Key Points
Autoantigens bind to and induce proliferation of CLL cells, supporting chronic antigenic stimulation as an important pathomechanism in CLL.
Abstract
Antigenic targets of the B-cell receptor (BCR) derived from malignant cells in chronic lymphocytic leukemia (CLL) might play a role in the pathogenesis of this neoplasm. We screened human tissue–derived protein macroarrays with antigen-binding fragments derived from 47 consecutive cases of CLL. An autoantigenic target was identified for 12/47 (25.5%) of the cases, with 3 autoantigens being the target of the BCRs from 2 patients each. Recombinantly expressed autoantigens bound specifically to the CLL cells from which the BCR used for the identification of the respective autoantigen was derived. Moreover, binding of the autoantigen to the respective leukemic cells induced a specific activation and proliferation of these cells. In conclusion, autoantigens are frequent targets of CLL-BCRs. Their specific binding to and induction of proliferation in the respective leukemic cells provide the most convincing evidence to date for the long-time hypothesized role of autoantigens in the pathogenesis of CLL.
Introduction
Chronic lymphocytic leukemia of the B-cell type (B-CLL) is the most common leukemia in adults in the Western world1 and has a heterogeneous clinical course. It consists of B cells expressing common B-cell markers, including monoclonal immunoglobulins on their cell surface, which function as the antigen receptor of B cells (BCR).2 In approximately half of cases, the leukemic cell clone displays somatically mutated immunoglobulin (Ig) heavy (H) chain variable (IgHV) genes.3 Patients with CLL expressing mutated IgHV BCR (M-CLL) have a better prognosis than patients with unmutated IgHV genes (U-CLL).4-7 Even though the exact cell of origin is not known, it is assumed on the basis of phenotypic analyses8 and gene expression profiling9,10 that both U-CLL and M-CLL derive from antigen-experienced B cells,11 and clonal selection by antigen is probably responsible for a biased usage of the IgHV repertoire of B cells of both U-CLL and M-CLL compared with normal B cells.4,12 In CLL, certain IgHV genes frequently recombine with distinct human immunoglobulin heavy diversity (IGHD) and heavy chain joining (IGHJ) segments, resulting in highly similar H chain complementarity determining region 3 (CDR3) sequences. These “stereotyped” receptors13 often associate with distinct Ig light chain variable κ (IgKV) or λ (IgLV) genes with characteristic κ-CDR3 or λ-CDR3 sequences. Twenty to 30% of patients with CLL display stereotyped BCRs that belong to 1 of approximately 150 different subsets with homologous receptors.3,14-20 Taken together, these data suggest that the functional status of the immunoglobulin and its interaction with antigens may play a role in CLL pathogenesis and progression.
The antigens bound by CLL BCRs are largely unknown, although some autoantigens have been defined.21-26 BCRs of U-CLL have been reported to be more promiscuous than those of M-CLL.25 Moreover, many CLL-BCRs have been reported to display structural features that resemble antibodies reactive with microbial antigens.14 Finally, stereotypical BCRs from CLL have been reported to recognize survival promoting antigens such as vimentin and calreticulin found on stromal cells27 or apoptotic cells,28,29 supporting the role of the microenvironment in the pathogenesis and/or progression of B-CLL.30,31
The aim of the present study was to perform an unbiased search for autoantigens targeted by CLL-BCRs by using a modification of the SEREX32 approach, which has proven successful in defining antigenic targets of paraproteins from patients with monoclonal gammopathy of underdetermined significance (MGUS) and multiple myeloma.33,34 The results of this study demonstrate that autoantigens are the antigenic targets of CLL-BCRs in a considerable proportion of CLL cells, bind specifically to, and induce proliferation of the respective leukemic cells.
Materials and methods
This study was approved by the local ethical review board (“Ethikkommission der Ärztekammer des Saarlandes”) and conducted according to the Declaration of Helsinki. Recombinant DNA work was performed with permission of and according to the regulations of local authorities (Government of Saarland). Human materials were obtained during routine diagnostic or therapeutic procedures and immediately used or stored at −80°C. Written informed consent was obtained from patients.
Study population
Peripheral blood samples of 51 consecutive patients with B-CLL treated at the Department of Internal Medicine I of Saarland University were included in this study if they contained >65% CD5+/CD19+ B cells (supplemental Table 1). Additional CLL cases with predefined stereotypes were obtained from the Department of Hematology, Ulm University, Germany (supplemental Table 2).
Preparation of Fab fragments derived from CLL cells
Fresh EDTA blood of patients with >65% CD5+/CD19+ malignant B cells was used for the preparation of antigen-binding fragments (Fabs). In detail, blood was drawn and centrifuged (10 minutes with 270g). Plasma was removed and stored at −70°C for further use. The cell pellet was washed with phosphate-buffered saline (PBS) and subjected to Ficoll separation using a standard protocol. Cells were removed, washed again with PBS, and suspended in RPMI at 1 × 107 cells/mL. After the addition of papain (final concentration 100 µg/mL, Sigma-Aldrich, Taufkirchen, Germany; supplemental Table 3), and cysteine (final concentration 0.1 mM, Sigma-Aldrich), the mixture was incubated for 2.5 h at 37°C with gentle shaking. Iodoacetamide (Sigma-Aldrich) was added to a final concentration of 20 mM for the inactivation of papain. After centrifugation, the clear supernatant was dialyzed against PBS followed by concentration using Amicon 10-kD cartridges (Millipore, Darmstadt, Germany). Final concentration of the Fabs was set at 10 µg/mL for screening. Preparation and purification was checked by sodium dodecyl sulfate polyacrylamide gel electrophoresis on native and denaturing gels followed by immunodetection of the Fabs using anti-human Fab antibodies (Dianova, Hamburg, Germany).
Screening of Fab preparations for immunoreactivity
High-density protein arrays Unipex 1 and Unipex 2 were obtained from Source Bioscience Life Sciences (Nottingham, UK; for the description of membranes, see http://www.lifesciences.sourcebioscience.com/clone-products/proteomic-resources/protein-arrays.aspx). For screening, the filters were blocked in 10% (weight to volume ratio) non-fat dry milk powder in TBST (TBS, 0.1% [volume to volume ratio] Tween 20) at 4°C overnight, washed twice in TBST, and incubated for 1 h with the Fab preparations at 10 µg/mL. Following 3 30-min TBST washes and subsequent incubation with 1:2500 (volume to volume ratio) diluted rabbit anti-human κ- and λ-light chain (Dako, Copenhagen, Denmark) and anti-rabbit IgG-HRP (1:5000, Biorad, Munich, Germany) in 2% (weight to volume ratio) milk/TBST, the filters were washed 3 times for 10 minutes in TBST. This was followed by detection using a Pharmacia ECL system (Freiburg, Germany). Positive signals were localized according to the manufacturer’s protocol. Corresponding clones were obtained from Source Bioscience Life Sciences.
Analysis of IgHV, IgKV, and IgLV genes
Genomic DNA was extracted from blood using a Qiagen blood DNA extraction kit (Qiagen, Hilden, Germany). Primers and protocols used for the amplifications had been described by van Dongen et al.35 Products were analyzed by electrophoresis on agarose gels and further sequenced by automated sequencing. Sequencing results were analyzed online using IMGT/V-QUEST on IMGT, the international ImMunoGeneTics information system (http://www.imgt.org/).36
BCR cloning and expression
Peripheral blood from patients was used for the isolation of genomic DNA using a Qiagen Blood DNA purification kit. Variable regions of Ig heavy and light chains were amplified as described previously. Polymerase chain reaction (PCR) products were sequenced and adapted to a phagemid vector (pCES) for expression of His6-tagged proteins in Escherichia coli TG1 as has been described previously.37 After lysis with PBS, the Fab products were purified by IMAC chromatography (Qiagen) and concentrated and stored at −20°C until use.
ELISA
Each region coding for an antigenic BCR target was recombinantly expressed in HEK293 cells under control of a cytomegalovirus (CMV) promoter introducing a FLAG tag at the carboxyl terminus of the protein. Total cell extracts were prepared and coated indirectly to Nunc Maxisorb plates using anti-FLAG mAb. Enzyme-linked immunosorbent assay (ELISA) was performed according to standard protocols with Fabs (obtained by papain digestion of CLL cells or by recombinant expression of the cloned BCR, respectively), goat anti-human IgG (Fab)2-biotin (Dianova), and rabbit anti-human λ- and anti human κ-light chain (Dako). For the determination of the antibody-binding epitope, recombinant fragments were constructed and expressed as described for the full-length protein. All ELISA experiments were run in at least 3 independent assays.
Western blot
For western blot analyses, proteins were purified from 500 mL of bacterial cultures grown at 37°C. Expression of recombinant proteins fused with a His6-tag was induced in the cultures with 1 mM isopropyl-d-thiogalactopyranoside (Fermentas, St. Leon-Rot, Germany) at an optical density at 578 (OD578) of 0.6-0.7. After 4 hours, cells were pelleted, resuspended in 10 mL of buffer B (20 mM Tris-HCl pH 8.0, 0.05% Triton ×100, 0.4 µM PMSF [Sigma], 10 µM leupeptin [Sigma], 1 mg/mL lysozyme [Roche, Mannheim, Germany]), and incubated at 4°C for 20 minutes. Alternatively, total blood lysates from patients and controls were used as a source for the antigenic target.
Identification of Fab-binding epitopes
Epitope specificity was determined by ELISA. Recombinant fragments of the identified antigenic targets were produced by PCR, expressed in HEK293, and purified and used as a coat. These fragments were screened for reactivity with patients’ natural and recombinant Fabs by ELISA.
Staining of CLL cells with recombinant BCR target proteins
Peripheral blood of the respective patients was subjected to Ficoll separation according to standard protocols. CLL cells were first stained with the recombinant antigen and, in a second step, with CD5/CD19 (BD, Heidelberg, Germany). In detail, the mononuclear cells were stained with the patient-specific His6-tagged antigen (5 µg/mL), followed by mouse anti-His (1:500, Qiagen), anti-mouse IgG (Fab)2-biotin (1:200, Dianova) and Strept-APC (1:500, BD). All staining steps were done for 30 minutes at 4°C, each followed by 3 washing steps with PBS. After the first staining and an intense washing, the cells were stained with CD5/CD19 following the instructions of the manufacturer. Cells were analyzed on a FACS Canto Analyzer (BD, Heidelberg, Germany).
Activation of CLL cells
Intracellular calcium changes were assessed using Fluo-4/AM dye and analyzed by flow cytometry analysis on a FACS Canto Analyzer. CLL cells and B cells derived from healthy donors were isolated by Ficoll separation followed by an intensive washing with PBS. Cells were resuspended in calcium- and magnesium-free PBS and loaded with Fluo-4/AM dye (final concentration 2 µM, Invitrogen, Karlsruhe, Germany) for 30 minutes at room temperature. Antigen was added followed by flow cytometry of the cells. The calcium ionophore ionomycin (10 ng/μL, Sigma-Aldrich) was used as a positive control for the release of calcium from internal stores. Time course of [Ca2+]intern was analyzed by adding the antigen to the dye-loaded cells, mixing, and immediately starting repeated measuring of the samples in intervals of 30 seconds.
Induction of Ki67 and c-myc protein expression and proliferation
Blood was drawn, subjected to Ficoll separation, and followed by purification of CD5+/CD19+ CLL cells using MACS cell separation technology (Miltenyi, Cologne, Germany). Then, 4 × 103 Ficoll-separated peripheral blood mononuclear cells (PBMC) and purified CLL cells (CD5/CD19 positive cells >95%) were seeded and cultured in RPMI 1640/10% fetal calf serum for 4 hours before a specific BCR target (10 µg/mL), an irrelevant BCR target (10 µg/mL), and anti-IgM (10 µg/mL) was added. After 3 days of culture, cells were analyzed for the expression of Ki67 by flow cytometry and c-myc by western blot, using a monoclonal antibody against MYC (clone 9E10, 2 µg/mL [homemade]). In addition, cell proliferation was measured using the EZ4U kit (Biomedica, Vienna, Austria) according to the manufacturer’s instructions.
Results
Immunoscreening of protein macroarrays with CLL-derived Fab fragments
Qualified peripheral blood samples (ie, samples with >65% CD5+/CD19+ cells) from 51 consecutive patients with CLL treated at our institution were included in this study. The characteristics of these 51 patients are summarized in supplemental Table 1. Patient 7 was excluded from the study; he did not qualify because of too low a percentage of CLL cells in his peripheral blood, leaving 50 patients from whom we tried to obtain Fabs. Fabs were obtained by papain digestion of PBMC from these patients and checked for integrity by gel electrophoresis (data not shown). The Fab preparation was successful in 47/50 patients. The Fabs were used at a concentration of 10 µg/mL to screen for reactivity with proteins represented in 2 high-density protein macroarrays: 1 derived from human fetal brain complementary DNA and a second derived from a mixture of expression libraries derived from activated T cells, lung, and colon. Twelve of the 47 Fab preparations (25.5%) showed a reaction with a protein represented in 1 of the 2 macroprotein arrays (Table 1 and supplemental Figure 1). Three antigens (pyruvate carboxylase, the hypothetical protein Fam32A, and calcyclin-binding protein) were targeted by the Fabs from 2 patients each, whereas the remaining 6 antigens were targeted by the Fabs derived from the CLL cells of 1 patient only.
Summary of antigens targeted by CLL-derived Fab, binding epitope, IG heavy/light chain usage, mutational status, and stereotyped subsets
| No. . | Antigen . | Accession no. . | BCR-binding epitope . | Recognized by patient no. . | Heavy chain . | Light chain . | Germline homology (IGHV/IGKV/IGLV) (%) . | Mutations . | Subset . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IGHV . | IGDV . | Frame . | IGJV . | Type . | IGKV/IGLV . | IGKJ/IGLJ . | ||||||||
| 1 | Pyruvate carboxylase | NM_000920 | aa1031-1084 | 24 | 4-34*01 | 1-14*01 | 1 | 4*02 | IGKV | 3-20*01 | 1*01 | 98.6/98.58 | Unmut./unmut. | None |
| aa1124-1181 | 26 | 3-7*03 | 3-3*01 | 1 | 4*02 | IGLV | 4-69*01 | 2*01 | 100/100 | Unmut./unmut. | None | |||
| 2 | LLP homolog | NM_032338 | aa38-91 | 20 | 5-51*01 | 3-3*01 | 2 | 6*02 | IGKV | 1-5*01 | 4*01 | 100/96.77 | Unmut./mut. | None |
| 3 | Fam32A | NM_014077 | aa1-32 | 21 | 1-69*01 | 3-10*01 | 3 | 6*03 | IGLV | 3-21*02 | 2*01 | 100/100 | Unmut./unmut. | 5 |
| aa69-79 | 31 | 3-23*04 | 3-3*01 | 2 | 1*01 | IGKV | 3-20*01 | 1*01 | 98.26/98.23 | Unmut./unmut. | None | |||
| 4 | Notch2 | NM_024408 | aa495-505 | 51 | 3-23*01 | 4-23*01 | 3 | 4*02 | IGKV | 3-15*01 | 4*01 | 97.1/100 | Mut./unmut. | None |
| 5 | CACYBP | NM_014412 | aa92-102 | 44 | 4-34*01 | 3-10*01 | 2 | 6*02 | IGKV | 2-30*01 | 2*01 | 96.14/97.96 | Mut./mut. | 4 |
| aa1-102 | 46 | 3-30*03F | 3-3*01F | 2 | 4*02 | IGKV | 3-11*01 | 2*01 | 91.67/96.42 | Mut./mut. | None | |||
| 6 | SMCHD1 | NM_015295 | aa1897-1907 | 45 | 3-30*03 | 3-09*01 | 1 | 4*02 | IGLV | 2-14*02 | 3*01 | 100/100 | Unmut./unmut. | None |
| 7 | MAZ | NM_001042539 | aa239-254 | 27 | 4-34*03F | 3-3*01F | 2 | 6*02 | IGKV | 3-20*01F | 1*01F | 95.77/96.1 | Mut./mut. | None |
| 8 | Actin-γ | NM_001614.21 | aa55-67 | 47 | 3-13*01 | 6-19*01 | 1 | 2*01 | IGKV | 3-11*01 | 3*01 | 100/100 | Unmut./unmut. | None |
| 9 | MTUS1 | NM_001001924 | aa1146-1162 | 48 | 1-24*01 | 6-19*01 | 1 | 6*02 | IGKV | 3-11*01 | 1*01 | 100/100 | Unmut./unmut. | None |
| No. . | Antigen . | Accession no. . | BCR-binding epitope . | Recognized by patient no. . | Heavy chain . | Light chain . | Germline homology (IGHV/IGKV/IGLV) (%) . | Mutations . | Subset . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IGHV . | IGDV . | Frame . | IGJV . | Type . | IGKV/IGLV . | IGKJ/IGLJ . | ||||||||
| 1 | Pyruvate carboxylase | NM_000920 | aa1031-1084 | 24 | 4-34*01 | 1-14*01 | 1 | 4*02 | IGKV | 3-20*01 | 1*01 | 98.6/98.58 | Unmut./unmut. | None |
| aa1124-1181 | 26 | 3-7*03 | 3-3*01 | 1 | 4*02 | IGLV | 4-69*01 | 2*01 | 100/100 | Unmut./unmut. | None | |||
| 2 | LLP homolog | NM_032338 | aa38-91 | 20 | 5-51*01 | 3-3*01 | 2 | 6*02 | IGKV | 1-5*01 | 4*01 | 100/96.77 | Unmut./mut. | None |
| 3 | Fam32A | NM_014077 | aa1-32 | 21 | 1-69*01 | 3-10*01 | 3 | 6*03 | IGLV | 3-21*02 | 2*01 | 100/100 | Unmut./unmut. | 5 |
| aa69-79 | 31 | 3-23*04 | 3-3*01 | 2 | 1*01 | IGKV | 3-20*01 | 1*01 | 98.26/98.23 | Unmut./unmut. | None | |||
| 4 | Notch2 | NM_024408 | aa495-505 | 51 | 3-23*01 | 4-23*01 | 3 | 4*02 | IGKV | 3-15*01 | 4*01 | 97.1/100 | Mut./unmut. | None |
| 5 | CACYBP | NM_014412 | aa92-102 | 44 | 4-34*01 | 3-10*01 | 2 | 6*02 | IGKV | 2-30*01 | 2*01 | 96.14/97.96 | Mut./mut. | 4 |
| aa1-102 | 46 | 3-30*03F | 3-3*01F | 2 | 4*02 | IGKV | 3-11*01 | 2*01 | 91.67/96.42 | Mut./mut. | None | |||
| 6 | SMCHD1 | NM_015295 | aa1897-1907 | 45 | 3-30*03 | 3-09*01 | 1 | 4*02 | IGLV | 2-14*02 | 3*01 | 100/100 | Unmut./unmut. | None |
| 7 | MAZ | NM_001042539 | aa239-254 | 27 | 4-34*03F | 3-3*01F | 2 | 6*02 | IGKV | 3-20*01F | 1*01F | 95.77/96.1 | Mut./mut. | None |
| 8 | Actin-γ | NM_001614.21 | aa55-67 | 47 | 3-13*01 | 6-19*01 | 1 | 2*01 | IGKV | 3-11*01 | 3*01 | 100/100 | Unmut./unmut. | None |
| 9 | MTUS1 | NM_001001924 | aa1146-1162 | 48 | 1-24*01 | 6-19*01 | 1 | 6*02 | IGKV | 3-11*01 | 1*01 | 100/100 | Unmut./unmut. | None |
Mut., mutated; unmut., unmutated.
*Samples with less than 2% nucleotide differences to published germline sequences were defined as unmutated.
Demonstration of the CLL-derived BCR origin of natural CLL-Fabs
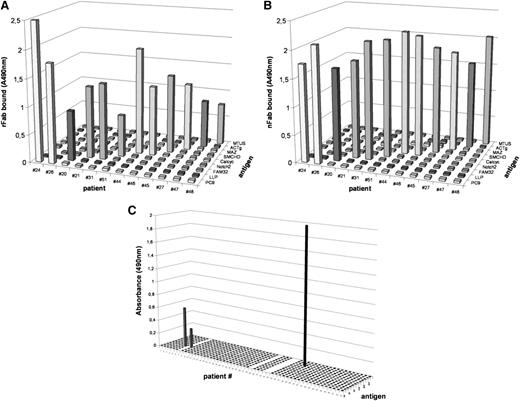
To demonstrate that the observed reactions were indeed mediated by Fabs derived from the BCR on the surface of the respective CLL clone and not from contaminating immunoglobulins or other membrane proteins, the IG heavy/light chain usage regions of the CLL clones of all 12 patients, whose nFabs had targeted an antigen in the immunoscreening with the two macroarrays were amplified and sequenced (Table 1). The immunoglobulin genes (IgHV, IgKV, and IgLV) were amplified by PCR and cloned into a phagemid vector (pCES) to produce recombinant Fab fragments in TG1 E coli cells. The resulting recombinant Fab fragments (rFab) were purified by chromatography and characterized by protein gel electrophoresis and western blot. As a next step, the expressed rFab and the “natural” Fab (nFab obtained by digestion of CLL cells) were tested by ELISA using the respective recombinant antigens as a coat. Identical specificity for and exclusive reactivity with the respective autoantigen was confirmed for all 12 pairs of rFab and nFab derived from the CLL cells by demonstrating reactivity with the identical protein of the macroarray (supplemental Figure 1) and identical binding pattern of nFab and corresponding rFabs to the 9 autoantigens identified in this study (Figure 1). Definitive proof that all 12 pairs of nFab and rFab recognized identical antigens (and epitopes) was provided by competition ELISA using specific antigens and control antigens (supplemental Figure 2) that demonstrated the displacement of the specific rFab by increasing concentrations of their respective nFab derived from the same CLL clone (supplemental Figure 3).
Reactivity and specificity of rFab and nFab derived from CLL cells from 12 patients tested for all identified CLL antigens. (A) rFabs obtained by BCR cloning and expression and (B) CLL-derived nFabs obtained by papain digestion show identical specificity in the ELISA using the recombinant CLL antigens as antigenic coats. (C) Reactivity and specificity of nFab derived from CLL cells from 47 patients tested for previously identified CLL antigens. On the x-axis, all 51 patients are shown (patients 29, 33, and 34 failed during Fab preparation; patient 7 does not qualify); the antigens tested are listed on the y-axis and included (from front to back: BSA, acetylated BSA, LDL, oxidized LDL (oxLDL), Streptococcus pneumoniae, calreticulin, cofilin, vimentin, myosin heavy chain 2A (MYH2A), U266 cells before and after induction of apoptosis with methotrexate, and CMV-derived pUL32 protein. Only 3 reactivities were detected: oxLDL was recognized by BCRs from patients 6 and 8 and MYH2A was recognized by patient 38.
Reactivity and specificity of rFab and nFab derived from CLL cells from 12 patients tested for all identified CLL antigens. (A) rFabs obtained by BCR cloning and expression and (B) CLL-derived nFabs obtained by papain digestion show identical specificity in the ELISA using the recombinant CLL antigens as antigenic coats. (C) Reactivity and specificity of nFab derived from CLL cells from 47 patients tested for previously identified CLL antigens. On the x-axis, all 51 patients are shown (patients 29, 33, and 34 failed during Fab preparation; patient 7 does not qualify); the antigens tested are listed on the y-axis and included (from front to back: BSA, acetylated BSA, LDL, oxidized LDL (oxLDL), Streptococcus pneumoniae, calreticulin, cofilin, vimentin, myosin heavy chain 2A (MYH2A), U266 cells before and after induction of apoptosis with methotrexate, and CMV-derived pUL32 protein. Only 3 reactivities were detected: oxLDL was recognized by BCRs from patients 6 and 8 and MYH2A was recognized by patient 38.
Characterization of CLL-derived BCR antigens
We had previously shown that antigenic targets of BCRs from multiple myeloma and MGUS are hyperphosphorylated in the respective patients compared with normal controls. We therefore analyzed the antigenic targets of CLL-BCRs by gel electrophoresis and isoelectric focusing. As shown in supplemental Figure 4, the antigens detected in our study by CLL-BCRs showed identical migration patterns when derived from patients and controls, excluding posttranslational modifications, deletions, or insertions as a cause for their autoimmunogenicity. Similarly, the sequencing of genomic and complementary DNA showed no differences between patients and controls, excluding mutations or single-nucleotide polymorphisms as the underlying mechanism inducing their immunogenicity (data not shown).
Determination of the Fab-binding epitopes
To determine the Fab-binding epitopes, recombinant fragments of the identified antigenic targets were produced by PCR, expressed in HEK293, and purified and used as a coat for ELISA. Detection was done using both nFab and rFab derived from the respective patients. Surprisingly, the Fabs from patients, which recognized the same antigen, bound to different epitopes of these antigens in all 3 pairs (Table 1). For example, both nFab and rFab from patient 24 bound to amino acids 1031-1084 of the antigenic target pyruvate carboxylase, whereas the Fabs from patient 26 bound to amino acids 1124-1181 of this antigen. Similarly, the epitopes of the hypothetical protein Fam32A and calcyclin-binding protein, which were targeted by the Fabs derived from the CLL cells of 2 different patients, were not identical (Table 1). None of the epitopes showed homology to known bacterial or viral proteins deposited in the data bank.
IG heavy and light chain usage, mutational status, and CLL stereotypes of autoantigen-targeting CLL-BCRs
The IgHV and IgLV usage of the CLL-derived rFabs from 12 patients binding to 1 of the 9 antigens detected in this study were determined by sequencing the corresponding PCR products from 8/12 patients. The results are summarized in Table 1. No prevalence for a specific IG heavy and light chain usage was observed. Different IgHV and IGLV genes were used by Fabs targeting the same antigen: for example, pyruvate carboxylase 9 was recognized by the CLL-BCRs from patients 24 and 26, respectively. CLL-BCR from patient 24 used IgHV 4-34 and IgKV 3-20, whereas the CLL-BCR from patient 26 used IgHV 3-7*03 and IgLV 4-69*01. Moreover, the same antigen reacted both with the CLL-BCR from patient 26, which was encoded by 100% unmutated IgHV/IgLV genes, and with the CLL-BCR from patient 24, which was encoded by IgHV/IgLV genes, which were mutated to a degree of 1.4% each.
According to the classification of stereotype subsets as proposed by Stamatopoulos et al19 and Lanemo Myrhinder et al,38 the CLL-BCRs from 2 of the 12 patients targeting an autoantigen completely fulfilled the criteria for a defined stereotype subset: the BCR from patient 21 belonged to subset 5 and the BCR from patient 44 belonged to subset 4. The CLL-BCRs from all other patients for which an autoantigenic target had been identified in the original immunoscreen could not be assigned to a defined stereotype subset (Table 1).
Reactivity of Fab with previously described autoantigens
We tested the nFab obtained from our 47 CLL patients for reactivity with BCR targets molecularly defined and published in the literature. These antigens included bovine serum albumin (BSA), acetylated BSA, low-density lipoprotein (LDL) and oxidized LDL,38 Streptococcus pneumonieae,38 calreticulin,38 cofilin,38 vimentin,38 myosin heavy chain 2A (MYH2A),29 U266 cells before and after induction of apoptosis (by treatment with methotrexate 2 mM for 48 hours at 37°C),38 and the CMV-derived pUL32 protein.26 We could confirm only 3 reactivities: oxLDL was recognized by patients 6 and 8 (both belonging to subset 1) and MYH2A by patient 38 (subset 6), whereas all other antigens tested could not be confirmed and no binding of these previously reported BCR targets to CLL cells belonging to the respective subsets could be demonstrated (Figure 1C).
Antigenic targets of CLL-BCRs belonging to the same stereotype
Among the CLL-BCRs for which an antigenic target was identified, one belonged to stereotype subset 4 and one to subset 5, respectively. The BCR from patient 21 belonged to subset 5 and bound to Fam32 protein, whereas the CLL-BCR from patient 44 belonged to subset 4 and bound calcyclin-binding protein (Table 1). The CLL-BCRs from patients 21 and 31 both recognized Fam32A protein (Table 1), but only the BCR from patient 21 could be assigned to subset 5; the BCR from patient 31 could not be assigned to any of the known subsets (Table 1). The same was shown for the 2 CLL-BCRs recognizing calcyclin-binding protein. Only the CLL-BCR from patient 44 belonged to subset 4, whereas the BCR from the other patient belonged to none of the known subsets.
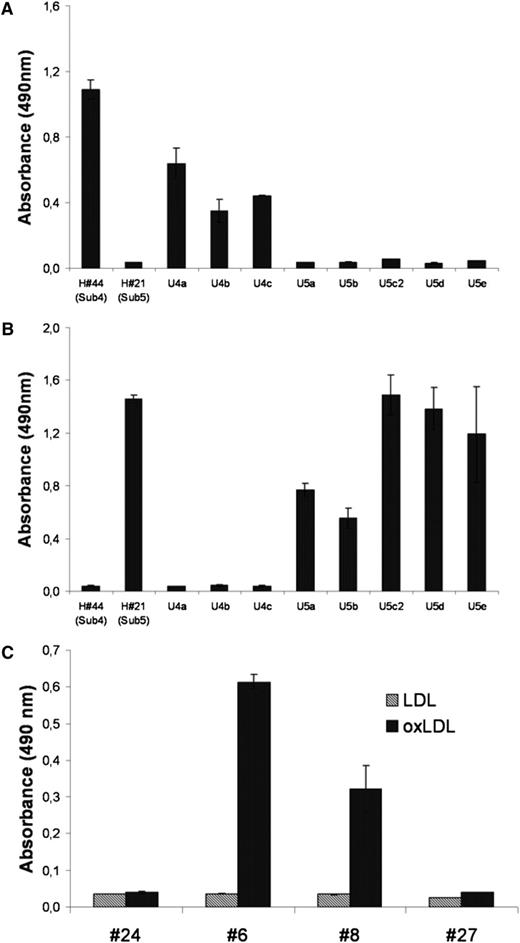
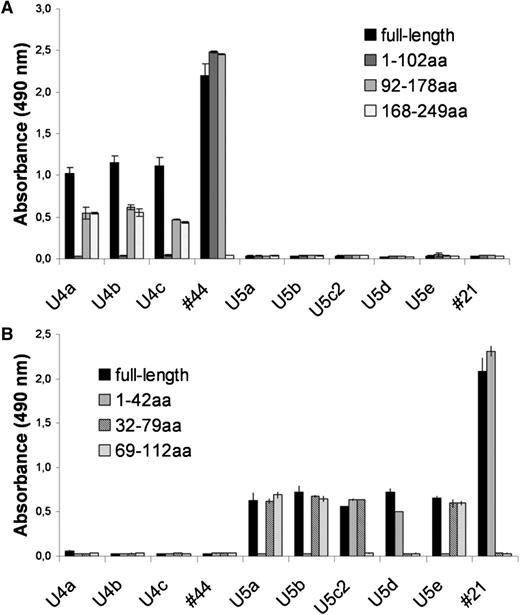
To determine whether BCRs belonging to the same stereotype do indeed recognize the same antigen, we checked the specificity of BCRs derived from additional CLL patients (U4a-c, U5a-e, see supplementary table ST2) treated at Ulm University that had not been included in the original immunoscreen, but had previously been shown to belong to stereotyped subsets 4 and 5, respectively. Calcyclin-binding protein was demonstrated to be the common antigen for all 4 subset-4 CLL-BCRs, whereas all 6 CLL-BCRs belonging to subset 5 targeted the Fam32A protein (Figure 2A-B). Interestingly, even though the CLL-BCRs belonging to the same subset targeted the same autoantigen, they bound to different epitopes of the respective antigens (Figure 3A-B).
Antigenic specificity of CLL-derived BCR belonging to the same stereotype subset. In the ELISA nFabs derived from subsets 4 and 5, samples were incubated with Fam32A protein (A) or calcyclin-binding protein (B), respectively. The 4 subset-4 derived BCRs reacted specifically with CACYBP, whereas the 6 subset-5 derived BCRs bound specifically to Fam32A protein. (C) Fabs derived from subset-1 BCR reacted specifically with oxLDL. Gray columns, LDL; black columns, oxLDL.
Antigenic specificity of CLL-derived BCR belonging to the same stereotype subset. In the ELISA nFabs derived from subsets 4 and 5, samples were incubated with Fam32A protein (A) or calcyclin-binding protein (B), respectively. The 4 subset-4 derived BCRs reacted specifically with CACYBP, whereas the 6 subset-5 derived BCRs bound specifically to Fam32A protein. (C) Fabs derived from subset-1 BCR reacted specifically with oxLDL. Gray columns, LDL; black columns, oxLDL.
Binding of Fabs belonging to the same stereotype to different epitopes of the same antigen. (A) Binding of subset 4-derived nFab to recombinant fragments of the subset-4 specific antigen calcyclin-binding protein. (B) Binding of subset 5-derived nFab to subset-5 specific antigen Fam32A. Antigens were coated on an ELISA and incubated with patients’ nFab. Samples U4a-c and 44 represent nFab from 4 patients with a CLL-BCR belonging to subset 4. Samples U5a-e and 21 represent nFab from 6 patients with a CLL-BCR belonging to subset 5. The numbers indicate the amino acid (aa) positions of recombinant antigen fragments.
Binding of Fabs belonging to the same stereotype to different epitopes of the same antigen. (A) Binding of subset 4-derived nFab to recombinant fragments of the subset-4 specific antigen calcyclin-binding protein. (B) Binding of subset 5-derived nFab to subset-5 specific antigen Fam32A. Antigens were coated on an ELISA and incubated with patients’ nFab. Samples U4a-c and 44 represent nFab from 4 patients with a CLL-BCR belonging to subset 4. Samples U5a-e and 21 represent nFab from 6 patients with a CLL-BCR belonging to subset 5. The numbers indicate the amino acid (aa) positions of recombinant antigen fragments.
Binding of recombinant BCR targets to CLL cells ex vivo
To check whether the Fab-binding antigens bind to the surface of the respective CLL cells, FLAG-tagged recombinant antigens were incubated with their respective CLL cells from 5 patients and analyzed by flow cytometry. As shown in Figure 4 and supplemental Figure 5.1-5.5, in all 5 cases from whom enough CLL cells were available for the binding studies, the CLL cells bound specifically their respective recombinant antigen, whereas no binding or cross-reactivity was observed with control antigens or antigens demonstrated to bind to BCRs with a different binding specificity in the original immunoscreen.
Specific binding of the autoantigenic BCR targets to the surface of the respective CLL cells. Staining of patient 45’s CLL cells using the specific (autologous) antigen SMCHD1 and an antigen (PC9) targeted by another patient’s CLL-BCR. R1, viable cells; R2, dead cells. (A) Unstained cells; (B) staining with CD5-FITC/CD19-PE; (C) staining with specific antigen SMCHD1 (antigen-presenting cell [APC] channel); (D) staining with unspecific antigen PC9 (APC channel); (E) overlay of the histograms shown in panels A-D. The green line represents the specific antigen as shown in panel C.
Specific binding of the autoantigenic BCR targets to the surface of the respective CLL cells. Staining of patient 45’s CLL cells using the specific (autologous) antigen SMCHD1 and an antigen (PC9) targeted by another patient’s CLL-BCR. R1, viable cells; R2, dead cells. (A) Unstained cells; (B) staining with CD5-FITC/CD19-PE; (C) staining with specific antigen SMCHD1 (antigen-presenting cell [APC] channel); (D) staining with unspecific antigen PC9 (APC channel); (E) overlay of the histograms shown in panels A-D. The green line represents the specific antigen as shown in panel C.
Activation and induction of proliferation of CLL cells by binding of the BCR-specific antigen
To determine whether binding of the identified BCR targets to the respective leukemic cells induces their activation, intracellular calcium levels of CLL cells and healthy B cells were determined by flow cytometry before and after binding of their specific antigenic BCR target. As shown in Figure 5 and supplemental Figure 6, only the antigen with specificity for the respective BCR induced a strong increase of the internal Ca2+ concentration followed by a slow decrease (Figure 5 and supplemental Figure 7) and increased the expression of the MYC protein and proliferation of the CLL cells. To exclude that increased expression of Ki67 and c-myc as well as the observed proliferation were not caused by contaminating T cells, the experiments were repeated with purified CD5+/CD19+ cells. As shown in Figure 6 and supplemental Figures 8 and 9, the results were practically identical, demonstrating that the response to stimulation with the BCR target antigens was (nearly) exclusively mediated by the leukemic B-cell population. The specific induction of Ki67 expression, the increase in MYC expression, and the proliferation induced after binding of the specific autoantigenic BCR target were consistently observed in all 4 cases from whom enough CLL cells were available for these experiments and that included both U-CLL and M-CLL cells (Figure 6 and supplemental Figures 8 and 9).
Activation of CLL cells by binding of their autoantigenic BCR target. (A) Binding of the BCR-specific autoantigenic target SMCHD1 from patient 45 to his CLL cells induced an increase of cytoplasmic calcium concentration. [Ca2+]intern was determined by flow cytometry using Fluo-4 dye (final concentration 2 µM). 1, PBMC (negative control); 2, PBMC + αIgM (positive control); 3, PBMC + unspecific antigen PC9; 4, PBMC + specific antigen SMCHD1; 5: PBMC + specific antigen SMCHD1 + ionomycin. (B) Time course of activation of CLL cells after binding of their autoantigenic BCR target. Binding of the BCR-specific autoantigenic target SMCHD1 from patient 45 to his CLL cells induced a fast increase of cytoplasmic calcium concentration followed by a slow decrease. [Ca2+]intern was determined by flow cytometry in intervals of 30 seconds after addition of the antigen SMCHD1 (time [t] = 0). 1, dye-loaded cells without antigen; 2, 30 seconds; 3, 60 seconds; 4, 90 seconds; 5, 120 seconds; 6, 150 seconds. (C) Number of Fluo-4–responding cells after binding of their autoantigenic BCR target. Data taken from panel B.
Activation of CLL cells by binding of their autoantigenic BCR target. (A) Binding of the BCR-specific autoantigenic target SMCHD1 from patient 45 to his CLL cells induced an increase of cytoplasmic calcium concentration. [Ca2+]intern was determined by flow cytometry using Fluo-4 dye (final concentration 2 µM). 1, PBMC (negative control); 2, PBMC + αIgM (positive control); 3, PBMC + unspecific antigen PC9; 4, PBMC + specific antigen SMCHD1; 5: PBMC + specific antigen SMCHD1 + ionomycin. (B) Time course of activation of CLL cells after binding of their autoantigenic BCR target. Binding of the BCR-specific autoantigenic target SMCHD1 from patient 45 to his CLL cells induced a fast increase of cytoplasmic calcium concentration followed by a slow decrease. [Ca2+]intern was determined by flow cytometry in intervals of 30 seconds after addition of the antigen SMCHD1 (time [t] = 0). 1, dye-loaded cells without antigen; 2, 30 seconds; 3, 60 seconds; 4, 90 seconds; 5, 120 seconds; 6, 150 seconds. (C) Number of Fluo-4–responding cells after binding of their autoantigenic BCR target. Data taken from panel B.
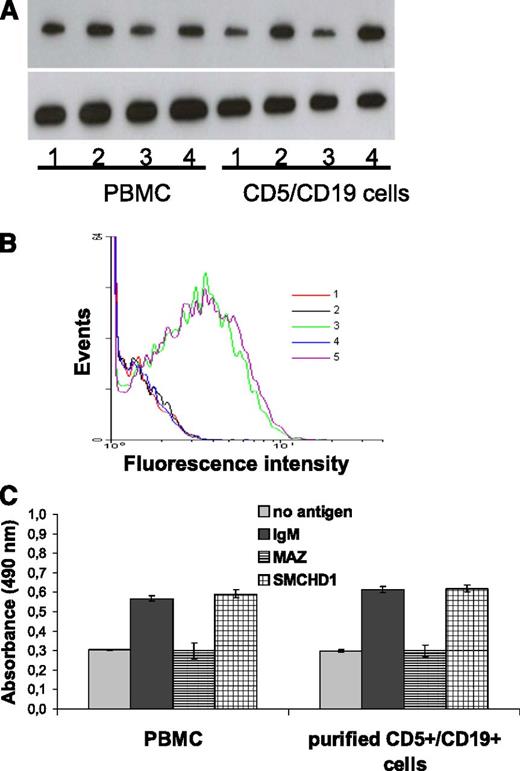
Induction of MYC and Ki67 protein expression as well as proliferation of leukemic CLL cells by binding of their autoantigenic BCR target. Shown is the analysis of PBMC from CLL patients (containing 82% CD5+/CD19+ cells) and purified (>95%) CD5+/CD19+ CLL cells derived from patient 45 after binding of the autoantigenic CLL-BCR target SMCHD1, the irrelevant CLL-BCR target MAZ, and αIgM. (A) Induction of MYC protein expression. 1, without antigen; 2, cells + αIgM; 3, cells + irrelevant antigen MAZ; 4, cells + specific antigen SMCHD1. (B) Flow cytometric analysis: Ki67 staining of purified CD5+/CD19+ cells. 1, unstained cells; 2: cells without antigen; 3, cells + αIgM; 4, cells with irrelevant antigen MAZ; 5, cells with specific antigen SMCHD1. (C) Proliferation of CLL cells as measured by EZ4U assay. PBMCs from CLL patient (left); purified CD5+/CD19+ CLL cells (right). The 4 columns represent, from left to right, no antigen, αIgM, unspecific antigen MAZ, and specific antigen SMCHD1.
Induction of MYC and Ki67 protein expression as well as proliferation of leukemic CLL cells by binding of their autoantigenic BCR target. Shown is the analysis of PBMC from CLL patients (containing 82% CD5+/CD19+ cells) and purified (>95%) CD5+/CD19+ CLL cells derived from patient 45 after binding of the autoantigenic CLL-BCR target SMCHD1, the irrelevant CLL-BCR target MAZ, and αIgM. (A) Induction of MYC protein expression. 1, without antigen; 2, cells + αIgM; 3, cells + irrelevant antigen MAZ; 4, cells + specific antigen SMCHD1. (B) Flow cytometric analysis: Ki67 staining of purified CD5+/CD19+ cells. 1, unstained cells; 2: cells without antigen; 3, cells + αIgM; 4, cells with irrelevant antigen MAZ; 5, cells with specific antigen SMCHD1. (C) Proliferation of CLL cells as measured by EZ4U assay. PBMCs from CLL patient (left); purified CD5+/CD19+ CLL cells (right). The 4 columns represent, from left to right, no antigen, αIgM, unspecific antigen MAZ, and specific antigen SMCHD1.
Discussion
To the best of our knowledge, this study has the largest number of CLL patients using an unbiased approach to detect autoantigenic targets of the BCR of CLL cells. It has yielded several important results: first, autoantigens are the target of CLL-BCRs in a considerable proportion (12/47 or 25.5%) of patients; second, although hypothesized for a long time, our results are the first formal proof that BCRs belonging to the same stereotype subset indeed target the identical antigen, even though, surprisingly, the epitopes targeted by the CLL-BCRs of the same stereotype and with the same antigen-specificity derived from different patients were not identical; third, to the best of our knowledge, specific binding of the BCR antigens to the surface of the respective CLL cell ex vivo has been demonstrated for the first time; and fourth, and perhaps most important, we showed that the specific binding of the autoantigenic BCR target to its respective receptor on the surface of CLL cells induces a specific activation and proliferation of the leukemic cells in all 4 cases where enough CLL cells were available for these experiment. From the remaining 8 CLL patients for whom a BCR target was identified, not enough CLL cells were available, either because patients had undergone treatment of their CLL or had died.
The observation that a considerable number of CLL patients share stereotypic BCRs has suggested a role of the antigenic BCR target in the pathogenesis of this disease for a long time. However, only a few of these antigenic targets have been molecularly defined to date. In our unbiased search for autoantigenic targets of CLL-BCRs in 47 consecutive patients, we found an autoantigen as the BCR target in approximately one fourth of cases. Although no antigen was detected with a predominance similar to the one of paratartg-7, which is the antigenic target of 15% of the paraproteins/BCRs in multiple myeloma of European patients,34 it remains elusive that 2 antigens, the hypothetical protein Fam32A and calcyclin-binding protein, were the target of the BCRs from 2 patients each, even though the respective BCRs belonged to different stereotypes. That we could show that pyruvate carboxylase was the target of 2/47 patients included in our screening study, similar to the calcyclin-binding protein and the hypothetical protein Fam32A, which in addition to the 2/47 consecutive patients were the target from other CLL patients belonging to stereotype subset 4 and 5, respectively, demonstrates that these antigens are autoimmunogenic beyond stereotype boundaries. The reason for this extraordinary autoimmunogenicity remains to be determined, because sequence analysis of the genes coding for these 2 antigens revealed no single-nucleotide polymorphisms or mutations; similarly, posttranslational modifications such as hyperphosphorylation, the obvious mechanism for the autoimmunogenicity of the MGUS/multiple myeloma–associated BCR target paratarg-7, could be excluded because the respective proteins derived from the patients and healthy controls showed identical migration in the gel electrophoresis and in the isoelectric focusing.
Although hypothesized for a long time, we prove for the first time that BCRs belonging to the same stereotype do indeed target the same antigen. Interestingly, however, the epitopes binding the BCRs from different patients belonging to the same stereotype were not identical. The reason for this remains to be investigated, but none of the identified epitopes showed homologies with known bacterial or viral peptides (data no shown), rendering a crossreactivity of the autoantigens with these infectants unlikely.
Of the few BCR targets molecularly defined and published in the literature, only a few could be confirmed: oxidized LDL38 reported to be target of BCR using IgHV 1-69 and subset 1 and the nonmuscle myosin heavy chain IIA, reported to be the target of BCRs using IgHV 1-69 and subset 6, whereas all other antigens tested could not be confirmed as a BCR target (Figure 1C). The reason for this discrepancy can only be speculated on, with different antibody and antigen concentrations used for the detection of the respective reactivity being the most likely reason. Because of these inconsistencies in the screening for BCR targets, we suggest that a molecular structure detected by a recombinant BCR should not be considered a bona fide BCR target unless its binding to the respective CLL cell can be demonstrated ex vivo.
All BCR targets identified in this study are physiologically located in the cytoplasm of a broad spectrum of cells, and the question arises whether and how CLL-BCR can encounter their specific antigen in vivo. That this can indeed be the case with cytoplasmic antigens, has been shown by cytoplasmic antigens being translocated to surface membrane blebs and apoptotic bodies.28 This would enable binding to the surface of CLL cells, with a BCR targeting the respective autoantigen, thus suggesting a central role for apoptosis in selecting and expanding CLL cells and their precursors.
Besides the demonstration of binding of the putative BCR targets to CLL cells ex vivo, our demonstration that such a binding activates the leukemic cells and induces their proliferation is the most important finding of this study. That binding of the antigenic BCR target to the surface of CLL cells induces activation of the respective CLL cells is not only the strongest experimental evidence to date to support a role of the antigenic target in the pathogenesis of CLL; the ongoing autoantigenic stimulation in vivo can also explain the extensive intraclonal diversification observed in subgroups of CLL patients.39,40 However, although it has been hypothesized that only CLL cells with an unmutated IgHV rely on and respond to antigen binding with activation and proliferation,41 we observed this activation also after binding of the antigenic target (MAZ) to the mutated CLL-BCR from patient 27. Even though autoreactivity of the CLL-BCRs could also be expected to lead to a negative selection rather than to a growth advantage, it has recently been shown that overexpression of the autoimmunity-associated phosphatase PTPN22 promotes survival of antigen (anti-IgM)-stimulated CLL cells by selectively blocking pro-apoptotic and enhancing anti-apoptotic BCR signals, which allows these cells to escape from negative selection.42 As this article was under revision, 2 new studies were published. The first43 identified a novel signal-initiating interaction between the third complementary determining region of the Ig heavy chain variable domain (HCDR3) and an epitope in the second framework region (FR2) that appears to be unique to CLL B cells, questioning the need for classical antigen binding for the activation and expansion of CLL cells. After having proven in this study that binding of the specific antigenic CLL-BCR target induces proliferation, it will now be interesting to dissect the contribution of either mechanism to the malignant growth in CLL. In the second publication44 a new M-CLL subset was defined expressing stereotypic BCRs highly specific for β-(1,6)-glucan, a major determinant of yeast and filamentous fungi.
In summary, the results reported here suggest autoreactivity of CLL cells via the BCR as a general mechanism not only for the pathogenesis, but also for the evolution and growth of this disease. Our results also favor a role for persistent antigenic stimulation rather than clonal expansion promoted by nonspecific stimuli in a considerable proportion of patients with CLL. Targeting the specific crosstalk between malignant B cells and their antigenic target presented by the microenvironment represents a promising strategy for therapeutic interventions in those patients where the BCR target is molecularly defined.7 The availability of molecularly defined BCR antigens opens completely new avenues for the further elucidation of the pathomechanisms involved in the development and the evolution of CLL.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Kostas Stamatopoulos for the assignment of the B-cell receptors analyzed in this study to defined stereotypes.
This work was supported by a grant from Sander-Stiftung (a charity foundation in Munich, Germany).
Authorship
Contribution: C.Z., K.-D.P., and M.P. designed the study; C.Z., N.F., E.R., and M.K. performed the immunoscreen and recombinant expression of the antigens; S.S. and A.B. provided stereotyped chronic lymphocytic leukemia cases and clinical information; and all authors participated in writing the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Michael Pfreundschuh, Department of Internal Medicine I, Saarland University Medical School, Kirrberger Strasse, D-66421 Homburg (Saar), Germany; e-mail: michael.pfreundschuh@uks.eu.
References
Author notes
M.P. and K.-D.P. contributed equally to this study.

![Figure 4. Specific binding of the autoantigenic BCR targets to the surface of the respective CLL cells. Staining of patient 45’s CLL cells using the specific (autologous) antigen SMCHD1 and an antigen (PC9) targeted by another patient’s CLL-BCR. R1, viable cells; R2, dead cells. (A) Unstained cells; (B) staining with CD5-FITC/CD19-PE; (C) staining with specific antigen SMCHD1 (antigen-presenting cell [APC] channel); (D) staining with unspecific antigen PC9 (APC channel); (E) overlay of the histograms shown in panels A-D. The green line represents the specific antigen as shown in panel C.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/121/23/10.1182_blood-2012-08-447904/4/m_4708f4.jpeg?Expires=1769232597&Signature=OFFir0VMez0aK0dm6k2YRwCzScRe5efXypRq4vrSzs0A48pLsNvwnGRYIlTn-rjuZQjhQ8xWlG-wApv~u7Y4dzE6ppSr99rWFpjsuTx4qEf64NpNJsuqdECyYisjux7ThWCZq0NUVHN-zdVR5GTg~2jClC60p70XXdIII4C7GfjpN9VxqHrOLBUU8sHv7zDrnuIkH4K3fgimI3xI~lSJ03bQcV5OiBXaEdKwOk2xKTHxqatF-KCQYpDB9S~FCbxQl9uNGOKQSt4SqQI2JZvJX9yfWIqbxQhc22clVKNu13AxGGIhmMT9RTayq13xolC0c3DXUoxexykyE7SX0nz5Rw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. Activation of CLL cells by binding of their autoantigenic BCR target. (A) Binding of the BCR-specific autoantigenic target SMCHD1 from patient 45 to his CLL cells induced an increase of cytoplasmic calcium concentration. [Ca2+]intern was determined by flow cytometry using Fluo-4 dye (final concentration 2 µM). 1, PBMC (negative control); 2, PBMC + αIgM (positive control); 3, PBMC + unspecific antigen PC9; 4, PBMC + specific antigen SMCHD1; 5: PBMC + specific antigen SMCHD1 + ionomycin. (B) Time course of activation of CLL cells after binding of their autoantigenic BCR target. Binding of the BCR-specific autoantigenic target SMCHD1 from patient 45 to his CLL cells induced a fast increase of cytoplasmic calcium concentration followed by a slow decrease. [Ca2+]intern was determined by flow cytometry in intervals of 30 seconds after addition of the antigen SMCHD1 (time [t] = 0). 1, dye-loaded cells without antigen; 2, 30 seconds; 3, 60 seconds; 4, 90 seconds; 5, 120 seconds; 6, 150 seconds. (C) Number of Fluo-4–responding cells after binding of their autoantigenic BCR target. Data taken from panel B.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/121/23/10.1182_blood-2012-08-447904/4/m_4708f5.jpeg?Expires=1769232597&Signature=HlZVYPOnjfDdvnrB8cDKs-68hUXH-kmfUcZRfCdOfFsFhVg8R0rl8NA5SB3xiJ0CSsoC8mtv9B5rBN2s-PoIRcrBSoRiBzSiqH7FWpvYIBwGGHlffyVwELqI4L290Cy6AxshQR~vAaCY4mrQnkD9AJIyHKE~4Ok5xp2Qacwapbkeg1UmvMIFq56vMlNhsBkxhArZWLorHfRD~jyT1ep4hn3c4G9pex-6vwtQOKyjuH9-ixvehJ8~CbHy8gRkdMrH8kIvRa7kxFKjrtfp469Q~ejrl3~SQBoBZAUjmku7sGQysI3gSz8kz7mUlZOeoGQ0OVa3odU5ttCA7gM4CeJ2wA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)