Key Points
TCR stimulation increases IL-7 responsiveness.
CD4+SPT proliferate more to IL-7 therapy.
Abstract
Interleukin-7 (IL-7) is currently used in clinical trials to augment T-cell counts. Paradoxically, elevated systemic IL-7 found in lymphopenic humans is typically insufficient for CD4+ T-cell regeneration, and thymopoiesis becomes critical in this process. Here we show that the proliferative effect of IL-7 is more pronounced on CD4+CD8− thymocytes compared with peripheral CD4+ T cells. These cells express miR181a at higher levels and respond to lower concentrations of IL-7. As single-positive CD4+ thymocytes (CD4+SPT) exit the thymus, they rapidly diminish their proliferation to IL-7 therapy, and this is mediated, at least in part, by major histocompatibility complex class II distribution outside the thymus. Interestingly, increasing T-cell receptor (TCR) stimulation augments IL-7 responsiveness and proliferation of peripheral CD4+ T cells, whereas failure to stimulate TCR abrogates proliferation induced by IL-7. Finally, we demonstrated that IL-7 enhances the proliferation of CD4+ T cells that undergo “slow proliferation” in lymphopenic hosts. To date, our results indicate that TCR signaling is a major controlling factor for CD4 responsiveness and proliferation to IL-7 therapy.
Introduction
Homeostasis keeps T-cell numbers constant in the periphery throughout the entire life of an individual.1-3 During fetal development and in the early postnatal period, thymopoiesis is critical for T-cell production, T-cell receptor (TCR) diversity, and acquisition of immunocompetence. With aging, thymic senescence occurs and peripheral mechanisms ensure maintenance of T-cell numbers and TCR diversity. Mouse models demonstrate that TCR triggering and interleukin-7 (IL-7) are necessary for naive CD8+ and CD4+ T-cell maintenance in the periphery.4 As a result, preventing TCR contact with self-peptide major histocompatibility complexes (MHCs) or limiting in vivo IL-7 availability diminishes cell survival.5-7 Together, this suggests that maintenance of T cells in the periphery requires 2 signals: a tonic interaction between the TCR and self-MHC and continual availability of IL-7.
Postnatal T-cell depletion in humans, which typically occurs following high-dose chemotherapy or after bone marrow transplantation, is generally associated with rapid normalization of CD8+ T cells but long lasting CD4 lymphopenia.8 Regeneration of T lymphocytes can follow 2 distinct pathways: thymic production that recapitulates T-cell production as it occurs early in life and homeostatic peripheral expansion (HPE), which consists of exaggerated proliferation of residual T lymphocytes in response to the lymphopenic environment. While HPE can efficiently regenerate CD8+ T cells, this pathway is inefficient for CD4+ lymphocytes.
Because of the critical role played by IL-7 in T-cell homeostasis, several models were developed to evaluate the use of this cytokine to increase T-cell counts.9-17 In wild-type mice, studies have demonstrated that B, T, natural killer cells and monocyte macrophages were modulated by IL-7. IL-7 therapy can increase T-cell recovery after T-cell–depleted bone marrow transplantation, and this is generally accompanied with diminished TCR excision circle (TREC) levels among total T cells.16,18 Other studies confirmed the dilution of TRECs in IL-7–treated mice, suggesting that IL-7 works primarily by expanding mature lymphocytes.13 Recent clinical studies have demonstrated that IL-7 can significantly improve immune reconstitution in lymphopenic humans.19-21 However, similar to observations made in mice, CD8+ T cells were also expanded to a greater extent than CD4+ lymphocytes. Although recombinant human IL-7 (rhIL-7) therapy induces significant increases in the naive T-cell subset, changes in thymic size have not been observed. Together, the data support a model wherein IL-7 effects clearly involve enhanced homeostatic expansion. However, a direct effect on the thymus cannot be ruled out with current data.
Materials and methods
Animals and administration of IL-7 or Flt3L
The Animal Committee of Centre de Recherche de l'Hopital Maisonneuve-Rosemont (CRHMR) approved all experiments. C57BL/6Ly5.1, C57BL/6Ly5.2, C57BL/6Ly5Thy1.1, and Rag1−/− mice (the Jackson Laboratory); B6Abb mice (Taconic Farms); and B6 RAG2p-GFP mice22 were used. B6IL-7−/− mice were bred at CRHMR. rhIL-7 was administered at concentration of 0, 1, 2.5, 5, or 10 μg for 6 days as a daily intraperitoneal injection. IL-7 was supplied by Cytheris. Recombinant human Flt3L (BioXcell) was administered intraperitoneally (10 μg/day for 10 day). For parking experiments in MHC class II (MCHII)−/− and wt recipients, mice were treated immediately with IL-7 (no parking) or 3 days post transfer for 6 days.
CFSE staining and adoptive transfer of lymphocytes
Peripheral T lymphocytes were enriched by negative selection as described in the mouse T-cell enrichment kit (StemCell Technologies). For enrichment of thymic single-positive T cells, negative selection on CD4 and CD8 cells was performed (StemCell Technologies). Enriched lymph node (LN) or thymic single-positive T cells were stained with carboxyfluorescein diacetate succinimidyl ester (CFSE) as previously described23 (Molecular Probes). Mice received 1 × 106 CFSE-labeled polyclonal T cells (LN T cells) or enriched thymocytes at a CD4:CD8 1:1 ratio or, where indicated, 2 × 106 cells of mixed CD45.1+ LN T cells and CD90.1+ thymic single-positive cells at a 1:1 ratio or, where indicated 5 × 105 GFP+ T cells. After 7 days, mice were sacrificed and CFSE content was analyzed with a 4-laser LSR II (Becton Dickinson). FlowJo software (TreeStar) was used for analyses. Absolute number of cells recovered per LN and spleens were calculated based on forward-scatter (FSC) and side-scatter (SSC) profiles. GFP+ and CFSE+ cells were sorted using a FACS Aria III (Becton Dickinson).
Flow cytometry
T lymphocytes were incubated for 30 minutes on ice with diluted monoclonal antibodies (mAbs). The following mAbs were used: pacific blue–anti-CD4 (RM4.5), phycoerythrin (PE)–anti-CD8 (53-6.7), fluorescein isothiocyanate (FITC)–anti-CD8 (5H10-1), PE–anti-CD45.2 (104), Allophycocyanin-Cy7 (APC)-Cy7–anti-CD62L (MEL-14), PE–anti-CD45RB (C363-16A), phycoerythrin-Cy7 (PECy7)–anti-CD69 (H1.2F3), and allophycocyanin (APC)–anti-β7 (FIB504) PE–anti-CD132 (TUGm2); and PECy7–anti-CD11c (N418) (all from Biolegend); APC-conjugated anti-CD45.1 (A20), PE–anti-Stat5, and FITC–Bcl-2 (all from BD Biosciences; and APC–anti-CD127 (A7R34) (from ebiosciences). PE-anti-Annexin V and peridinine chlorophyll protein (PerCP)-anti-7AAD (BD Bioscience) were used, and staining was performed according to the manufacturer’s instructions.
STAT5 and Bcl-2 staining
For in vitro detection of phosphorylated STAT5, cells were stimulated for 30 minutes with different concentrations of rhIL-7. For in vivo detection, mice were treated with IL-7 for 6 days. For detection following anti-CD3 (145-2c11; BioXCell) stimulation, cells were starved for 1 hour, incubated with 1 μg/mL anti-CD3 for 15 minutes, cross-linked with anti-hamster (immunoglobulin-G; Sigma) at 2 μg/mL for 15 minutes, and then incubated with varying concentrations of IL-7 for 30 minutes. Then cells were fixed in hot (37°C) lyse/fix buffer, washed twice in phosphate-buffered saline (PBS), and made permeable in iced Perm Buffer III (BD Biosciences). Cells were then stained for surface receptors. For Bcl-2 intracellular staining, cells were starved for 1 hour and then incubated with different concentrations of rhIL-7 at 37°C for 24 hours. Cells were washed with 0.1% saponin in PBS and stained for Bcl-2. Cells were then washed once in saponin buffer, then in PBS, and stained for CD4 and CD8.
Quantitative polymerase chain reaction for bcl2 and miR181a expression analysis
ABI TaqMan assays were used to measure gene expression in various T-cell populations. For miR181a, CD4+GFP+ and CD4+GFP- were sorted from LN and thymus of a Rag2-GFP mouse. For Bcl2, CD4+SPT and CD4+PERI were sorted and plated with varying concentrations of IL-7 for 6 hours. Total RNA samples were extracted using Trizol. miR181a expression was compared with an internal control (snoRNA202), and Bcl2 was compared with glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Quantification was determined using standard curve methods following the ABI TaqMan quantitative polymerase chain reaction protocol.
Statistical analyses
Prism 5.0 (GraphPad Software) was used for statistical analyses. A 2-tailed, unpaired Student t test with 95% confidence bounds was used for statistical analysis unless otherwise indicated. A P value ≤ 0.05 was considered significant.
Results
IL-7 therapy preferentially expands CD8+ T lymphocytes
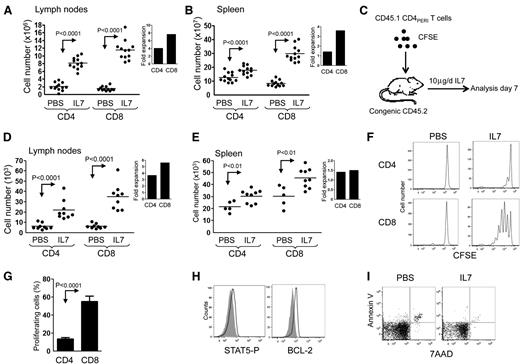
In humans, primates, and mice, IL-7 therapy has been shown to induce global T-cell expansion.11-13,19,20,24 However, a careful analysis of T-cell expansion during IL-7 therapy indicates that CD8+ and CD4+ T cells respond differently to supraphysiologic levels of IL-7. Following a 6-day course of IL-7 therapy, we observed enlarged spleen and lymphadenopathy associated with augmented numbers of splenocytes (P < .0001) and LN cells (P < .0001; supplemental Figure 1). The magnitude of expansion of T cells was higher in the LN than in the spleen, with expansion of both CD8+ (LN: P < .0001, spleen: P < .0001) and CD4+ T cells (LN: P < .0001, spleen: P < .001; Figure 1A-B). Consistent with previous studies in mice,13-15 IL-7 therapy induces stronger expansion of CD8+ compared with CD4+ T cells. These results demonstrate that exogenous administration of IL-7 presents a natural bias toward the expansion of CD8+ T cells.
IL-7 therapy induces T-cell expansion in the spleen and LNs with preferential homeostatic proliferation of CD8+ T cells. Wild-type adult mice (8 to 12 weeks old) received daily intraperitoneal injections of either PBS or rhIL-7 (10 μg per mouse) for 6 days. (A-B) Graphic summary of mean absolute number of LN and spleen T cells of mice treated with IL-7 or PBS (2 axillary and 2 inguinal LNs were used for LN cell count). Inlets represent the fold increase compared with PBS controls. (C) Schematic representation of the experimental design where enriched congenic T cells were administered to congenic recipients treated with PBS or IL-7. (D-E) Mean absolute number of CD45.1+CD4+ and CD45.1+CD8+ T cells in the LN and spleen of PBS- and IL-7–treated CD45.2+C57BL/6 animals. Inlets represent the fold increase compared with PBS controls. (F) Representative flow cytometric analysis of CFSE-labeled CD45.1+CD4+ and CD45.1+CD8+ T cells found in the LN of PBS- and IL-7–treated CD45.2+ C57BL/6 recipients. (G) Mean percentage of congenic CD45.1+CD4+ and CD45.1+CD8+ T cells proliferating after 6 days of IL-7 treatment based on CFSE dilution. (H) STAT5-P and BCL-2 expression of transferred CD4+PERI after PBS (gray) or IL-7 (black) treatment. Data show between 5 and 9 mice per group pooled from 2 independent experiments. (I) Dot plot showing Annexin V and 7 AAD of transferred CD4+PERI. Data are representative of 2 independent experiments.
IL-7 therapy induces T-cell expansion in the spleen and LNs with preferential homeostatic proliferation of CD8+ T cells. Wild-type adult mice (8 to 12 weeks old) received daily intraperitoneal injections of either PBS or rhIL-7 (10 μg per mouse) for 6 days. (A-B) Graphic summary of mean absolute number of LN and spleen T cells of mice treated with IL-7 or PBS (2 axillary and 2 inguinal LNs were used for LN cell count). Inlets represent the fold increase compared with PBS controls. (C) Schematic representation of the experimental design where enriched congenic T cells were administered to congenic recipients treated with PBS or IL-7. (D-E) Mean absolute number of CD45.1+CD4+ and CD45.1+CD8+ T cells in the LN and spleen of PBS- and IL-7–treated CD45.2+C57BL/6 animals. Inlets represent the fold increase compared with PBS controls. (F) Representative flow cytometric analysis of CFSE-labeled CD45.1+CD4+ and CD45.1+CD8+ T cells found in the LN of PBS- and IL-7–treated CD45.2+ C57BL/6 recipients. (G) Mean percentage of congenic CD45.1+CD4+ and CD45.1+CD8+ T cells proliferating after 6 days of IL-7 treatment based on CFSE dilution. (H) STAT5-P and BCL-2 expression of transferred CD4+PERI after PBS (gray) or IL-7 (black) treatment. Data show between 5 and 9 mice per group pooled from 2 independent experiments. (I) Dot plot showing Annexin V and 7 AAD of transferred CD4+PERI. Data are representative of 2 independent experiments.
Since previous work has suggested a potential contribution from thymopoiesis to the overall T-cell expansion induced by IL-7 therapy, we used an adoptive transfer strategy to study the effect of IL-7 treatment on peripheral CD4+ (CD4+PERI) and CD8+ T cells (Figure 1C-G). Following the adoptive transfer of highly enriched CD4+CD45.1+ and CD8+CD45.1+ into IL-7–treated CD45.2+ recipients, we found a significant increased number of CD4+ and CD8+ T cells in the spleen and LN (Figure 1D-E). While the fold expansion was relatively similar between CD4+ and CD8+ T cells in the spleen, we noted a preferential accumulation of CD8+ T lymphocytes in the LNs.
We then used CFSE labeling of CD45.1+CD4+ and CD45.1+CD8+ T cells to compare the proliferation of CD4+ cells with CD8+ T cells. As predicted, we found that IL-7 therapy induced stronger proliferation of CD8+ cells compared with CD4+ T cells (Figure 1F-G). Despite inefficient proliferation of CD4+ T cells in response to IL-7 treatment, cells expressed higher levels of Bcl-2, while the proportion of annexin V–positive cells was diminished, indicating an effect of IL-7 therapy on survival (Figure 1H-I). Together, our data support a model wherein IL-7 therapy augments CD8 counts by increasing proliferation and possibly survival, while the rise in CD4 counts is driven mainly by survival.
CD4+ recent thymic emigrants are not preferentially expanded by IL-7 therapy
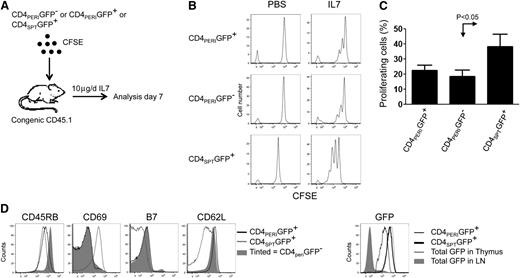
Previous work showing diminished TREC numbers and increased CD4+CD31+CD27+ cells in the peripheral blood suggests that IL-7 therapy affects CD4+ recent thymic emigrants (CD4+RTE) to a greater extent.20 Since RTEs are thought to respond vigorously to systemic IL-7,25 we postulated that the few CD4+ cells undergoing proliferation during IL-7 therapy might be RTEs. To determine how RTEs respond to IL-7 therapy, we used the well-characterized C57BL/6RAG-GFP transgenic mouse model in which peripheral RTEs express the green fluorescent protein (GFP).22 We sorted peripheral CD4+CD45.2+GFP+ and CD4+CD45.2+GFP− cells and transferred them into IL-7–treated congenic recipients (Figure 2A). We also sorted CD4+CD8−CD45.2+GFP+ thymocytes and transferred them into congenic mice treated with IL-7. Seven days later, cells were harvested and fixed with paraformaldehyde to bleach the GFP protein, and CFSE analysis was performed. While we anticipated extensive proliferation of GFP+ RTEs in response to IL-7 therapy, the difference with CD4+CD45.2+GFP− was not significant (Figure 2B-C). However, we did find that thymic CD4+CD8−GFP+ were vigorously proliferating in response to IL-7 therapy, raising the prospect that greater responsiveness to IL-7 in RTEs might be only transient.
Recent thymic emigrants CD4+ do not proliferate more than peripheral CD4+ T cells during IL-7 therapy. (A) Schematic of the experimental design where CD45.2+CD4+PERIGFP+, CD45.2+CD4+PERIGFP−, and thymic CD45.2+CD8−CD4+SPTGFP+ cells were sorted from LNs or thymuses of CD45.2+Rag−/−GFP mice and stained with CFSE prior to their transfer into CD45.1+C57BL/6 mice. Recipient mice were then treated with PBS (vehicle) or rhIL-7 (10 μg/day) for 6 days. (B) Representative flow cytometric analysis of CFSE (CTV)–labeled CD4+PERIGFP+ and CD4+PERIGFP− and CD4+SPTGFP+ found in the LN of PBS- and IL-7–treated animals 7 days post transfer. (C) Graphic summary of the percentage of proliferating cells ± standard error of CD4+PERIGFP+, CD4+PERIGFP−, and CD4+SPTGFP+ based on the dilution of CFSE staining after 6 days of IL-7 therapy. (D) Phenotypic analysis of CD4+PERIGFP+, CD4+PERIGFP−, and CD4+SPTGFP+. Data show 2 animals per group from 2 experiments.
Recent thymic emigrants CD4+ do not proliferate more than peripheral CD4+ T cells during IL-7 therapy. (A) Schematic of the experimental design where CD45.2+CD4+PERIGFP+, CD45.2+CD4+PERIGFP−, and thymic CD45.2+CD8−CD4+SPTGFP+ cells were sorted from LNs or thymuses of CD45.2+Rag−/−GFP mice and stained with CFSE prior to their transfer into CD45.1+C57BL/6 mice. Recipient mice were then treated with PBS (vehicle) or rhIL-7 (10 μg/day) for 6 days. (B) Representative flow cytometric analysis of CFSE (CTV)–labeled CD4+PERIGFP+ and CD4+PERIGFP− and CD4+SPTGFP+ found in the LN of PBS- and IL-7–treated animals 7 days post transfer. (C) Graphic summary of the percentage of proliferating cells ± standard error of CD4+PERIGFP+, CD4+PERIGFP−, and CD4+SPTGFP+ based on the dilution of CFSE staining after 6 days of IL-7 therapy. (D) Phenotypic analysis of CD4+PERIGFP+, CD4+PERIGFP−, and CD4+SPTGFP+. Data show 2 animals per group from 2 experiments.
IL-7 therapy induces stronger proliferation of CD4+SPT
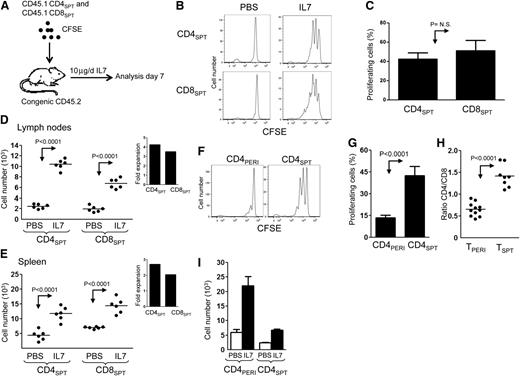
The finding that thymic CD4+SPT proliferate more than peripheral CD4+ RTEs in response to IL-7 therapy prompted us to further investigate the mechanism that regulates this process in vivo. Therefore, we transferred highly enriched CFSE-labeled single-positive thymocytes (CD4+CD45.1+SPT and CD8+CD45.1+SPT) into congenic hosts treated with IL-7 for 6 days (Figure 3A). Surprisingly, CD4+SPT and CD8+SPT thymocytes show similar rates of IL-7–induced proliferation (Figure 3B-C and H). Furthermore, IL-7 treatment promoted greater accumulation of CD4+SPT compared with CD8+SPT cells in the LNs (P < .0001), with similar but nonsignificant findings in the spleen (Figure 3D-E). Despite stronger proliferation of CD4+SPT in comparison with CD4+PERI (Figure 3F-G), the absolute number of cells recovered post transfer in both PBS- and IL-7–treated animals remained below the values obtained for CD4+PERI (Figure 3I). This may relate to the immaturity of some CD4+SPT that could present defective homing toward LNs, as CD62L expression is lower compared with CD4+PERI (Figure 2D). Nonetheless, our results are consistent with previous work showing that RTEs can be easily outcompeted by mature T cells.26 Thus far, our data show that IL-7 therapy works on 2 levels; on one hand, it provides a survival benefit for mature CD4+ cells in the periphery and on the other, it induces stronger proliferation of thymocytes as they exit the thymus.
IL-7 therapy induces stronger proliferation of CD4+SPT compared with CD4+PERI. (A) Schematic representation of the experimental design where CFSE-labeled CD45.1+CD4+CD8− (CD4+SPT) and CD45.1+CD4−CD8+ (CD8+SPT) thymocytes enriched from the thymus of CD45.1+B6SJL mice were mixed at a ratio of 1:1 prior to their adoptive transfer into CD45.2+C57BL6 recipients. Recipient mice were then treated daily with PBS (vehicle) or rhIL-7 for 6 days. (B) Representative flow cytometric analysis of transferred thymocytes CD45.1+CD4+CD8− and CD45.1+CD4−CD8+ found in the LN of PBS- and IL-7–treated mice. (C) Graphic summary of the mean percentage ± standard error of proliferating CD4+SPT and CD8+SPT in response to IL-7 therapy according to CFSE dilution. (D-E) Graphical mean of the absolute number of CD4+SPT and CD8+SPT found in LN and spleen of PBS- and IL-7–treated recipients. Inlets show fold expansion of CD4+SPT and CD8+SPT in LN and spleen of IL-7 vs PBS-treated animals. (F) Comparison of CFSE-labeled CD4+SPT vs CD4+PERI 6 days after their transfer into CD45.2+B6. (G) Graphic summary of the mean proliferation ± standard error of CD4+PERI and CD4+SPT after 6 days of IL-7 treatment. (H) Graphic summary of CD4/CD8 ratio of TPERI and TSPT after 6 days of IL-7 treatment. (I) Graphic representation of the mean number ± standard error of CD4+PERI and CD4+SPT recovered in the LN of PBS- and IL-7–treated mice. Data show between 6 and 8 mice pooled from 2 to 3 independent experiments.
IL-7 therapy induces stronger proliferation of CD4+SPT compared with CD4+PERI. (A) Schematic representation of the experimental design where CFSE-labeled CD45.1+CD4+CD8− (CD4+SPT) and CD45.1+CD4−CD8+ (CD8+SPT) thymocytes enriched from the thymus of CD45.1+B6SJL mice were mixed at a ratio of 1:1 prior to their adoptive transfer into CD45.2+C57BL6 recipients. Recipient mice were then treated daily with PBS (vehicle) or rhIL-7 for 6 days. (B) Representative flow cytometric analysis of transferred thymocytes CD45.1+CD4+CD8− and CD45.1+CD4−CD8+ found in the LN of PBS- and IL-7–treated mice. (C) Graphic summary of the mean percentage ± standard error of proliferating CD4+SPT and CD8+SPT in response to IL-7 therapy according to CFSE dilution. (D-E) Graphical mean of the absolute number of CD4+SPT and CD8+SPT found in LN and spleen of PBS- and IL-7–treated recipients. Inlets show fold expansion of CD4+SPT and CD8+SPT in LN and spleen of IL-7 vs PBS-treated animals. (F) Comparison of CFSE-labeled CD4+SPT vs CD4+PERI 6 days after their transfer into CD45.2+B6. (G) Graphic summary of the mean proliferation ± standard error of CD4+PERI and CD4+SPT after 6 days of IL-7 treatment. (H) Graphic summary of CD4/CD8 ratio of TPERI and TSPT after 6 days of IL-7 treatment. (I) Graphic representation of the mean number ± standard error of CD4+PERI and CD4+SPT recovered in the LN of PBS- and IL-7–treated mice. Data show between 6 and 8 mice pooled from 2 to 3 independent experiments.
Thymic CD4+SPT cells are more responsive to IL-7 than CD4+PERI
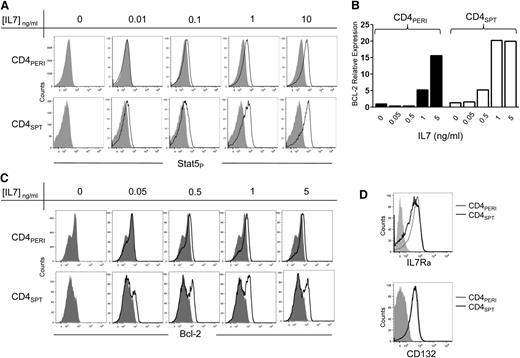
Because CD4+SPT undergo stronger proliferation in response to IL-7 therapy, we postulated that these cells might be more responsive to IL-7. IL-7 belongs to the common γc cytokine family and signals through JAK1-3 and STAT5, which in turn regulates Bcl-2 anti-apoptotic factor expression. Therefore we first evaluated the phosphorylation of STAT5 in CD4+PERI and CD4+SPT exposed in vitro to varying concentrations of IL-7. As predicted, lower concentrations of IL-7 were sufficient to induce STAT5 phosphorylation in CD4+SPT in comparison with CD4+PERI (Figure 4A). We then evaluated Bcl-2 expression following in vitro stimulation with IL-7 and found upregulation of Bcl-2 expression occurring at lower concentrations of IL-7 in CD4+SPT compared with CD4+PERI (Figure 4C). We also used real-time quantitative polymerase chain reaction to confirm the induction of bcl-2 occurring at lower concentrations of IL-7 in CD4+SPT (Figure 4B). To determine if enhanced IL-7 signaling on single-positive thymocytes was related to the expression levels of the IL-7Rα or γC chain, we measured CD127 and CD132 by flow cytometry. However, we did not find a significant difference in levels of expression between CD4+SPT and CD4+PERI (Figure 4D). Together, our data confirm that CD4+SPT respond to lower concentrations of IL-7, and this is not due to a difference in expression levels of IL-7Rα or γc chain receptors.
CD4+SPT are more sensitive to IL-7 therapy than CD4+PERI. (A) STAT5 phosphorylation in CD4+PERI vs CD4+SPT exposed to varying concentrations of rhIL-7. Results are representative of 3 independent experiments. (B) Quantification of bcl2 RNA expression on CD4+PERI and CD4+SPT exposed to varying IL-7 concentrations for 6 hours. Data show 1 mouse representative of 3 independent experiments. (C) Bcl-2 expression in CD4+PERI vs CD4+SPT exposed to varying concentrations of rhIL-7. (D) Representative flow cytometric analysis of IL-7Rα (CD127) and CD132 (common γC chain) evaluated on fresh CD4+CD8− thymocytes (CD4+SPT) and peripheral CD4+ T cells. Data are representative of 5 mice pooled from 3 independent experiments.
CD4+SPT are more sensitive to IL-7 therapy than CD4+PERI. (A) STAT5 phosphorylation in CD4+PERI vs CD4+SPT exposed to varying concentrations of rhIL-7. Results are representative of 3 independent experiments. (B) Quantification of bcl2 RNA expression on CD4+PERI and CD4+SPT exposed to varying IL-7 concentrations for 6 hours. Data show 1 mouse representative of 3 independent experiments. (C) Bcl-2 expression in CD4+PERI vs CD4+SPT exposed to varying concentrations of rhIL-7. (D) Representative flow cytometric analysis of IL-7Rα (CD127) and CD132 (common γC chain) evaluated on fresh CD4+CD8− thymocytes (CD4+SPT) and peripheral CD4+ T cells. Data are representative of 5 mice pooled from 3 independent experiments.
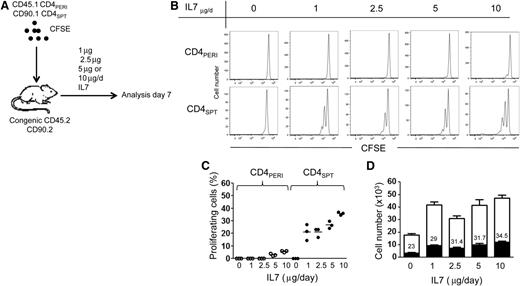
The in vitro finding that CD4+SPT respond to lower concentrations of IL-7 suggests that these cells might proliferate to lower doses of IL-7 in vivo. To test this, we transferred CFSE-labeled CD4+PERICD45.1+CD90.2+ and CD4+SPTCD45.2+CD90.1+ T cells (1:1 ratio) into the same congenic recipients and treated mice with varying doses of rhIL-7 (0, 1, 2.5, 5, or 10 μg; Figure 5A). Consistent with our in vitro data, we found that lower doses of IL-7 were sufficient to induce the proliferation of CD4+SPT compared with CD4+PERI (Figure 5B). Despite lack of proliferation of CD4+PERI observed at lower doses of IL-7 (Figure 5C), we found a significant rise in cell counts, confirming the response of CD4+PERI to IL-7 (Figure 5D). We also compared the recovery of CD4+SPT with that of CD4+PERI at day 7 in the LNs and found that CD4+SPT were diminished compared with CD4+PERI, suggesting that these cells are perhaps outcompeted by CD4+PERI. Importantly, however, as we augmented the dose of IL-7, we found substantial increases in CD4+SPT recovery, which is consistent with a cumulative effect of IL-7 on cell survival (Figure 5D).
Lower dose of IL-7 induces CD4+SPT proliferation. (A) Schematic representation of the experimental design where CD90.2+CD45.1+CD4+PERI and CD90.1+CD45.2+CD4+SPT were transferred simultaneously into the same congenic recipients (CD90.2+CD45.2+). Mice were treated daily with either PBS (vehicle) or different doses of IL-7 for 6 days (1, 2.5, 5, or 10 μg/d). (B) Representative flow cytometric analysis of CFSE dilution in CD90.2+CD45.1+CD4+PERI and CD90.1+CD45.2+CD4+SPT cells isolated from LNs of PBS- or IL-7–treated mice. (C) Graphic representation of the percentage of proliferating cells in function of IL-7 concentration for CD90.2+CD45.1+CD4+PERI and CD90.1+CD45.2+CD4+SPT at day 7 of IL-7 treatment. (D) Graphic summary of the absolute number of CD90.2+CD45.1+CD4+PERI (white) and CD90.1+CD45.2+CD4+SPT (black). The numbers inside the white boxes represent the proportion of CD90.1+CD45.2+CD4+SPT cells recovered from LN of mice treated with indicated concentration of IL-7. Three mice per group were analyzed in this experiment.
Lower dose of IL-7 induces CD4+SPT proliferation. (A) Schematic representation of the experimental design where CD90.2+CD45.1+CD4+PERI and CD90.1+CD45.2+CD4+SPT were transferred simultaneously into the same congenic recipients (CD90.2+CD45.2+). Mice were treated daily with either PBS (vehicle) or different doses of IL-7 for 6 days (1, 2.5, 5, or 10 μg/d). (B) Representative flow cytometric analysis of CFSE dilution in CD90.2+CD45.1+CD4+PERI and CD90.1+CD45.2+CD4+SPT cells isolated from LNs of PBS- or IL-7–treated mice. (C) Graphic representation of the percentage of proliferating cells in function of IL-7 concentration for CD90.2+CD45.1+CD4+PERI and CD90.1+CD45.2+CD4+SPT at day 7 of IL-7 treatment. (D) Graphic summary of the absolute number of CD90.2+CD45.1+CD4+PERI (white) and CD90.1+CD45.2+CD4+SPT (black). The numbers inside the white boxes represent the proportion of CD90.1+CD45.2+CD4+SPT cells recovered from LN of mice treated with indicated concentration of IL-7. Three mice per group were analyzed in this experiment.
TCR stimulation modulates IL-7 responsiveness in CD4+ T cells
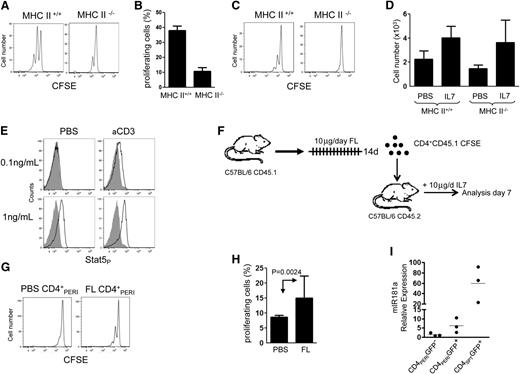
Current models hold that TCR triggering and IL-7 work together to maintain T-cell numbers in the periphery. Whether proliferation of CD4+SPT induced by IL-7 is dependent on TCR stimulation received in the periphery remains unknown. To investigate the requirement of TCR stimulation received by CD4+SPT to undergo proliferation during IL-7 therapy, we transferred CD4+SPT into MHCII−/− hosts and treated mice with IL-7 (Figure 6A). As predicted, we found a partial but significant diminution of cell proliferation in CD4+SPT transferred into IL-7–treated MHCII−/− hosts (Figure 6B). In contrast, cell proliferation induced by IL-7 was completely absent when CD4+PERI cells were transferred into MHCII−/− hosts (supplemental Figure 2A). The thymic medulla is rich in MHC class-II molecules,27,28 and engagement with these, prior to transfer, could potentially provide sufficient TCR stimulation for CD4+SPT to proliferate outside the thymus. We repeated the same experiment but parked CD4+SPT for 3 days into an MHCII−/− host before initiating IL-7 therapy. Despite the fact that IL-7 signaling occurred in this setting (Figure 6D and supplemental Figure 2B), IL-7–induced proliferation of CD4+SPT was completely abrogated (Figure 6C). Thus far, our data support a model wherein TCR stimulation is instrumental to IL-7 effects on CD4+ proliferation and raise the possibility that IL-7 responsiveness could be, in part, controlled by TCR stimulation.
Thymic CD4+ single-positive thymocytes require MHCII contact to proliferate during IL-7 therapy. Enriched CD45.1+CD4+SPT cells from thymuses were labeled with CFSE prior to their transfer into CD45.2+C57BL/6-MHC+/+ or CD45.2+C57BL/6-MHC−/− hosts. (A) Representative flow cytometric analysis of transferred CD4+SPT into MHC+/+ or MHC−/− hosts after 6 days of IL-7 therapy. (B) Histogram analysis showing the absolute number of CD45.1+CD4+SPT cells. (C) Experiment in (A) was repeated and cells were parked for 3 days in their appropriate recipient prior to initiating IL-7 therapy. (D) Histogram analysis showing the absolute number of CD45.1+CD4+SPT recovered in MHCII+/+ and MHCII−/− hosts after parking and 6 days of IL-7 therapy. (E) Flow cytometric analysis showing STAT5-phosphorylation in CD4+PERI exposed to varying concentrations of IL-7 +/− anti-CD3 stimulation. Data are representative of 2 mice per group. (F) Schematic representation of the experimental design where mice were conditioned with FLT3 ligand for 14 days and peripheral FL-CD4+PERI or PBS-CD4+PERI isolated and transferred into wild-type mice treated with IL-7 for 6 days. (G) Flow cytometric analysis showing CFSE profile of FL-CD4+PERI or PBS-CD4+PERI after 6 days of IL-7 treatment. (H) Graphic representation of the percentage of FL-CD4+PERI or PBS-CD4+PERI proliferating cells. Data show 6 mice pooled from 3 independent experiments. (I) Graphic summary of the relative expression of miR181a transcript in peripheral CD4+GFP−, CD4+RTE GFP+, and CD4+SPT GFP+. Data show 3 mice per group pooled from 3 independent experiments.
Thymic CD4+ single-positive thymocytes require MHCII contact to proliferate during IL-7 therapy. Enriched CD45.1+CD4+SPT cells from thymuses were labeled with CFSE prior to their transfer into CD45.2+C57BL/6-MHC+/+ or CD45.2+C57BL/6-MHC−/− hosts. (A) Representative flow cytometric analysis of transferred CD4+SPT into MHC+/+ or MHC−/− hosts after 6 days of IL-7 therapy. (B) Histogram analysis showing the absolute number of CD45.1+CD4+SPT cells. (C) Experiment in (A) was repeated and cells were parked for 3 days in their appropriate recipient prior to initiating IL-7 therapy. (D) Histogram analysis showing the absolute number of CD45.1+CD4+SPT recovered in MHCII+/+ and MHCII−/− hosts after parking and 6 days of IL-7 therapy. (E) Flow cytometric analysis showing STAT5-phosphorylation in CD4+PERI exposed to varying concentrations of IL-7 +/− anti-CD3 stimulation. Data are representative of 2 mice per group. (F) Schematic representation of the experimental design where mice were conditioned with FLT3 ligand for 14 days and peripheral FL-CD4+PERI or PBS-CD4+PERI isolated and transferred into wild-type mice treated with IL-7 for 6 days. (G) Flow cytometric analysis showing CFSE profile of FL-CD4+PERI or PBS-CD4+PERI after 6 days of IL-7 treatment. (H) Graphic representation of the percentage of FL-CD4+PERI or PBS-CD4+PERI proliferating cells. Data show 6 mice pooled from 3 independent experiments. (I) Graphic summary of the relative expression of miR181a transcript in peripheral CD4+GFP−, CD4+RTE GFP+, and CD4+SPT GFP+. Data show 3 mice per group pooled from 3 independent experiments.
To test this hypothesis, we used an in vitro system to study the effect of TCR stimulation on IL-7 response in CD4+PERI. We incubated CD4+PERI with αCD3 and varying concentrations of IL-7 and measured pSTAT5. While TCR stimulation alone had no effect on pSTAT5, when combined with IL-7 it can induce phosphorylation of STAT5 at IL-7 doses that are normally insufficient to induce pSTAT5 (Figure 6E). However, we could not demonstrate an effect of TCR stimulation on IL-7–induced pSTAT5 in CD4+SPT cells, perhaps because cells receive sufficient TCR stimulation inside the thymus and IL-7 responsiveness cannot be further increased (data not shown).
Given that TCR stimulation appears to modulate IL-7 responsiveness, we artificially increased the number of MHCII-expressing cells in the periphery by treating mice with FLT3 ligand (FL) prior to the transfer of CD4+PERI from these mice into IL-7–treated hosts (Figure 6F-H).23 As predicted, we found more proliferation induced by IL-7 in CD4 cells isolated from FL-treated mice. A direct effect of FL on CD4+ cells was ruled out since these cells do not express FLT3R29 (supplemental Figure 3). Based on these results, we conclude that accessibility to MHCII molecules seems to be a chief factor that can modulate IL-7 responsiveness and limit the proliferation of CD4+ T cells induced by IL-7 therapy.
Finally, we sought to determine whether TCR sensitivity might differ between CD4+PERI and CD4+SPT. The importance of miR-181a during positive selection has been documented and it serves primarily to increase TCR sensitivity to self-peptide MHC complexes by regulating several phosphatases known to attenuate TCR signaling.30 Using RAG-GFP mice, we compared the level of miR181a in peripheral mature CD4+ T cells (CD4+GFP−), peripheral CD4+RTEs (CD4+GFP+), and single positive CD4+ thymocytes (CD4+GFP+SPT). We found miR181a levels per cell to be the lowest in peripheral CD4+GFP- T cells, with intermediate levels in CD4+GFP+RTE and the highest levels in thymic-derived CD4+GFP+SPT (Figure 6I). Based on these data, we concluded that CD4+GFP+SPT are perhaps more sensitive to TCR stimulation.
IL-7 therapy expands CD4+ T cells that undergo inefficient HPE in lymphopenic Rag−/− hosts
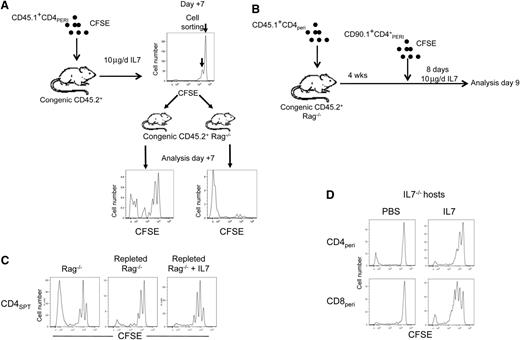
In lymphopenic Rag−/− mice, CD4 HPE is characterized by 2 distinct patterns of proliferation; some cells undergo “brisk proliferation” while others undergo “slow proliferation.” As a result, brisk-proliferating CD4+ T cells outcompete slow-proliferating cells, and this explains the severe skewing and diminished TCR diversity that normally occurs in this setting. Given that IL-7 therapy has been shown to increase TCR diversity,20 we postulated that the effect of IL-7 might be more pronounced on CD4+ cells that undergo slow proliferation under lymphopenic conditions. Following adoptive transfer of CD4+PERI into wt mice and IL-7 therapy, CD4+ T cells were sorted based on CFSE dilution and transferred into Rag−/− recipients to evaluate their proliferation (Figure 7A). Surprisingly, most of the CD4+ T cells that proliferated during IL-7 therapy proliferated slowly in Rag−/− hosts. In contrast, CD4+ cells that failed to undergo proliferation during IL-7 therapy exhibited a pattern of brisk proliferation in Rag−/− recipients (Figure 7B), supporting a model wherein IL-7 therapy induces the proliferation of CD4+ T cells that normally undergo slow proliferation under lymphopenic conditions.
IL-7 therapy preferentially expands CD4+ T cells with low affinity TCR for self-peptide–MHCII complexes. (A) Schematic representation where diluted and undiluted CFSE peaks from CD45.1+CD4+PERI obtained after 6 days of IL-7 therapy were sorted and transferred into distinct Rag−/− recipients for another 7 days. At the bottom, representative flow cytometric analysis 7 days after the transfer of each CFSE peaks into Rag−/− recipients. Left histogram represents cells that have initially undergone proliferation induced by IL-7. Right histogram represents cells that have not initially undergone proliferation during IL-7 therapy. Experiment was repeated twice with 2 mice for each experiment. (B) Schematic representation of the experimental design where Rag−/− mice were first transplanted with 1 × 106 CD45.1+CD90.2+ T cells. Five weeks post transfer, mice received 1 × 106 CFSE-labeled CD45.2+CD90.1+ T cells. Mice were then treated with either PBS or IL-7 for 8 days. Mice were then sacrificed and CD45.2+CD90.1+CD4+ T cells were analyzed for evidence of proliferation. Control consisted of empty Rag−/− mice. (C) Flow cytometric analysis of CFSE dilution of cells described in (B). Data are representative of 6 to 9 mice per group pooled from 3 independent experiments. (D) Representative flow cytometric analysis of CFSE dilution of peripheral CD4+ T cells transferred into IL-7−/− recipients treated with PBS or IL-7. Data are representative of 6 mice per group pooled from 2 independent experiments.
IL-7 therapy preferentially expands CD4+ T cells with low affinity TCR for self-peptide–MHCII complexes. (A) Schematic representation where diluted and undiluted CFSE peaks from CD45.1+CD4+PERI obtained after 6 days of IL-7 therapy were sorted and transferred into distinct Rag−/− recipients for another 7 days. At the bottom, representative flow cytometric analysis 7 days after the transfer of each CFSE peaks into Rag−/− recipients. Left histogram represents cells that have initially undergone proliferation induced by IL-7. Right histogram represents cells that have not initially undergone proliferation during IL-7 therapy. Experiment was repeated twice with 2 mice for each experiment. (B) Schematic representation of the experimental design where Rag−/− mice were first transplanted with 1 × 106 CD45.1+CD90.2+ T cells. Five weeks post transfer, mice received 1 × 106 CFSE-labeled CD45.2+CD90.1+ T cells. Mice were then treated with either PBS or IL-7 for 8 days. Mice were then sacrificed and CD45.2+CD90.1+CD4+ T cells were analyzed for evidence of proliferation. Control consisted of empty Rag−/− mice. (C) Flow cytometric analysis of CFSE dilution of cells described in (B). Data are representative of 6 to 9 mice per group pooled from 3 independent experiments. (D) Representative flow cytometric analysis of CFSE dilution of peripheral CD4+ T cells transferred into IL-7−/− recipients treated with PBS or IL-7. Data are representative of 6 mice per group pooled from 2 independent experiments.
Given that brisk-proliferating CD4+ T cells rapidly outcompete slow-proliferating CD4+ T cells during lymphopenia, we wanted to determine which population would be affected the most by IL-7 therapy. While empty Rag−/− hosts efficiently supported HPE of brisk-proliferating CD4+SPT thymocytes, Rag−/− mice replete with polyclonal T cells prior to the adoptive transfer of congenic CD4+SPT supported, almost exclusively, HPE of slow-proliferating CD4+SPT thymocytes. Consistent with data presented in Figure 7A, we found the proliferation of slow-proliferating CD4+SPT to be further enhanced by IL-7 therapy (Figure 7C). To confirm the specificity of IL-7 therapy toward slow-proliferating CD4+ T cells, we used IL-7−/− mice that do not support HPE of CD4+ T cells with the exception of a few CD4+ T cells that undergo brisk proliferation but fail to accumulate as they normally do in Rag−/− hosts (Figure 7D). In these mice, IL-7 treatment induced a slow proliferation of CD4+ T cells without inducing accumulation of CD4+ cells that extensively dilute their CFSE, confirming that IL-7 therapy works primarily by enhancing the proliferation of slow-proliferating CD4+ T cells in the setting of lymphopenia.
Discussion
During the last decade, progress has been made in understanding the biology of T-cell depletion, allowing the identification of IL-7 as the principal mediator of T-cell homeostasis in humans and mice.3 Given the critical role of IL-7 in thymopoiesis, HPE, and maintenance of T lymphocytes in the periphery, scientists have postulated that IL-7 therapy could be used to accelerate immune reconstitution. In humans, clinical trials have indicated that IL-7 therapy presents a stronger bias toward the expansion of CD8+ T cells compared with CD4+ T cells.19-21 Our study was undertaken to understand how IL-7 therapy works on CD4+ T cells. As in humans, IL-7 therapy preferentially expands CD8+ T cells in mice. Despite weak IL-7–induced proliferation of CD4+ T cells, IL-7 therapy does expand this subset, suggesting that the IL-7–mediated effect is primarily on survival. The proliferation induced by IL-7 therapy depends on signals mediated by self-peptides presented by MHCII molecules (Figure 6). A substantial effect of MHCII on CD4 proliferation is consistent with preclinical models that show the requirement of TCR signaling in addition to IL-7 in order to support CD4 HPE.6,7,31,32 For CD8+ T cells, CD11c+ dendritic cells appear important but not essential for HPE, as lack of CCR7 expression by CD8+ T cells has no effect on HPE of these cells.33,34 These fundamental differences, which are based on the requirement that antigen-presenting cells provide TCR stimulation for CD4+ T cells, could explain the greatest difference in the effects of IL-7 on CD8 and CD4 proliferation.
Some data obtained in mice and humans suggest a thymopoietic effect of IL-7 therapy.14,20,21,35-38 Studies have demonstrated substantial changes in the naive T-cell compartment that is often accompanied by diminished proportions or increased TREC numbers as well as increased TCR diversity, all consistent with production and/or preferential expansion/retention of RTEs during IL-7 therapy.13,15,20,26,39 Therefore we originally postulated that CD4+ T cells that undergo proliferation during IL-7 therapy were possibly CD4+RTEs. Surprisingly, it was not the case, and proliferation induced by IL-7 therapy was essentially the same in RTE and non-RTE CD4+ cells (Figure 2B-C). Unexpectedly, we found the effects of IL-7 to be the most potent on single-positive CD4+ thymocytes, as these cells proliferate more than CD4+PERI and CD4+RTEs during IL-7 therapy. Both CD4+SPTs and peripheral CD4+ T cells express similar levels of IL-7Rα, but CD4+ thymocytes respond to lower concentrations of IL-7. While TCR triggering is dispensable for RTE proliferation in response to IL-7,25,40,41 proliferation of CD4+SPT is largely dependent on TCR signaling that is mediated by self-peptide/MHCII complexes. Importantly, some CD4+SPT cells can still proliferate in MHCII−/− hosts. However, TCR-independent proliferation during IL-7 therapy is of short duration, and, once in the periphery, CD4+SPTs rapidly diminish their response to IL-7. Because the thymic microenvironment is rich in MHCII molecules, we postulated that TCR signaling received inside the thymus modulates responsiveness to IL-7 and residual proliferation in MHCII−/− hosts.27,28,42,43 A substantial effect of TCR signaling on cytokine responsiveness has been shown for IL-2 and IL-15, and our data provide compelling evidence that TCR stimulation also modulates IL-7 response.44-46 The finding that CD4+ T cells isolated from FL-treated animals undergo stronger proliferation when exposed to IL-7 therapy confirms that the bioavailability of MHCII-expressing cells and the influence of TCR stimulation are both critical for CD4 proliferation during IL-7 therapy. The role of phosphoinositide 3-kinase (PI3K) in lymphocyte homeostasis is well known and serves primarily to control cell proliferation and cell death.36,41,47 TCR signaling occurs through PI3K, and IL-7 can signal via STAT5 or PI3K. Therefore a synergistic/additive effect between IL-7 and TCR signaling on PI3K levels could be required to trigger CD4 proliferation during lymphopenia or IL-7 therapy. Finally, although we observed higher levels of miR181a in CD4+SPT compared with CD4+PERI, its contribution to TCR signaling in these cells remains unknown. However, a positive effect of miR181a on TCR sensitivity would be consistent with increased proliferation induced by IL-7. Additional studies are needed to clarify the physiological role of miR181a on TCR signaling and IL-7 response of CD4+SPT.
Several studies have demonstrated that thymic insults occur in most clinical settings of T-cell depletion in humans, and HPE represents the main pathway by which T lymphocytes recover. Following immune reconstitution by HPE, we showed that slow-proliferating CD4+ cells, but not brisk-proliferating cells, can still undergo HPE, suggesting the existence of 2 distinct CD4 niches that minimally overlap each other. Given that brisk-proliferating CD4+ T cells can recover by HPE and that IL-7 therapy has no effect on their proliferation, our data support a model wherein the primary benefit of IL-7 therapy occurs through the enhanced proliferation of CD4+ cells that typically undergo slow proliferation during lymphopenia. Interestingly, slow-proliferating CD4+ T cells are more abundant inside the thymus (supplemental Figure 4). Therefore, as IL-7 rises following T-cell depletion, it is tempting to speculate that as CD4+RTEs exit the thymus, they will be the first to respond and undergo proliferation. Given that CD4 proliferation can be further increased by IL-7 treatment, it is likely that patients with residual thymic function will benefit the most from IL-7 therapy.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Mrs Catherine Mauger for mice husbandry, Dr Crystal Mackall and Dr Claude Perreault for reviewing the manuscript, and Dr Claude Perreault who kindly provided the RAG2p-GFP mice. The authors also thank Cytheris for providing rhIL-7.
This work was supported by grants from the Canadian Institutes of Health Research and Le fond de la Recherche en Santé du Québec to M.G; S.-D.G. received a scholarship from the Canadian Institutes of Health Research.
Authorship
Contribution: O.H.-T. and D.L. performed experiments, analyzed data, and wrote the manuscript; S.-D.G. and S.T. provided scientific input to the design of some experiments and reviewed the manuscript; M.D. performed cell sorting; B.A. and A.G. reviewed the manuscript and provided IL-7; and M.G. designed the project, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: B.A. and A.G. work for Cytheris. The remaining authors declare no competing financial interests.
Correspondence: Martin Guimond, Department of Microbiology-Immunology, Faculty of Medicine, University of Montreal, Department of Hematology-Oncology, Maisonneuve-Rosemont Hospital, 5415, Boulevard de l’Assomption, Montreal, H1T 2M4, Canada;e-mail: martin.guimond@umontreal.ca.
References
Author notes
O.H.-T. and D.L. are co-first authors.