Key Points
Patient-derived iPSCs recapitulate juvenile myelomonocytic leukemia.
MEK inhibition normalizes GM-CSF independence and hypersensitivity in myeloid precursors from JMML iPSCs.
Abstract
Juvenile myelomonocytic leukemia (JMML) is an aggressive myeloproliferative neoplasm of young children initiated by mutations that deregulate cytokine receptor signaling. Studies of JMML are constrained by limited access to patient tissues. We generated induced pluripotent stem cells (iPSCs) from malignant cells of two JMML patients with somatic heterozygous p.E76K missense mutations in PTPN11, which encodes SHP-2, a nonreceptor tyrosine phosphatase. In vitro differentiation of JMML iPSCs produced myeloid cells with increased proliferative capacity, constitutive activation of granulocyte macrophage colony-stimulating factor (GM-CSF), and enhanced STAT5/ERK phosphorylation, similar to primary JMML cells from patients. Pharmacological inhibition of MEK kinase in iPSC-derived JMML cells reduced their GM-CSF independence, providing rationale for a potential targeted therapy. Our studies offer renewable sources of biologically relevant human cells in which to explore the pathophysiology and treatment of JMML. More generally, we illustrate the utility of iPSCs for in vitro modeling of a human malignancy.
Introduction
Juvenile myelomonocytic leukemia (JMML) is a pediatric myeloproliferative disorder associated with pathological expansion of clonal myelomonocytic cells (reviewed in Niemeyer et al1 ). Although JMML represents only 2% of pediatric hematologic malignancies, it is particularly difficult to treat and frequently fatal. Hematopoietic stem cell transplant, the only known curative modality, is associated with chronic late effects and relapse risks exceeding 45%.2 The disorder is caused by somatic and/or germline mutations in signaling genes, including NRAS, KRAS, PTPN11, NF1, and CBL.1,3 These mutations have an impact on multiple biochemical pathways, including JAK/STAT and Ras/MAPK, with the net effect being enhanced signaling that drives myelopoiesis. Indeed, myeloid progenitor hypersensitivity to granulocyte macrophage colony-stimulating factor (GM-CSF) is a key pathological feature of JMML and one diagnostic criterion.4
Recent advances in defining the genetics of JMML raise the possibility for new, more effective targeted therapies.5-8 However, because JMML mainly affects young children, pathological specimens for mechanistic studies and drug testing are difficult to obtain. To circumvent this problem, we created induced pluripotent stem cells (iPSCs) from two children with JMML caused by somatic p.E76K mutations in the PTPN11 gene. We show that in vitro hematopoietic differentiation of patient-derived iPSCs models JMML and provides relevant human cells for drug testing. Our studies provide a renewable source of pathological material for studying JMML and, more generally, they offer proof of principle for the utility of patient iPSCs to analyze blood disorders.
Study design
Generation of iPSCs
Primary JMML and control samples were obtained at the Benioff Children’s Hospital at the University of California San Francisco under a locally approved institutional review board research protocol. Ficoll-purified mononuclear cells from bone marrow or peripheral blood were reprogrammed by using the STEMCCA lentivirus9 expressing doxycycline-regulated OCT4, KLF4, MYC, and SOX2 as described.10,11 The reprogramming efficiencies of JMML and wild-type (WT) blood cells into iPSCs appeared to be similar, although we did not compare this formally. The cell lines described in this report are available upon request.
In vitro differentiation of iPSCs into hematopoietic lineages
Selected iPSC clones were cultured in serum-free medium with sequential combinations of cytokines that support the production of prehematopoietic mesoderm and hematopoietic progenitors (see Kennedy et al,10 Chou et al,11 and supplemental Methods). Methylcellulose colony assays were performed with human interleukin (IL)-3, erythropoietin, GM-CSF, and stem cell factor.
Enzyme inhibitor studies
Chemical inhibitors of MEK (PD0325901) and JAK1/2 (ruxolitinib) were purchased from LC Laboratories. Compounds were suspended in dimethylsulfoxide to a concentration of 10 mM, stored at −20°C, and diluted with cell medium immediately prior to use at the specified experimental concentrations.
Phosphoflow cytometry
Statistics
Results are graphed as mean values ± standard error of the mean. P values were calculated by using the two-tailed Student t test.
Results and discussion
iPSCs derived from somatic tissues can be propagated long term and manipulated in vitro to form various differentiated cell types, including hematopoietic cells (reviewed in Yoshida and Yamanaka14 and Panopoulos and Belmonte15 ). We generated iPSCs from circulating malignant cells of two unrelated male JMML patients with somatically acquired heterozygosity for p.E76K missense mutations in the PTPN11 gene (supplemental Table 1 and supplemental Figure 1A). This mutation constitutively activates the PTPN11-encoded SHP-2 phosphatase and is associated with increased GM-CSF receptor signaling.16-18 Both JMML samples exhibited a normal 46XY karyotype. Control iPSCs were derived from bone marrow, umbilical cord blood, or peripheral blood of unrelated healthy individuals (supplemental Table 1). All iPSC clones used in subsequent studies fulfilled standard quality control criteria, including expression of endogenous pluripotency markers, silencing of lentivirally encoded reprogramming genes, and the formation of three germ-cell layers in teratomas (supplemental Figure 1B-E and not shown). All JMML-derived iPSCs harbored the same heterozygous PTPN11 p.E76K mutation that was present in both patients at diagnosis. Cells were passaged at least 20 times to minimize lineage bias caused by potential memory effects that could be retained from the cells of origin.19 Interestingly, JMML iPSCs appeared to proliferate faster than WT control iPSCs (supplemental Figure 1F), although further studies are required to verify this finding and determine the underlying mechanism.
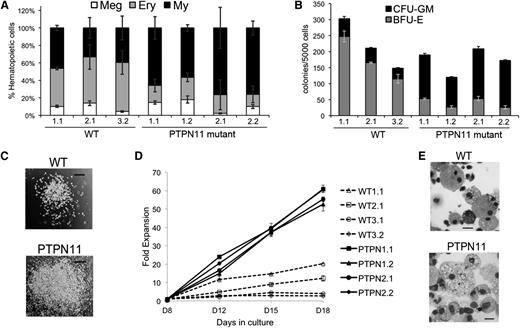
Control and JMML iPSCs were differentiated with cytokines that support multilineage hematopoiesis by using two different protocols: induced formation of embryoid bodies (EBs) and adherent monolayer cultures (supplemental Figure 2A, supplemental Table 2, supplemental Methods and Chou et al11 ). Seven to 8 days after initiation of differentiation, iPSC-derived EBs or monolayer cultures shed multipotent hematopoietic progenitors into the medium (supplemental Figure 2A-B). By days 12 to 14, these progenitors differentiated into erythroid, myeloid, and megakaryocytic cells as evidenced by characteristic cell surface markers and morphologies (supplemental Figure 2C, Figure 1E, and Chou et al11 ). The PTPN11-mutant progenitors produced by EBs generated an approximately twofold increased proportion of myeloid cells compared with controls, as detected by flow cytometric analysis of day 14 cultures (Figure 1A and supplemental Figure 2C). Methylcellulose colony assays demonstrated that day 8 PTPN11-mutant progenitors produced two- to threefold increased numbers of myeloid colonies compared with controls (Figure 1B). Interestingly, the number of erythroid colonies produced by PTPN11 p.E76K progenitors was reduced, suggesting that the mutation either inhibits the expansion of erythroid precursors or biases multipotential progenitors toward a myeloid fate. The JMML iPSC-derived myeloid colonies were generally larger than control colonies, reflecting increased proliferative capacity (Figure 1C). Similarly, myeloid progenitors generated from monolayer differentiation cultures exhibited approximately a fivefold enhanced rate of proliferation compared with control cells (Figure 1D). Myeloid colonies from control and JMML iPSCs contained predominantly granulocytes and macrophages, although occasional cells with eosinophilic granules were present (Figure 1E).
Increased myelopoiesis from PTPN11 p.E76K heterozygous iPSCs. (A) EBs generated from WT or PTPN11 p.E76K (SHP-2) heterozygous iPSCs were cultured in a multilineage cytokine cocktail to support myeloerythroid maturation (see supplemental Methods). The iPSC lines are numbered according to the individual donor followed by the clone from that individual. The relative proportion of myeloid (My; CD45+CD18+), erythroid (Ery; CD235+), and megakaryocytic (Meg; CD41+CD42+) cells produced by each clone in three independent experiments is shown. The PTPN11-mutant clones produced increased proportions of myeloid cells (P = .008) and reduced proportions of erythroid cells (P = .001). (B) Hematopoietic progenitors released from day 8 EBs were enumerated in methylcellulose colony assays. Each bar represents the results of 3 separate experiments, each with triplicate colony assays. Myeloid colonies produced by PTPN11 p.E76K iPSCs were increased (P = .004), and erythroid colonies were decreased (P = .001). (C) Myeloid colonies from methylcellulose assays. Scale bar represents 200 µm. (D) Hematopoietic progenitors from day 8 monolayer differentiation cultures of PTPN11 p.E76K or WT iPSCs were suspended at 25 000 cells/mL in medium containing stem cell factor, GM-CSF, and IL-3 and were quantified at the indicated time points. By day 18, all cells exhibited myeloid morphology, similar to those represented in panel (E). (E) May-Grünwald-Giemsa-stained cells from WT and PTPN11 p.E76K myeloid colonies represented in panels (B) and (C). Scale bars represent 20 µm.
Increased myelopoiesis from PTPN11 p.E76K heterozygous iPSCs. (A) EBs generated from WT or PTPN11 p.E76K (SHP-2) heterozygous iPSCs were cultured in a multilineage cytokine cocktail to support myeloerythroid maturation (see supplemental Methods). The iPSC lines are numbered according to the individual donor followed by the clone from that individual. The relative proportion of myeloid (My; CD45+CD18+), erythroid (Ery; CD235+), and megakaryocytic (Meg; CD41+CD42+) cells produced by each clone in three independent experiments is shown. The PTPN11-mutant clones produced increased proportions of myeloid cells (P = .008) and reduced proportions of erythroid cells (P = .001). (B) Hematopoietic progenitors released from day 8 EBs were enumerated in methylcellulose colony assays. Each bar represents the results of 3 separate experiments, each with triplicate colony assays. Myeloid colonies produced by PTPN11 p.E76K iPSCs were increased (P = .004), and erythroid colonies were decreased (P = .001). (C) Myeloid colonies from methylcellulose assays. Scale bar represents 200 µm. (D) Hematopoietic progenitors from day 8 monolayer differentiation cultures of PTPN11 p.E76K or WT iPSCs were suspended at 25 000 cells/mL in medium containing stem cell factor, GM-CSF, and IL-3 and were quantified at the indicated time points. By day 18, all cells exhibited myeloid morphology, similar to those represented in panel (E). (E) May-Grünwald-Giemsa-stained cells from WT and PTPN11 p.E76K myeloid colonies represented in panels (B) and (C). Scale bars represent 20 µm.
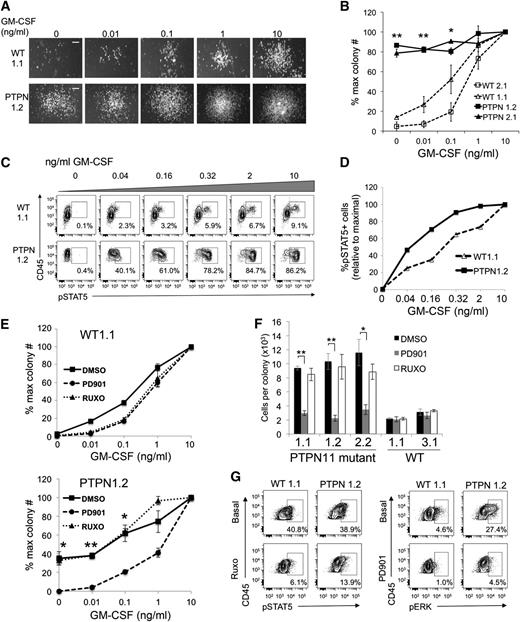
We plated fixed numbers of day 8 EB-derived hematopoietic progenitors from control and JMML iPSC clones in methylcellulose media with increasing concentrations of GM-CSF. In some experiments, small myeloid colonies (>50 cells) derived from JMML iPSCs formed without GM-CSF or at low doses (Figure 2A-B). The magnitude of this effect varied between different experiments (compare Figure 2B,E). However, within the same experiments, PTPN11-mutant progenitors always exhibited enhanced myeloid colony formation without GM-CSF and at subthreshold doses compared with controls (Figure 2A-B,E-F and supplemental Figure 2F), indicating constitutive activation of this cytokine signaling pathway. To investigate the associated mechanisms, rested iPSC-derived myeloid cells were stimulated with increasing concentrations of GM-CSF, fixed, permeabilized, and stained with antibodies against surface markers and pSTAT5 for phosphoflow cytometric analysis.12,13 After 16 hours of cytokine starvation, JMML iPSC-derived myeloid cells exhibited enhanced GM-CSF dose-responses for activation of STAT5 (Figure 2C-D and supplemental Figure 2D). Thus, iPSC-derived myeloid cells from primary JMML samples exhibit both GM-CSF independence and heightened responses to low doses of GM-CSF, as revealed by colony assays and STAT5 activation studies, recapitulating what is seen in human disease and genetically engineered mouse models.4,6,13,20
PTPN11 p.E76K iPSC-derived myeloid cells exhibit GM-CSF hypersensitivity that is ameliorated by MEK inhibition. (A) GM-CSF dose-response for myeloid colony formation from WT and PTPN11 p.E76K iPSCs. Day 8 EB-derived hematopoietic progenitors (3 × 103) were seeded into methylcellulose cultures with the indicated doses of GM-CSF. Representative photographs of myeloid colonies from one experiment are shown. Scale bar indicates 200 µm. (B) GM-CSF dose-response for myeloid colony formation from WT and PTPN11 p.E76K iPSCs, summarizing the results from multiple clones. Colonies were generated as described in panel (A). Myeloid colonies containing >50 cells were scored. The resultant colony numbers were normalized to the maximum colony number obtained at saturating GM-CSF concentration (10 ng/mL). Three independent experiments were performed with each clone. **P < .01 for PTPN11 p.E76K sample vs controls at 0 and 0.01 ng/mL GM-CSF. *At 0.1 ng/mL GM-CSF, P < .01 for PTPN11 p.E76K samples vs WT2.1 and P ≤ .05 vs WT1.1. (C) GM-CSF dose-responses for STAT5 activation. EB-derived hematopoietic progenitors were cultured for 4 days in GM-CSF, IL-3, and stem cell factor to generate myeloid cells. The cells were rested in cytokine- and serum-free medium for 16 hours and then stimulated with the specified concentrations of GM-CSF for 15 minutes. Cells were then fixed, permeabilized, stained with antibodies against surface markers and intracellular pSTAT5, and analyzed by phosphoflow cytometry. Cells are gated upon the CD45+CD18+ myeloid population for pSTAT5 analysis (not shown). (D) Summary of data from panel (C). Results at each GM-CSF concentration are normalized to the maximal response obtained at 10 ng/mL. (E) GM-CSF dose-response for myeloid colony formation from WT and PTPN11 p.E76K iPSCs with 100 nM PD0325901 (PD901) or 100 nM ruxolitinib (RUXO), representing the ED50 for each drug (supplemental Figure 2E). Representative experiments are shown for 1 clone of each genotype; similar results were obtained from additional clones (supplemental Figure 2F). **P < .01; *P ≤ .05 for dimethylsulfoxide (DMSO) vs PD901. (F) Average number of cells per myeloid colonies generated from WT and PTPN11 p.E76K iPSC clones at 10 ng/mL GM-CSF with PD0325901 (PD901) or ruxolitinib (RUXO), as depicted in panel (E). Methylcellulose cultures with myeloid colonies were solubilized in growth medium and individual cells were harvested and enumerated. Graphs show the results of three pooled dishes for each sample. Results are shown for multiple iPSC clones depicted in Figure 2E and supplemental Figure 2F. **P < .01; *P = .02. (G) Phosphoflow cytometric analysis of JAK-inhibited and MEK-inhibited iPSCs. iPSCs were rested for 1 hour in serum-free medium, incubated with 1 μM RUXO or 100 nM PD901, then fixed, permeabilized, stained, and analyzed as in Figure 2C. Basal levels of pSTAT5 are similarly increased in WT and PTPN11-mutant iPSCs (in comparison with isotype staining control; not shown), and RUXO modestly inhibits pSTAT5 in both samples. pERK is constitutively activated only in PTPN11-mutant iPSCs and inhibited by PD901. Cells are gated on the CD45+CD18+ population (not shown). Representative data from 1 experiment are shown.
PTPN11 p.E76K iPSC-derived myeloid cells exhibit GM-CSF hypersensitivity that is ameliorated by MEK inhibition. (A) GM-CSF dose-response for myeloid colony formation from WT and PTPN11 p.E76K iPSCs. Day 8 EB-derived hematopoietic progenitors (3 × 103) were seeded into methylcellulose cultures with the indicated doses of GM-CSF. Representative photographs of myeloid colonies from one experiment are shown. Scale bar indicates 200 µm. (B) GM-CSF dose-response for myeloid colony formation from WT and PTPN11 p.E76K iPSCs, summarizing the results from multiple clones. Colonies were generated as described in panel (A). Myeloid colonies containing >50 cells were scored. The resultant colony numbers were normalized to the maximum colony number obtained at saturating GM-CSF concentration (10 ng/mL). Three independent experiments were performed with each clone. **P < .01 for PTPN11 p.E76K sample vs controls at 0 and 0.01 ng/mL GM-CSF. *At 0.1 ng/mL GM-CSF, P < .01 for PTPN11 p.E76K samples vs WT2.1 and P ≤ .05 vs WT1.1. (C) GM-CSF dose-responses for STAT5 activation. EB-derived hematopoietic progenitors were cultured for 4 days in GM-CSF, IL-3, and stem cell factor to generate myeloid cells. The cells were rested in cytokine- and serum-free medium for 16 hours and then stimulated with the specified concentrations of GM-CSF for 15 minutes. Cells were then fixed, permeabilized, stained with antibodies against surface markers and intracellular pSTAT5, and analyzed by phosphoflow cytometry. Cells are gated upon the CD45+CD18+ myeloid population for pSTAT5 analysis (not shown). (D) Summary of data from panel (C). Results at each GM-CSF concentration are normalized to the maximal response obtained at 10 ng/mL. (E) GM-CSF dose-response for myeloid colony formation from WT and PTPN11 p.E76K iPSCs with 100 nM PD0325901 (PD901) or 100 nM ruxolitinib (RUXO), representing the ED50 for each drug (supplemental Figure 2E). Representative experiments are shown for 1 clone of each genotype; similar results were obtained from additional clones (supplemental Figure 2F). **P < .01; *P ≤ .05 for dimethylsulfoxide (DMSO) vs PD901. (F) Average number of cells per myeloid colonies generated from WT and PTPN11 p.E76K iPSC clones at 10 ng/mL GM-CSF with PD0325901 (PD901) or ruxolitinib (RUXO), as depicted in panel (E). Methylcellulose cultures with myeloid colonies were solubilized in growth medium and individual cells were harvested and enumerated. Graphs show the results of three pooled dishes for each sample. Results are shown for multiple iPSC clones depicted in Figure 2E and supplemental Figure 2F. **P < .01; *P = .02. (G) Phosphoflow cytometric analysis of JAK-inhibited and MEK-inhibited iPSCs. iPSCs were rested for 1 hour in serum-free medium, incubated with 1 μM RUXO or 100 nM PD901, then fixed, permeabilized, stained, and analyzed as in Figure 2C. Basal levels of pSTAT5 are similarly increased in WT and PTPN11-mutant iPSCs (in comparison with isotype staining control; not shown), and RUXO modestly inhibits pSTAT5 in both samples. pERK is constitutively activated only in PTPN11-mutant iPSCs and inhibited by PD901. Cells are gated on the CD45+CD18+ population (not shown). Representative data from 1 experiment are shown.
We investigated the utility of JMML iPSCs for drug screening by testing the sensitivity of their myeloid progeny to inhibitors of MEK (PD901) and JAK1/2 (ruxolitinib) kinases, both of which are hyperactivated in JMML.13 At a saturating GM-CSF concentration (10 ng/mL), myeloid colony formation by WT and JMML progenitors was inhibited similarly by PD901 or ruxolitinib, with a 50% effective dose (ED50) of approximately 100 nM for either drug (supplemental Figure 2E). At this concentration, PD901 normalized the GM-CSF dose response to myeloid colony formation from JMML iPSCs, blocked their formation of cytokine-independent colonies, and reduced the colony size to that of controls at saturating GM-CSF (Figure 2E-F and supplemental Figure 2F). These data from human PTPN11 p.E76K iPSCs are consistent with studies of Nf1- and Kras-mutant mice, in which JMML-like myeloproliferative disorders are attenuated by treatment with PD901.6,8 Together, these murine and human studies support clinical trials for MEK inhibition in patients with JMML. In contrast to MEK inhibition, ruxolitinib at its defined ED50 did not alleviate enhanced GM-CSF dose responses in the PTPN11-mutant iPSCs or appreciably inhibit pSTAT5 (Figure 2E-F, supplemental Figure 2F, and not shown). Incubation of PTPN11-mutant iPSCs with a higher ruxolitinib dose (1 μM) inhibited pSTAT5 below basal levels, although effects were similar in WT iPSC controls. However, after 1 hour of cytokine withdrawal, PTPN11-mutant iPSCs demonstrated constitutive ERK activation in comparison with controls, which was abrogated by incubation with 100 nM PD901 (Figure 2G). Thus MEK inhibition may provide a greater therapeutic window for treatment of PTPN11 p.E76K-associated JMML than does JAK1/2 inhibition. Nonetheless, different JMML-associated mutations may cause distinct biochemical effects with different drug sensitivity profiles requiring specific, individualized targeted therapies. Generating iPSCs from additional JMML mutational subtypes will provide valuable reagents for patient-specific drug testing platforms.
Prior studies have generated iPSCs from patients with numerous hematopoietic disorders, including myeloproliferative syndrome and leukemia.11,21-25 The blood cell–forming potential for many of these lines and the extent to which they recapitulate human diseases are not fully determined. Current studies demonstrate that patient-derived PTPN11 p.E76K-mutant iPSCs recapitulate key pathological features of JMML, including increased myelopoiesis and constitutive GM-CSF activation. These lines proliferate extensively and, upon induced hematopoietic differentiation, produce biologically relevant human myeloid cells for exploring JMML pathophysiology and treatment. Importantly, the availability of renewable human JMML iPSCs offers a critical resource for drug screening, since current therapies are inadequate for this patient population. More generally, our studies provide proof of principle that in vitro differentiation of patient-derived iPSCs represents a tractable approach for modeling human hematopoietic disorders, including clonal malignancies.
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Kevin Shannon, Benjamin Braun, members of the Children’s Hospital of Philadelphia Embryonic Stem Cell Core Facility, and members of the Weiss laboratory for helpful discussions. The authors thank Darrell Kotton and Gustavo Mostoslavsky for providing the STEMCCA reprogramming vectors.
This work was supported by National Institutes of Health grants RC2 HL101606 (M.J.W.), P30DK090969 (M.J.W.), U01 HL099656 (M.J.W.) and P30 CA082103 (M.L.L.), the Cookies for Kids’ Cancer Foundation (M.J.W.), The Leukemia and Lymphoma Society Translational Research Program 6059-09 (M.L.L.), and the Frank A. Campini Foundation (M.L.L.). M.J.W. and M.L.L. are former Scholars of the Leukemia and Lymphoma Society.
Authorship
Contribution: S.G.-B., P.P., S.K.S., J.W., C.A, S.B., L.L., and H.F. performed experiments; and S.G.-B., S.K.T., J.K.C., S.T.C., D.L.F., M.L.L., and M.J.W. designed experiments and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mitchell J. Weiss, The Children’s Hospital of Philadelphia, 3615 Civic Center Blvd, ARC 316B, Philadelphia PA 19104; email: weissmi@email.chop.edu.
References
Author notes
S.G.-B. and P.P. contributed equally to this study.