A (Sigma-Aldrich) was added to enable accumulation of intracellular cytokines. Following an additional 5-hour incubation, cells were fixed and permeabilized with Fix & Perm A/B buffers (Life Technologies). Cells were stained with the lineage and maturation markers CD4, CD8, CD45RA, and CD27 and intracellular cytokines (interferon γ [IFNγ] and TNFα) and assessed using an LSR-II cytometer (BD Biosciences).26
BM transplantation and selumetinib administration
BALB/c mice (Charles River) were ablatively conditioned (7.5 Gy total body irradiation) using a GammaCell 40 unit (33 cGy/min) 1 day prior to transplant. B6.SJL-PtprcaPepcb/BoyJ (B6-CD45.1) mice were bred at the University of Miami School of Medicine under the care of the Division of Veterinary Resources. Bone marrow (BM) cells were obtained from femurs, tibias, and vertebrae from sex-matched B6-CD45.1 (Thy1.2+) donor animals. A single-cell suspension of marrow cells was prepared by flushing bones with a 21-gauge needle and the cells were filtered through a 100-µm nylon mesh. Donor marrow cells were depleted of T cells via complement-mediated lysis using anti–T-cell–specific antibody H0-13-4 (hybridoma supernatant, mouse anti-Thy1.2 IgM; ATCC) generously provided by Dr Bruce Blazar (University of Minnesota) and rabbit complement (Cedarlane Laboratories). The marrow cells were incubated at 37°C for 45 minutes, washed twice in RPMI, and resuspended for hematopoietic cell transplant. Marrow T-cell depletion was routinely >99%. Donor T cells were prepared from peripheral and mesenteric lymph nodes obtained from B6-Thy1.1 animals (bred and maintained at the University of Miami). Donor cells were stained for T cells (anti-CD4, clone RM4-5; anti-CD8, clone 53-6-7), adjusted to 1.2 × 106 T cells per milliliter and were pretreated for 30 minutes with vehicle (0.1% DMSO) or selumetinib (10 µM in 0.1% DMSO) prior to mixing with BM. Recipient mice were transplanted (day 0) with T-depleted BM (TCD-BM) (3 × 106) and 6.0 × 105 T cells by tail vein (intravenous) injection in 0.5 mL. From day 0 through day 7 200 µL of vehicle (methocel/polysorbate buffer) or selumetinib (25 mg/kg) was administered orally once daily.
Institutional review board and institutional animal care and use committee approvals were obtained as required.
Statistics
Results
The RAS/MEK/ERK pathway is preferentially activated in naive and central memory T cells, and is susceptible to MEK inhibition
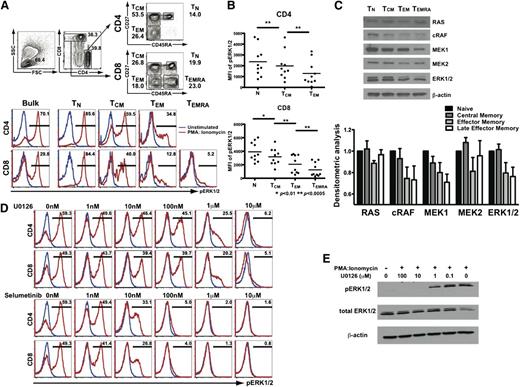
We optimized flow cytometric methods to facilitate the measurement of pERK1/2 in individual human CD4+ and CD8+ T cells. To examine the memory stage dependence of RAS/MEK/ERK activation, human PBMCs were stimulated using PMA:ionomycin and pERK1/2 analyzed within CD4+ and CD8+ T-cell subsets (naive/TN: CD45RA+CD27+; central memory/TCM; CD45RA−CD27+; effector memory/TEM: CD45RA−CD27−; and late effector memory/TEMRA; CD45RA+CD27−), using the gating strategy shown (Figure 1A top). Because CD45RA+ revertants (eg, TEMRA) cells are relatively rare in CD4+ T cells in most healthy human donors, only 2 memory T-cell subsets (TCM and TEM) were assessed in the CD4+ population, in contrast to CD8+ T cells.
T-cell activation via the RAS/MEK/ERK pathway is memory stage–dependent, and suppressed by MEK inhibition. (A) PBMCs were stimulated with PMA:ionomycin with ERK1/2 phosphorylation assessed within CD4+ and CD8+ T-cell maturation subsets. (B) Aggregate results (from n = 10 healthy donors) depicting ERK1/2 MFI within maturation subsets. MFI, mean fluorescence intensity. (C) Expression of RAS/MEK/ERK pathway members in CD8+ T cells by western blotting and collective results with densitometry values (from n = 3 healthy donors) standardized relative to expression in naive T cells. (D) ERK1/2 phosphorylation within PMA:ionomycin-activated CD4+ and CD8+ T cells with U0126 or selumetinib analyzed by phosphoflow cytometry. (E) Confirmation of dose-dependent inhibition of PMA:ionomycin-induced activation of purified CD3+ human T cells by U0126 by western blotting.
T-cell activation via the RAS/MEK/ERK pathway is memory stage–dependent, and suppressed by MEK inhibition. (A) PBMCs were stimulated with PMA:ionomycin with ERK1/2 phosphorylation assessed within CD4+ and CD8+ T-cell maturation subsets. (B) Aggregate results (from n = 10 healthy donors) depicting ERK1/2 MFI within maturation subsets. MFI, mean fluorescence intensity. (C) Expression of RAS/MEK/ERK pathway members in CD8+ T cells by western blotting and collective results with densitometry values (from n = 3 healthy donors) standardized relative to expression in naive T cells. (D) ERK1/2 phosphorylation within PMA:ionomycin-activated CD4+ and CD8+ T cells with U0126 or selumetinib analyzed by phosphoflow cytometry. (E) Confirmation of dose-dependent inhibition of PMA:ionomycin-induced activation of purified CD3+ human T cells by U0126 by western blotting.
To test the hypothesis that activation via the RAS/MEK/ERK pathway depends on differentiation of human T cells, we examined ERK1/2 phosphorylation within CD4+ and CD8+ T-cell subsets in PMA:ionomycin-stimulated healthy donor PBMCs. Using single-cell phosphoflow cytometry, we assessed pERK1/2 mean fluorescence intensity (MFI) within naive and memory subsets (gating on either bulk, naive, or progressively mature memory T-cell subsets, Figure 1A bottom). Consistently across donors, we found that activation of the RAS/MEK/ERK pathway, reflected by increased ERK1/2 MFI, was greatest in naive and decreased with maturation through memory stages in both CD4+ and CD8+ T cells (Figure 1B).
To determine whether differential ERK1/2 phosphorylation with T-cell maturation was associated with skewed expression of RAS/MEK/ERK family members, sort-purified memory CD8+ T-cell subsets were assessed for RAS, cRAF, MEK1, MEK2, and ERK1/2 expression by western blotting. The expression of cRAF, MEK1, and ERK1/2 was clearly higher in naive and central memory T cells, relative to effector memory CD8+ T cells (Figure 1C), with little apparent differences in RAS and MEK2 expression. These data demonstrated that the memory stage-dependent differential phosphorylation of ERK1/2 is associated with differences in expression of multiple RAS/MEK/ERK family members, suggesting that MEK inhibition could preferentially target naive and central memory T cells.
U0126 is a classical MEK inhibitor previously demonstrated to inhibit ERK1/2 phosphorylation in cancer cell lines.27 To assess the effects of MEK inhibition on T-cell activation, PBMCs were stimulated in the presence of increasing concentrations of MEK inhibitors, with ERK1/2 phosphorylation assessed in single cells by phosphoflow cytometry. Assessment of pERK1/2 within gated subpopulations of CD4+ and CD8+ T cells demonstrated dose-dependent suppression of ERK1/2 phosphorylation by U0126, a classical MEK inhibitor, and selumetinib, a second-generation MEK inhibitor. Selumetinib has been found to have minimal to no hematologic toxicity to date, and is being tested in ≥50 early phase trials in human cancers (www.clinicaltrials.gov).18,19,28,29 Significant suppression of ERK1/2 phosphorylation was evident in the presence of 1 µM U0126 in both CD4+ and CD8+ T cells (Figure 1D). Notably, selumetinib proved much more potent, with effective suppression observed at just 100 nM (Figure 1D). Results using single-cell phosphoflow cytometry were confirmed by experiments obtained using purified CD3+ human T cells assessed by western blotting, with decreased detection of pERK1/2 with no significant differences in native ERK1/2 expression, as expected (Figure 1E).
MEK inhibitors suppress T cells in a memory stage–dependent fashion
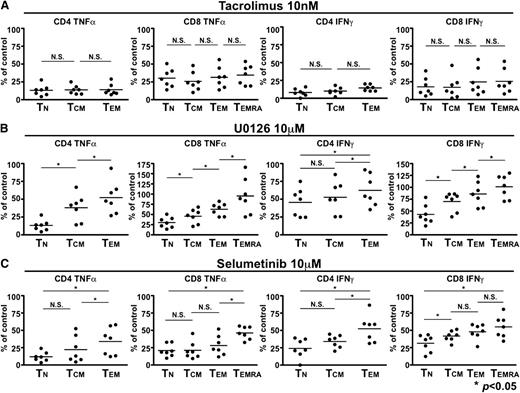
Given our demonstration of the differences in ERK1/2 phosphorylation with T-cell differentiation, we predicted that MEK inhibitors would exhibit stage dependence, in contrast to calcineurin inhibitors, which act in a nonselective fashion. We first confirmed that the calcineurin inhibitor tacrolimus suppressed CD4+ and CD8+ T cells in a memory stage-independent fashion by examining the production of 2 cytokines important in human GVHD (TNFα and IFNγ) in progressively differentiated superantigen-activated T-cell subsets (relative to the amount of cytokine production in the respective memory subset stimulated in the absence of tacrolimus, Figure 2A). Results across healthy donors were consistent, and demonstrated that tacrolimus was indiscriminate with respect to memory stage (Figure 2A). In contrast, when we assessed immunosuppression using the MEK inhibitor U0126 or the second generation agent selumetinib, we demonstrated profound suppression of SEB-induced cytokine production in naive T cells, but relative sparing of progressively differentiated T cells that was consistent across healthy donors (*P < .05 for all associations, Figure 2B).
MEK inhibition of T cells is memory stage–dependent, in contrast to calcineurin inhibition. (A) Tacrolimus inhibits T cells in a memory stage–independent fashion. PBMCs from healthy donors (n = 7) were stimulated with SEB in the presence of DMSO as a carrier control, and in the presence of tacrolimus. The extent of cytokine production (either TNFα at left, or IFNγ, right) was expressed with relative suppression expressed as a percentage of the DMSO control assessed within T-cell maturation subsets. Means for the degree of inhibition within progressively differentiated memory subsets are shown, with no significant difference seen across subsets. (B-C) MEK inhibition spares progressively differentiated CD4+ and CD8+ T cells. As above, the extent of cytokine production (either TNFα at left, or IFNγ, right) was assessed in the presence of the MEK inhibitor U0126 (10 µM, B) or selumetinib (10 μM, C), with relative suppression expressed as a percentage of the DMSO control assessed within T-cell maturation subsets. Means for the degree of inhibition within progressively differentiated memory subsets are shown, demonstrating profound inhibition in naive CD4+ and CD8+ T cells, with less significant inhibition seen (*P < .05) in each progressively differentiated memory CD4+ or CD8+ T-cell subset.
MEK inhibition of T cells is memory stage–dependent, in contrast to calcineurin inhibition. (A) Tacrolimus inhibits T cells in a memory stage–independent fashion. PBMCs from healthy donors (n = 7) were stimulated with SEB in the presence of DMSO as a carrier control, and in the presence of tacrolimus. The extent of cytokine production (either TNFα at left, or IFNγ, right) was expressed with relative suppression expressed as a percentage of the DMSO control assessed within T-cell maturation subsets. Means for the degree of inhibition within progressively differentiated memory subsets are shown, with no significant difference seen across subsets. (B-C) MEK inhibition spares progressively differentiated CD4+ and CD8+ T cells. As above, the extent of cytokine production (either TNFα at left, or IFNγ, right) was assessed in the presence of the MEK inhibitor U0126 (10 µM, B) or selumetinib (10 μM, C), with relative suppression expressed as a percentage of the DMSO control assessed within T-cell maturation subsets. Means for the degree of inhibition within progressively differentiated memory subsets are shown, demonstrating profound inhibition in naive CD4+ and CD8+ T cells, with less significant inhibition seen (*P < .05) in each progressively differentiated memory CD4+ or CD8+ T-cell subset.
MEK inhibition effectively suppresses alloreactivity
Based upon our initial studies demonstrating that MEK inhibitors suppress naive and central memory cells critical for alloreactivity, we predicted that allogeneic dendritic cell–stimulated T-cell proliferation would be effectively suppressed by MEK inhibitors. For clinical translation, we also hoped that complementary targeting using MEK and calcineurin inhibitors could yield a combination approach capable of maximally inhibiting alloreactivity.
To test these hypotheses, we analyzed the division profiles of human CD4+ and CD8+ T cells following potent allogeneic stimulation with HLA-mismatched DCs. Alloreactivity was assessed at baseline and in the presence of tacrolimus, U0126, and selumetinib. Notably, these experiments were conducted using clinically achievable concentrations of MEK inhibitors (eg, 1 and 10 µM for U0126 and selumetinib, achievable therapeutic concentrations in murine and human pharmacokinetic studies).19,27
As expected, stimulation of PBMCs with HLA-mismatched DC resulted in robust proliferation of both CD4+ and CD8+ T cells (as seen in the DMSO “no drug” control, Figure 3A and in data from multiple healthy donors, Figure 3B). As expected, tacrolimus effectively suppressed alloreactivity, as seen in individual (Figure 3A) and aggregate results (Figure 3B). As predicted by our earlier experiments (Figures 1-2), U0126 effectively inhibited alloreactivity, with suppression comparable to that of tacrolimus (Figure 3A-B, P < .01 relative to DMSO control but difference from tacrolimus, NS). Although tacrolimus and U0126 were effective, selumetinib consistently suppressed DC-stimulated alloreactivity more effectively than tacrolimus (representative data shown in Figure 3A; aggregate data in Figure 3B, P < .01 for selumetinib, relative to tacrolimus and P < .05 for CD8+ T cells).
MEK inhibitors effectively suppress human alloreactivity alone and synergistically with calcineurin inhibitors. (A) CFSE-labeled PBMC were cultured with allogeneic DCs with DMSO, tacrolimus, U0126, or selumetinib for 7 days, with division profiles measured by CFSE dye dilution. (B) Aggregate results (n = 6) depicting the percentage of CFSElow CD4+ and CD8+ T cells in the presence of DMSO, Tacrolimus (Tacro), U0126, and selumetinib (Sel). Results shown depict means + SD. *P < .05; **P < .01. (C) Naive (CD45RA+CD27+) CD4+ and CD8+ T cells were sorted, CFSE-labeled and cultured with allogeneic DCs in the presence of DMSO, tacrolimus, or selumetinib (Sel). In addition to CFSE dilution, functional differentiation was assessed by examining CD27 and CD45RA expression. Data representative of 5 consistent and independent experiments is shown. (D-E) CFSE-labeled PBMCs were cultured with DCs along DMSO (control), tacrolimus, and selumetinib (alone and in combination with suboptimal tacrolimus levels). (E) Aggregate results (n = 4) depicting the percentage of CFSElow CD4+ and CD8+ T cells demonstrate synergistic inhibition by tacrolimus (Tacro) and selumetinib (Sel). Results depict means + SD. *P < .05.
MEK inhibitors effectively suppress human alloreactivity alone and synergistically with calcineurin inhibitors. (A) CFSE-labeled PBMC were cultured with allogeneic DCs with DMSO, tacrolimus, U0126, or selumetinib for 7 days, with division profiles measured by CFSE dye dilution. (B) Aggregate results (n = 6) depicting the percentage of CFSElow CD4+ and CD8+ T cells in the presence of DMSO, Tacrolimus (Tacro), U0126, and selumetinib (Sel). Results shown depict means + SD. *P < .05; **P < .01. (C) Naive (CD45RA+CD27+) CD4+ and CD8+ T cells were sorted, CFSE-labeled and cultured with allogeneic DCs in the presence of DMSO, tacrolimus, or selumetinib (Sel). In addition to CFSE dilution, functional differentiation was assessed by examining CD27 and CD45RA expression. Data representative of 5 consistent and independent experiments is shown. (D-E) CFSE-labeled PBMCs were cultured with DCs along DMSO (control), tacrolimus, and selumetinib (alone and in combination with suboptimal tacrolimus levels). (E) Aggregate results (n = 4) depicting the percentage of CFSElow CD4+ and CD8+ T cells demonstrate synergistic inhibition by tacrolimus (Tacro) and selumetinib (Sel). Results depict means + SD. *P < .05.
To examine the effects of calcineurin and MEK inhibition free of effects potentially mediated by non–T-cell bystander populations present in bulk PBMCs, sorted naive (CD45RA+CD27+) CD4+ and CD8+ T cells were cocultured with HLA-mismatched DCs to examine whether tacrolimus and selumetinib could independently inhibit alloreactivity (Figure 3C). Both inhibitors effectively inhibited allogeneic DC-induced proliferation, shown by diminished CFSE dilution, and limited maturation of naive CD4+ T cells (CD45RA+CD27+) to progressively differentiated central memory-like (CD27+CD45RA−) T cells and effector memory (CD27−CD45RA−) cells, and of naive CD8+ T cells to central memory-like (CD27+CD45RA−) cells (CD4+ and CD8+ T cells, Figure 3C left and right panels, respectively). We next assessed whether synergistic inhibition could be achieved by simultaneously targeting the calcineurin and MEK pathways. Therefore, we examined the ability of selumetinib, at low or standard concentrations, to inhibit alloreactive proliferation alone or in combination with high (10 nM) or low (1 nM) concentrations of tacrolimus (representative data shown in Figure 3D; aggregate data in Figure 3E, P < .05). Near-complete inhibition of alloantigen-induced proliferation could be achieved by simultaneous targeting of both pathways, even when using suboptimal concentrations of tacrolimus.
Additional experiments demonstrated that selumetinib exposure during short-term culture did not significantly induce apoptosis in CD4+ or CD8+ T cells, either at baseline or following polyclonal stimulation (supplemental Figure 1, available on the Blood website). Furthermore, short-term exposure to selumetinib did not alter the proportion of CD4+CD25hiFOXP3+ regulatory T cells (supplemental Figure 2) or naive CD4+ or CD8+ T cells (data not shown), consistent with the hypothesis that the suppression of alloreactivity observed was due to direct effects of MEK inhibition on memory stage-dependent T-cell activation.
MEK inhibition spares polyfunctional CMV- and EBV-specific T cells
Our prior clinical studies demonstrated that CMV reactivation after SCT often occurs due to immunosuppression-associated dysfunction of CMV-specific T cells, rather than a simple quantitative deficiency.25 Most CMV-specific CD8+ T cells reside within subsets of effector memory (CD27−CD45RA−) and/or late effector memory T cells that re-express CD45RA (ie, TEMRA+, CD27−CD45RA+).26 We hypothesized that the segregation of alloreactivity (concentrated in early stages of the human T-cell maturation spectrum) and herpesvirus-specific immunity (contained largely in more differentiated memory cells) would result in greater selectivity by MEK inhibitors.
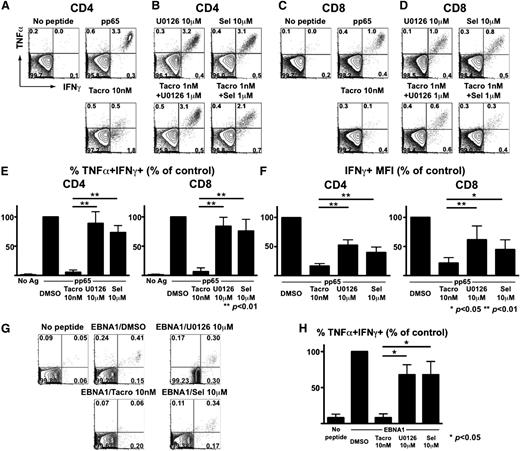
To test this, PBMCs from CMV-seropositive donors were stimulated with pp65 pentadecapeptide pools to assess the effects of calcineurin and MEK inhibition on the activation of CMV-specific T cells. We focused on polyfunctional (ie, TNFα+IFNγ+) subsets of CMV-specific T cells, given their importance in effective clearance of persistent viruses.30-32 Tacrolimus alone significantly impaired polyfunctional CMV-specific CD4+ (Figure 4A) and CD8+ (Figure 4C) T cells. In sharp contrast, only modest inhibition of polyfunctional CMV-specific CD4+ and CD8+ T cells was observed with U0126 or selumetinib (Figure 4B,D). Aggregate studies of healthy donors confirmed that polyfunctional (ie, TNFα+IFNγ+) CMV-specific T cells were relatively spared by MEK inhibitors (Figure 4E). Although tacrolimus spared some monofunctional cells producing IFNγ alone (Figure 4A,C), per-cell IFNγ production (correlated with protective immunity31 ) was markedly diminished (Figure 4F). In contrast, per-cell IFNγ production remained significantly higher following MEK inhibition (Figure 4F).
MEK inhibitors spare polyfunctional CMV- and EBV-specific T cells at doses inhibiting alloreactivity. (A-D) PBMCs from a healthy CMV-seropositive donor were stimulated with CMV pp65 peptide mixture along with DMSO (control), tacrolimus (Tacro), U0126, or selumetinib (Sel). (A) Tacrolimus-associated loss of polyfunctional CMV-specific CD4+ T cells. (B) Relative sparing of polyfunctional CMV-specific CD4+ T cells despite MEK inhibition. (C) Tacrolimus leads to loss of polyfunctional CMV-specific CD8+ T cells. (D) Relative sparing of polyfunctional CMV-specific CD8+ T cells despite MEK inhibition. (E) Aggregate results (n = 5 donors) depicting the frequencies of polyfunctional (TNFα+IFNγ+) CMV-specific cells are shown as mean + SD. Y-axis depicts the percentage of TNFα+IFNγ+ T cells, relative to control. (F) Results (n = 5) depicting the MFI of IFNγ-producing cells are shown as mean + SD. Y-axis depicts the percentage of IFNγ+ T cells, relative to control.*P < .01; **P < .05. (G-H) Healthy donor PBMCs were stimulated with an EBV-derived EBNA1 peptide mixture along with DMSO, tacrolimus, U0126, or selumetinib (Sel) and EBV-specific CD8+ T cells responses were analyzed. (H) Aggregate results (n = 3 donors) depicting the relative sparing of polyfunctional EBV-specific CD8+ T cells by MEK inhibitors. Differences are shown as means + SD. *P < .05.
MEK inhibitors spare polyfunctional CMV- and EBV-specific T cells at doses inhibiting alloreactivity. (A-D) PBMCs from a healthy CMV-seropositive donor were stimulated with CMV pp65 peptide mixture along with DMSO (control), tacrolimus (Tacro), U0126, or selumetinib (Sel). (A) Tacrolimus-associated loss of polyfunctional CMV-specific CD4+ T cells. (B) Relative sparing of polyfunctional CMV-specific CD4+ T cells despite MEK inhibition. (C) Tacrolimus leads to loss of polyfunctional CMV-specific CD8+ T cells. (D) Relative sparing of polyfunctional CMV-specific CD8+ T cells despite MEK inhibition. (E) Aggregate results (n = 5 donors) depicting the frequencies of polyfunctional (TNFα+IFNγ+) CMV-specific cells are shown as mean + SD. Y-axis depicts the percentage of TNFα+IFNγ+ T cells, relative to control. (F) Results (n = 5) depicting the MFI of IFNγ-producing cells are shown as mean + SD. Y-axis depicts the percentage of IFNγ+ T cells, relative to control.*P < .01; **P < .05. (G-H) Healthy donor PBMCs were stimulated with an EBV-derived EBNA1 peptide mixture along with DMSO, tacrolimus, U0126, or selumetinib (Sel) and EBV-specific CD8+ T cells responses were analyzed. (H) Aggregate results (n = 3 donors) depicting the relative sparing of polyfunctional EBV-specific CD8+ T cells by MEK inhibitors. Differences are shown as means + SD. *P < .05.
We also examined the effects of combination therapy using suboptimal tacrolimus and MEK inhibitors (Figure 4B,D). Although combinations of low-dose tacrolimus with these 2 MEK inhibitors consistently and completely inhibited HLA-mismatched DC-induced alloreactivity (Figure 3D), there was a distinct difference in the preservation of CMV-specific T cells, with persistent function of TNFα+IFNγ+ CMV-specific T cells despite combination immunosuppressive therapy.
To examine whether our findings extended to other herpesviruses, we also examined the effects of MEK inhibition on T cells specific for EBV, an important pathogen associated with posttransplant lymphoproliferative disease following allogeneic SCT. PBMCs from EBV-seropositive donors were stimulated with EBNA1 pentadecapeptide pools, and we assessed the effects of tacrolimus, U0126, and selumetinib. Quite similar to what we observed for CMV-specific T cells, we found that tacrolimus significantly impaired total and polyfunctional T cells, while both MEK inhibitors spared polyfunctional EBV-specific CD8+ T cells (representative data shown in Figure 4G, collective data in Figure 4H).
In aggregate, these data confirm that MEK inhibition, at doses capable of efficiently suppressing alloreactivity and even in combination with low-dose tacrolimus, spares polyfunctional CMV- and EBV-specific T cells. In addition to preserving increased numbers of cytokine-producing T cells, MEK inhibition also preserves per-cell cytokine production, providing further evidence of sparing of highly functional herpesvirus-specific T cells.
MEK inhibition delays the onset of experimental GVHD in vivo
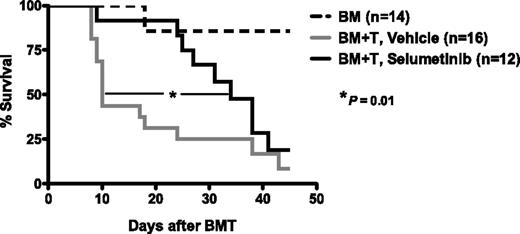
Given the impressive and selective inhibition of human alloreactivity in vitro, we sought to determine the safety and efficacy of MEK inhibition in murine hematopoietic SCT. We first administered the MEK inhibitor selumetinib to healthy mice by oral gavage, and observed no significant toxicities, consistent with prior published murine studies.33 We then confirmed that MEK inhibitor administration was safe in the setting of ablative conditioning and syngeneic SCT, demonstrating no impairment of hematopoietic engraftment (data not shown). These results demonstrated that short-term MEK inhibitor administration was unassociated with hematopoietic toxicity. We then examined the effects of MEK inhibition in a severe major histocompatibility complex (MHC) class I– and class II–mismatched murine model, wherein lethal GVHD uniformly develops in all transplanted animals. Selumetinib was administered following transplant of MHC-mismatched C57BL/6 (H-2b) marrow and T cells into BALB/c (H-2d) recipients. Prior to transplantation, B6 lymphocytes were incubated with selumetinib or DMSO for 30 minutes, then washed and infused into irradiated BALB/c recipients with B6 TCD-BM cells. Selumetinib was delivered orally (25 mg/kg) once daily from day 0 through day 7, with this short-term administration designed to limit the stress of oral gavage in lethally conditioned and transplanted recipients, and to model the approach used with methotrexate, routinely administered for a short period after SCT in humans. Selumetinib-treated recipients demonstrated significantly reduced GVHD-associated mortality and prolonged median survival by 3-fold (median 34 days for selumetinib vs 10 days for vehicle treatment, P = .01, Figure 5). As expected in this model, donor engraftment was consistently demonstrated in recipient spleen, BM, and thymus, unimpaired by selumetinib, and all deaths were GVHD-related.
Selumetinib inhibits GVHD in MHC-mismatched murine SCT. BALB/c recipients were irradiated (7.5Gy) on day −1 and infused (on day 0) with 5 × 106 B6 TCD-BM cells and 0.6 × 106 B6 T cells. B6 T cells were incubated with selumetinib or control (DMSO) for 30 minutes at 37°C before adoptive transfer. Murine recipients were administered vehicle or selumetinib (25 mg/kg) once daily via gavage from day 0 through day 7, and analyzed for survival. Engraftment of donor cells in recipient spleen, BM, and thymus was consistently demonstrated in all groups (not shown). Median survival was prolonged in mice receiving selumetinib (median of 34 days) vs control animals (median 10 days in vehicle-treated animals receiving T-replete grafts; P value 0.01 by log rank comparison using the Grehan-Breslow-Wilcoxon test). As expected, median survival was not reached in mice receiving marrow alone). Combined data from 2 independent experiments is shown, based on n = 14 animals (BM only), n = 16 (vehicle), and n = 12 (selumetinib) in total.
Selumetinib inhibits GVHD in MHC-mismatched murine SCT. BALB/c recipients were irradiated (7.5Gy) on day −1 and infused (on day 0) with 5 × 106 B6 TCD-BM cells and 0.6 × 106 B6 T cells. B6 T cells were incubated with selumetinib or control (DMSO) for 30 minutes at 37°C before adoptive transfer. Murine recipients were administered vehicle or selumetinib (25 mg/kg) once daily via gavage from day 0 through day 7, and analyzed for survival. Engraftment of donor cells in recipient spleen, BM, and thymus was consistently demonstrated in all groups (not shown). Median survival was prolonged in mice receiving selumetinib (median of 34 days) vs control animals (median 10 days in vehicle-treated animals receiving T-replete grafts; P value 0.01 by log rank comparison using the Grehan-Breslow-Wilcoxon test). As expected, median survival was not reached in mice receiving marrow alone). Combined data from 2 independent experiments is shown, based on n = 14 animals (BM only), n = 16 (vehicle), and n = 12 (selumetinib) in total.
Discussion
GVHD remains a major obstacle after T-replete allogeneic SCT, where it is a major cause of infectious mortality and impaired immune reconstitution, especially to pathogens including CMV. Although a wide variety of approaches are applied to prevent or treat GVHD, nearly all of these therapies are relatively nonselective, leading to the inhibition of pathogen-specific T cells along with alloreactive T cells. This effectively trades one potentially life-threatening condition (GVHD) for another (suppression of pathogen-specific T cells and more profound immunodeficiency), compounding the significant risk of GVHD-associated mortality.
In the past decade, we have witnessed significant advances in our understanding of how naive and memory T cells differ, with unique differences in their ability to respond to costimulatory signals, produce combinations of effector cytokines and chemokines, and to mediate alloreactivity.9,11-14,34 Knowing that the potential to mediate alloreactivity diminishes with memory differentiation, we tested the hypothesis that the RAS/MEK/ERK pathway would be differentially active within T-cell subsets by examined the signaling characteristics of naive and progressively differentiated human CD4+ and CD8+ T cells. We confirmed that activation of the RAS/MEK/ERK was memory stage dependent, with relatively robust signaling (assessed by ERK1/2 phosphorylation) in naive T cells, and a progressive decline in activation in central, effector, and late-effector memory cells.
We further confirmed maturation-dependent differences in expression of multiple RAS/MEK/ERK pathway members. Although others have demonstrated that progressively differentiated memory cells may differ in their signaling thresholds,35,36 no prior study has exploited these differences to confer selectivity of suppression of alloreactivity using targeted inhibitors, vis a vis more differentiated memory cells responsible for the control of pathogenic herpesviruses.
MEK inhibition, a clinically viable strategy with little apparent hematopoietic toxicity in human cancer trials,19,28,29 strongly suppresses human T-cell alloreactivity, with in vitro potency equal to or surpassing calcineurin inhibitors. Notably, and in marked contrast to comparatively indiscriminate inhibition by tacrolimus, MEK inhibition spares more differentiated CMV- and EBV-specific T cells, preserving their numbers and polyfunctionality. We speculate that the distinctive stage specificity of MEK inhibitors may be particularly well suited to allogeneic SCT due to their capacity to inhibit GVHD-mediating alloreactivity while sparing virus-specific T-cell responses.
It is important to acknowledge that MEK inhibitors would be expected to compromise primary immune responses derived from naive T cells present within donor grafts and adoptively transferred to recipients. Therefore, de novo immune responses (eg, priming of T cells to pathogens not previously seen by the donor) would likely be impaired during the duration of MEK inhibitor therapy. This would limit the use of this strategy in settings such as cord blood transplantation, wherein no memory T cells are transferred from donor to recipient. However, the vast majority of allogeneic SCT performed in the world use non–T-depleted grafts containing significant populations of memory T cells that are adoptively transferred to recipients and effectively contribute to immune reconstitution after allogeneic SCT. For example, most grafts from donors worldwide (>70% for CMV and >90% for EBV) contain herpesvirus-specific T cells that we predict would be spared by MEK inhibition, even when combined with low doses of tacrolimus (as shown in Figure 4).
The MEK inhibitors possess other attributes that increase their potential attractiveness as immunosuppressive agents that may be valuable in the immediate post-SCT setting. Because the RAS/MEK/ERK pathway is known to be important for cancer cell signaling and survival, MEK targeting may inhibit the growth of some malignant cells resistant to transplant conditioning in the critical early interval after transplantation. Indeed, recent studies of acute myeloid leukemia,37 multiple myeloma,38 cutaneous T-cell lymphomas39 and diffuse large B-cell lymphoma40 have demonstrated that MEK inhibition may have significant therapeutic value as antineoplastic agents in the setting of hematologic malignancies known to be responsive to allogeneic SCT.
An additional potential therapeutic benefit of MEK inhibitors is that they may favor the recovery of regulatory T cells further capable of inhibiting GVHD.41-44 Luo et al previously found that transforming growth factor-β–dependent induction of FOXP3 was dependent on transient inactivation of ERK signaling, and that pharmacologic inhibition of MEK using U0126 (also studied here) resulted in FOXP3 induction and suppressor functions of regulatory T cells.45 Others have confirmed that inhibition of either the mammalian target of rapamycin pathway or the RAS/MEK/ERK pathway enhances the expression of FOXP3 in a transforming growth factor-β–dependent manner.46 While beyond the scope of our current studies, these data suggest that MEK inhibition may have additional therapeutic benefits that may limit GVHD without adversely impacting graft-versus-malignancy responses important for favorable outcomes after SCT.43
These findings will require confirmation in human clinical trials of GVHD prevention. It will also be extremely valuable to confirm our findings in vivo in additional murine models of GVHD and graft-versus-leukemia responses. Additional murine studies should address how to optimally combine these agents with calcineurin inhibitors, and define dosing schedules that could inhibit alloreactivity in the critical initiation phase of GVHD, without compromising the long-term recovery of primary immune responses dependent on naive T cells as well as the functional capacity of adoptively transferred pathogen-specific memory T cells. Additionally, knockdown of RAS/ERK/MEK pathway members in murine and human T cells will also better define the precise mechanisms of the effects observed here. At this time, a number of potent MEK inhibitors are being developed and assessed in clinical trial settings, increasing the likelihood that clinical trials of MEK inhibitor-based immunomodulation should prove feasible in the immediate future.20-22,47 In summary, we have outlined a novel proof-of-concept strategy by demonstrating that MEK inhibitors effectively facilitate memory stage–specific inhibition of alloreactive human T cells, suggesting an obvious translational pathway to more selective, and consequently safer, immunosuppressive strategies in humans.
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Lisa S. St. John for efforts to develop experimental approaches used in this study, Despina Kolonias for technical expertise, and Qing Ma for helpful intellectual discussions.
This work was supported, in part, by grants from the National Institutes of Health (National Cancer Institute RO1 CA109326 [K.V.K.]) and support from the Kalish Family Foundation.
Authorship
Contribution: T.S. performed the primary experiments, analyzed the data, and wrote the manuscript; T.K.K. performed primary experiments, analyzed data, and contributed to the manuscript; C.L.B. contributed to experimental design, produced primary data, and contributed to data analysis and manuscript editing; E.D.W. helped to design flow cytometry approaches, interpreted data, and contributed to manuscript editing; R.B.L. developed and performed experiments in the murine transplantation model, assisted in data interpretation, and contributed to manuscript editing; and K.V.K. conceived the primary hypotheses, contributed to experimental design and data analysis, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Krishna V. Komanduri, Adult Stem Cell Transplant Program, University of Miami Sylvester Cancer Center, 1501 NW 10th Ave, Suite 916, Miami, FL 33136; e-mail: kkomanduri@med.miami.edu.
References
Author notes
T.S. and T.K.K. equally contributed to this work.