Abstract
Natural killer (NK)/T-cell lymphomas and NK-cell leukemias are aggressive malignancies. Occurring worldwide, they show a predilection for Asian and South American populations. Neoplastic cells are surface CD3−, cytoplasmic CD3ε+, CD56+, cytotoxic-molecule positive, Epstein-Barr virus (EBV) positive, with germline T-cell receptor gene. Lymphomas occur commonly in the nasal and upper aerodigestive region. Occasional cases present in the skin, salivary gland, testis, and gastrointestinal tract. Rare cases are disseminated with lymphadenopathy, hepatosplenomegaly, and a leukemic phase. Positron emission tomography computed tomography is useful in staging, as lymphomas are 18-fluorodeoxyglucose avid. Quantification of circulating EBV DNA is an accurate biomarker of tumor load. Nasal NK/T-cell lymphomas present mostly with stage I/II disease. Concomitant/sequential chemotherapy and radiotherapy is standard treatment. Radiotherapy alone is inadequate because of high systemic failure rate. For stage III/IV nasal, nonnasal, and disseminated lymphomas, systemic chemotherapy is indicated. Regimens containing l-asparaginase and drugs unaffected by P-glycoprotein are most effective. Hematopoietic stem cell transplantation (HSCT) is not indicated for early-stage nasal lymphomas. HSCT for lymphomas not in remission has poor results. In advanced-stage nasal, nonnasal, disseminated, or relapsed lymphomas, HSCT may be considered when remission is achieved. Prognostic modeling and EBV DNA monitoring may be useful in risk stratification for HSCT.
Introduction
Natural killer (NK) cells are cytolytic cells targeting tumor cells and bacteria- or virus-infected cells.1 NK cells and T cells share a common ontogeny and express T-lineage–associated antigens, including CD2 and CD7. Different from T cells, they are negative for surface CD3 but express cytoplasmic CD3 ε (ε). NK cells also express “NK-associated” antigens, including CD16, CD56, and CD57,2 with CD56 being the most consistently expressed.3 Molecularly, the T-cell receptor (TCR) genes are in germline configuration.
Malignancies of putative NK-cell origin
Two decades ago, 2 groups independently described a distinct subtype of CD3+CD56+ lymphoma4,5 characterized by extranodal distribution and an aggressive course. Originally classified as T-cell lymphomas, the development of monoclonal anti-CD3 antibodies showed that these lymphomas were in fact surface CD3− and cytoplasmic CD3ε+.6 Therefore, these angiocentric T-cell lymphomas7 are actually of putative NK-cell origin.
Pathology of NK/T-cell lymphomas
The World Health Organization (WHO) classification distinguishes between 2 histopathological subtypes of NK-cell malignancies: extranodal NK/T-cell lymphoma, nasal type8 and aggressive NK cell leukemia.9 Cytologically, neoplastic cells are small to medium-sized lymphoid cells possessing pale cytoplasm with azurophilic granules. Histopathologically, lymphoma cells may be admixed with a polymorphic population of small lymphocytes, plasma cells, eosinophils, and histiocytes, hence the old terminology “polymorphic reticulosis.” Lymphomatous infiltrate may show angiocentricity and angiodestruction, leading to coagulative necrosis. Marrow hemophagocytosis may occur. In aggressive NK-cell leukemia, circulating neoplastic cells can vary from large granular lymphocytes to frank blasts.10,11
Immunophenotypically, lymphoma cells are typically CD2+, cytoplasmic CD3ε+, CD56+, and express perforin, granzyme B, and TIA-1.8,9 Neoplastic cells are invariably infected by Epstein-Barr virus (EBV) in a clonal episomal form,11 which is detected most reliably by in situ hybridization (ISH) for EBV-encoded RNA (EBER), constituting a diagnostic requisite.8,9 However, rare cases might be CD2+, CD3ε+, cytotoxic-molecule positive, EBV positive, but CD56−, and exceptional ones might show TCR gene rearrangement. These cases are rare, being included as the “T-cell” part of the WHO nomenclature of NK/T-cell lymphoma.
Epidemiology of NK/T-cell lymphomas
NK/T-cell lymphomas occur worldwide, with a strong geographic predilection for Asian populations from China, Japan, Korea, and Southeast Asia11 and for Central and South American populations from Mexico,12 Peru,13 Argentina,14 and Brazil,15 constituting 5% to 15% of lymphomas in these countries. Occasional case series have also been reported from Europe16 and North America.17
Clinical presentations
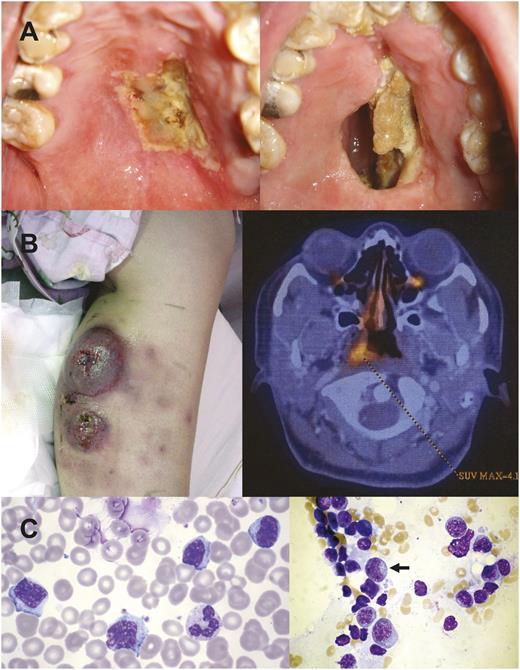
NK/T-cell lymphomas are almost exclusively extranodal.11 Initial sites involved are often the nose and nasopharynx and occasionally the paranasal sinuses, tonsil, Waldeyer ring, and oropharynx. When nasal lymphomas destroy the floor of the nasal cavity, a characteristic hard-palate perforation is found (Figure 1A). Clinically, these lymphomas are referred to as nasal NK/T-cell lymphomas. Usually localized, nasal NK/T-cell lymphomas may disseminate to the skin, salivary glands, testis, and gastrointestinal tract. Interestingly, the lymphoma occasionally presents primarily in these sites without an apparent nasal primary. Previously referred to as “nonnasal” NK/T-cell lymphomas and thought to be more aggressive,18 modern imaging technology, particularly positron emission tomography computed tomography (PET/CT),19,20 has shown that most if not all nonnasal lymphomas are associated with occult nasal primaries,19-22 implying that they are disseminated nasal lymphomas (Figure 1B). A strict definition of nonnasal NK/T-cell lymphoma requires absence of nasal involvement, shown by random nasopharyngeal biopsies and PET/CT.23 Such stringent definition was not used in previous series of nonnasal NK/T-cell lymphomas,18 which probably described many cases of disseminated nasal lymphomas. With improved treatment strategies, primary sites of presentation are no longer an independent prognostic indicator.24 Rarely, the lymphoma can present fulminantly with widespread systemic dissemination and involvement of the marrow and peripheral blood (Figure 1C).11,22,25 These cases, referred to as disseminated NK-cell lymphoma/leukemia or aggressive NK-cell leukemia according to WHO classification,9 are catastrophic with an extremely poor survival measured only in weeks.
Different clinical forms of NK/T-cell lymphomas. (A) Nasal lymphoma showing an initial ulcer (left) that ultimately perforated into the oral cavity, creating a communication between the oral cavity and the nasal cavity. In the past, such a lesion was referred to as “lethal midline granuloma.” (B) Lymphoma initially localized to the calf, presenting as nonhealing ulcers. PET/CT showed occult nasal involvement. This case could be erroneously classified as “nonnasal” lymphoma. (C) Aggressive NK-cell leukemia showing neoplastic cells that were large granular lymphocyte in morphology in the peripheral blood and in the bone marrow (arrow).
Different clinical forms of NK/T-cell lymphomas. (A) Nasal lymphoma showing an initial ulcer (left) that ultimately perforated into the oral cavity, creating a communication between the oral cavity and the nasal cavity. In the past, such a lesion was referred to as “lethal midline granuloma.” (B) Lymphoma initially localized to the calf, presenting as nonhealing ulcers. PET/CT showed occult nasal involvement. This case could be erroneously classified as “nonnasal” lymphoma. (C) Aggressive NK-cell leukemia showing neoplastic cells that were large granular lymphocyte in morphology in the peripheral blood and in the bone marrow (arrow).
Molecular pathogenesis
Early studies by our group showed specific karyotypic and genomic aberrations.26-28 Based on the observations that 6q21 is frequently deleted,26-29 genes residing in this region, including FOXO3, PRDM1, and HACE1, have been shown to be putative tumor-suppressor genes.30-32 The gene PRDM1 (alias BLIMP1) is particularly interesting because it regulates the maturation, homeostasis, and proliferation of NK cells.33 Gene expression profiling has shown patterns different from normal NK cells and other T-cell lymphomas.34-36 Several oncogenic pathways are activated, including Notch-1, Wnt, JAK/STAT, AKT, and nuclear factor κB.34-36 In addition to gene expression profiling, microRNA expression profiling has also identified a distinct microRNA signature,37 leading to dysregulation of p53, cell-cycle, and MAPK signaling pathways. Recently, whole-exome sequencing has identified somatic-activating mutations of the JAK3 gene in 35% of NK/T-cell lymphomas,38 resulting in cytokine-independent constitutive JAK/STAT activation. These findings are important to clinicians as they may provide therapeutic opportunities targeting overexpressed proteins including survivin and aurora kinase A35,36 or activated signaling pathways such as Notch-1 and JAK/STAT.35,36,38
Diagnosis
Extensive necrosis often hampers histopathological diagnosis, so surgeons should obtain biopsy specimens as large as feasible. The specimen should be sent fresh for cryostat sectioning to distinguish between surface- and cytoplasmic-CD3 expression and hence T-cell and NK-cell lymphomas. If only paraffin material is available, the diagnosis can still be made, provided that CD56, EBER, and cytotoxic molecules are positive.8 In doubtful cases, TCR rearrangement studies should be performed. In metastatic sites, lymphoma cells may lose CD56,39 so that EBER-ISH is more reliable for defining lymphoma infiltration.40 EBV is a diagnostic requisite, and EBV-negative cases should be diagnosed as peripheral T-cell lymphoma, not otherwise specified.8 Cases that are EBV positive but negative for CD56 or cytotoxic molecules should also be classified as peripheral T-cell lymphoma, not otherwise specified.8
Diseases requiring differentiation from NK/T-cell lymphomas
Chronic active EBV infection is a rare disease observed in Asian populations,41 characterized by chronic illness of >3 months, unremitting pyrexia, lymphadenopathy, cytopenia, and hepatosplenomegaly. There are high titers of anti–virus-capsid-antigen and anti–early antigen antibodies. EBV-infected cells are mostly T cells and occasionally NK cells. The disease is relentless, progressing from polyclonal to monoclonal, when an EBV-positive T-cell lymphoma develops. Occasionally, an EBV-positive NK-cell lymphoma develops, indistinguishable from de novo NK/T-cell lymphoma except for a prolonged antecedent illness.
Mosquito-bite hypersensitivity is an interesting disorder observed in Asian and South American populations.42 Patients report papulovesicular skin lesions attributed to bites from mosquito or other arthropods (but whether they really are insect bites has never been confirmed scientifically) that progress to ulceration and scarring. In serious cases, fever, lymphadenopathy and hepatosplenomegaly occur. Most prevalent in childhood, it overlaps with the entity hydroa vacciniforme-like lymphoma.43 Pathologically, atypical EBV-infected lymphoid cells, usually cytotoxic T cells and occasionally NK cells, infiltrate the epidermis and subcutis. Skin lesions subside and recur for up to 10 to 15 years before a lesion fails to heal and develops into an EBV-infected T-cell lymphoma. Very rarely, an EBV-positive NK-cell lymphoma occurs, again indistinguishable from de novo NK/T-cell lymphoma except for an antecedent history of recurring skin lesions (Figure 2A).
Clinical images of NK/T-cell lymphoma. (A) A large ulcer that on biopsy showed NK/T-cell lymphoma. Scars (arrows) were attributed by the patient to be due to ulcers that healed from previous mosquito bite hypersensitivity, occurring over a period of decades. There was almost complete healing of the ulcer after 2 courses of SMILE (right side). (B) Typical PET/CT image of a nasal lymphoma. (C) Relapse of nasal NK/T-cell lymphoma in the mediastinum. (D) Disseminated NK-cell lymphoma showing diffuse splenic uptake. (E) Relapsed cutaneous NK/T-cell lymphoma. The lesion was very subtle and showed up on PET/CT with a low SUVmax. This patient presented with multiple skin lesions.
Clinical images of NK/T-cell lymphoma. (A) A large ulcer that on biopsy showed NK/T-cell lymphoma. Scars (arrows) were attributed by the patient to be due to ulcers that healed from previous mosquito bite hypersensitivity, occurring over a period of decades. There was almost complete healing of the ulcer after 2 courses of SMILE (right side). (B) Typical PET/CT image of a nasal lymphoma. (C) Relapse of nasal NK/T-cell lymphoma in the mediastinum. (D) Disseminated NK-cell lymphoma showing diffuse splenic uptake. (E) Relapsed cutaneous NK/T-cell lymphoma. The lesion was very subtle and showed up on PET/CT with a low SUVmax. This patient presented with multiple skin lesions.
Lymphomatoid gastropathy44 and NK-cell enteropathy45 are benign conditions that may be mistaken as lymphomatous. They may be discovered incidentally during endoscopy as small (about 1 cm) edematous lesions with superficial ulcerations or hemorrhage, occurring most often in the stomach, followed by the duodenum and small and large bowel. Histologically, there is mucosal infiltration by atypical cells with an NK-cell phenotype (surface CD3−, cytoplasmic CD3ε+, CD56+, cytotoxic-molecules positive, TCR−). EBV is not present. The lesions self-regress, but may recur. It is a benign disease.
Initial evaluation and staging
A nasal panendoscopy is performed irrespective of the initial site(s) of presentation,23 and biopsy specimens are taken from suspicious lesions. In our center, random nasopharyngeal biopsies are performed even when no lesions are seen. As hepatitis B virus carrier rates are high in populations at risk of NK/T-cell lymphoma, hepatitis B virus testing should be routine and antiviral prophylaxis administered to carriers during chemotherapy.46 In bilateral marrow trephine biopsies, EBER-ISH is a must for detecting occult involvement.39,40
NK/T-cell lymphomas are 18-fluorodeoxyglucose avid (Figure 2B-E),19,20 and PET/CT is the recommended imaging modality. The standard uptake value maximum (SUVmax) is lower in NK/T-cell lymphoma than aggressive B-cell lymphomas. An SUVmax > 10 should prompt examination for coexisting neoplastic or infective processes.19,20 When PET/CT is unavailable, CT or magnetic resonance imaging (MRI) may be acceptable.23 In nasal lesions, careful initial radiologic evaluation is imperative for accurate planning of subsequent radiotherapy.
Quantification of circulating plasma EBV DNA
EBV infection is latent and not lytic in the lymphoma cells. During tumor cell apoptosis, EBV genomic fragments are released into the circulation, which can be quantified by polymerase chain reaction. As circulating lymphoma cells are not found, the appropriate material to be examined is plasma. Whole blood is not ideal because it contains EBV-infected memory B cells, which can introduce considerable and unpredictable errors. Early studies by us showed that plasma EBV DNA was correlated with tumor load, with high EBV DNA correlating negatively with survivals.47 These observations have since been confirmed.48 Serial EBV DNA monitoring is useful for assessing response and detecting recurrence.23
General principles of treatment
Current treatment strategies are adopted from retrospective analyses and phase 2 studies. For localized disease, radiotherapy together with chemotherapy is the standard approach.11,22,23 Meticulous radiotherapy planning with MRI or PET/CT is necessary to cover all involved areas, and a dose not less than 50 Gy should be delivered. Lower doses results in high in-field failure rates. In disseminated NK/T-cell lymphomas, systemic chemotherapy remains the mainstay of treatment. The role of hematopoietic stem cell transplantation (HSCT) has not been prospectively evaluated.49
Treatment of localized early-stage nasal lymphomas
Combined chemotherapy-radiotherapy is standard. NK/T-cell lymphoma is radiosensitive. In studies employing radiotherapy alone (typically around 50 Gy), overall response rates (ORRs) vary between 77% and 100%, with complete response (CR) rates ranging from 52% to 100%.50-53 The dose and the radiation field are important considerations. Radiation dose of ≥50 Gy gave better response rates.50-54 An extended field encompassing all macroscopic lesions, paranasal sinuses, palate, and nasopharynx also resulted in lower local failure rates when radiotherapy was employed alone.54 The toxicity of such an extended field might be alleviated by intensity-modulated radiotherapy, without affecting the treatment results.55 However, the systemic relapse rates were as high as 25% to 40%,50 suggesting that dissemination might have happened in these apparently localized nasal NK/T-cell lymphomas. These observations were made before the advent of PET/CT. Whether patients with localized nasal lymphoma defined by PET/CT may fare better with radiotherapy alone is undefined. Given the current data, radiotherapy alone is inadequate and chemotherapy is needed to decrease systemic failure.
Chemoradiotherapy with anthracycline-based chemotherapy
Anthracycline-based regimens followed by involved-field radiotherapy are unsatisfactory. In an earlier retrospective study using 4 cycles of CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisolone) followed by involved-field radiotherapy (45 Gy) for stage I/II nasal lymphoma, the CR rate was merely 58%, with a 3-year overall survival (OS) of 59%.56 Another disappointing observation was that 65% of patients had disease progression during CHOP.56 Further attempts to improve the efficacy of CHOP-based regimens by increasing cycle numbers and drug dosages, or by adding more chemotherapeutic agents, had not been successful.50 Hence, CHOP-based chemotherapy is not recommended for NK/T-cell lymphoma.
The poor response may be due to intrinsic properties of the lymphoma. NK-cells express very high P-glycoprotein concentrations. This property is retained in NK/T-cell lymphomas, resulting in a multidrug resistance (MDR) phenotype.57 Therefore, non–MDR-dependent drugs are now incorporated in protocols specifically designed for NK/-T cell lymphomas.
Concurrent chemoradiotherapy
Two recent prospective trials studied chemotherapy given concurrently with radiotherapy, trying to exploit the additional benefits of radiosensitization.58,59 In 27 patients with stage I/II nasal lymphomas, concurrent radiotherapy (50 Gy) and 3 cycles of 2/3DeVIC (dexamethasone, etoposide, ifosfamide, and carboplatin) (Table 1) gave an ORR of 81%, with CR achieved in 77%.56 The 5-year OS was 70%, and the 5-year progression-free survival (PFS) was 63%.60 Main toxicities were myelosuppression (grade 3/4 neutropenia: 93%) and radiation-related mucositis (grade 3: 30%). In another study, 30 patients with localized disease were treated with concurrent radiotherapy (40 Gy) and cisplatin followed by 3 cycles of VIPD (etoposide, ifosfamide, cisplatin, and dexamethasone) (Table 1).59 The ORR was 83%, with CR achieved in 80%. The 3-year OS and PFS were 86% and 85%, respectively. Local and systemic relapse was found at the same rate of 6.6%. Two patients died of infections.
Novel chemotherapeutic regimens for NK/T-cell lymphomas
| Regimen . | Protocol . | Reference . |
|---|---|---|
| AspaMetDex | Escherichia colil-asparaginase: 6000 U/m2 IM, days 2, 4, 6, and 8 | 16 |
| Methotrexate: 3000 mg/m2 IV, day 1 | ||
| Dexamethasone: 40 mg orally, days 1-4 | ||
| 2/3DeVIC | Dexamethasone: 40 mg IV, days 1-3 | 56 |
| Etoposide: 67 mg/m2 IV, days 1-3 | ||
| Ifosfamide: 1000 mg/m2 IV, days 1-3 | ||
| Carboplatin: 200 mg/m2 IV, day 1 | ||
| VIPD | Etoposide: 100 mg/m2 IV, days 1-3 | 57 |
| Ifosfamide: 1200 mg/m2 IV, days 1-3 | ||
| Cisplatin: 33 mg/m2 IV, days 1-3 | ||
| Dexamethasone: 40 mg IV or orally, days 1-4 | ||
| LVP | l-asparaginase: 6000 IU/m2 IV, days 1-5 | 59 |
| Vincristine: 1.4/m2 IV, day 1 | ||
| Prednisolone: 100 mg orally, days 1-5 | ||
| GELOX | Gemcitabine: 1000 mg/m2 IV, days 1 and 8 | 60 |
| E. colil-asparaginase: 6000 units/m2 IM, days 1-7 | ||
| Oxaliplatin: 130 mg/m2 IV, day 1 | ||
| SMILE | Dexamethasone: 40 mg IV or orally, days 2-4 | 62 |
| Methotrexate: 2000 mg/m2 IV, day 1 | ||
| Ifosfamide: 1500 mg/m2 IV, days 2-4 | ||
| E. colil-asparaginase: 6000 U/m2 IV, days 8, 10, 12, 14, 16, 18, and 20 | ||
| Etoposide: 100 mg/m2 IV, days 2-4 |
| Regimen . | Protocol . | Reference . |
|---|---|---|
| AspaMetDex | Escherichia colil-asparaginase: 6000 U/m2 IM, days 2, 4, 6, and 8 | 16 |
| Methotrexate: 3000 mg/m2 IV, day 1 | ||
| Dexamethasone: 40 mg orally, days 1-4 | ||
| 2/3DeVIC | Dexamethasone: 40 mg IV, days 1-3 | 56 |
| Etoposide: 67 mg/m2 IV, days 1-3 | ||
| Ifosfamide: 1000 mg/m2 IV, days 1-3 | ||
| Carboplatin: 200 mg/m2 IV, day 1 | ||
| VIPD | Etoposide: 100 mg/m2 IV, days 1-3 | 57 |
| Ifosfamide: 1200 mg/m2 IV, days 1-3 | ||
| Cisplatin: 33 mg/m2 IV, days 1-3 | ||
| Dexamethasone: 40 mg IV or orally, days 1-4 | ||
| LVP | l-asparaginase: 6000 IU/m2 IV, days 1-5 | 59 |
| Vincristine: 1.4/m2 IV, day 1 | ||
| Prednisolone: 100 mg orally, days 1-5 | ||
| GELOX | Gemcitabine: 1000 mg/m2 IV, days 1 and 8 | 60 |
| E. colil-asparaginase: 6000 units/m2 IM, days 1-7 | ||
| Oxaliplatin: 130 mg/m2 IV, day 1 | ||
| SMILE | Dexamethasone: 40 mg IV or orally, days 2-4 | 62 |
| Methotrexate: 2000 mg/m2 IV, day 1 | ||
| Ifosfamide: 1500 mg/m2 IV, days 2-4 | ||
| E. colil-asparaginase: 6000 U/m2 IV, days 8, 10, 12, 14, 16, 18, and 20 | ||
| Etoposide: 100 mg/m2 IV, days 2-4 |
IM, intramuscularly.
l-asparaginase–based chemotherapy with sandwiched radiotherapy
l-asparaginase as a single agent was found to be effective for relapsed/refractory NK/T-cell lymphomas.61 Based on these observations, l-asparaginase has been incorporated into several regimens. A recent phase 2 study examined 26 patients with stage I/II nasal lymphoma treated with the LVP regimen (l-asparaginase, vincristine, and prednisolone) (Table 1) for 6 courses, with sandwiched radiotherapy after 2 courses.62 After 2 cycles, the ORR was 92%, with CR achieved in 42%. After completion of radiotherapy (56 Gy) and 2 to 4 cycles of LVP, the ORR was 89%, with CR achieved in 81%. Grade 3 leucopenia was observed in 2.7% of patients, and grade 3 radiation-related mucositis in 23.1%. With a median follow-up of 27 months, the 2-year OS and PFS were 88.5% and 80.6%.
A slightly more intense regimen, GELOX (gemcitabine, l-asparaginase, and oxaliplatin) (Table 1), had been evaluated in a prospective study.63 In 27 stage I/II patients, 2 cycles of treatment resulted in an ORR of 93%, with CR achieved in 56%. After sandwiched radiotherapy (56 Gy) and 2 to 4 cycles of GELOX, the ORR was 96%, with CR achieved in 74%. With a median follow-up of 27 months, the 2-year OS and PFS were both 86%. The local and systemic relapse rates were 15% and 11%. Grade 3/4 leucopenia developed in 33.3% of patients and grade 3 radiation-related mucositis in 15%.
The most intense protocol in early-stage nasal lymphomas is the regimen SMILE (dexamethasone, methotrexate, ifosfamide, l-asparaginase, and etoposide). In a prospective study of 29 patients with newly diagnosed nasal lymphomas,24 interim analysis after 2 to 3 courses showed an ORR of 86%, with CR achieved in 69%. After sandwiched involved-field radiotherapy (50 Gy) and 3 to 4 cycles of SMILE, the ORR was increased to 89.7%, with CR rates unchanged. These results showed that response to SMILE was rapid and seen even after 2 cycles. Furthermore, remission was durable, with 90% of patients remaining in CR during follow-up. Grade 3/4 neutropenia occurred in 61% of patients. There was a regimen-related mortality of 7%.
Allergic reactions to l-asparaginase and even anaphylactic shock may occur. Therefore, a skin test is recommended before each infusion.24 In patients developing allergic reactions, Erwinia asparaginase should be used. Other precautions of using l-asparaginase–containing regimens have been presented elsewhere.24
Rational choice of therapy in localized early-stage nasal lymphomas
Current data indicate that regimens employing non–MDR-dependent drugs together with radiotherapy give very good ORR, CR rates, and survivals (Table 2). There is no clear evidence that concomitant radiotherapy/chemotherapy is necessary, as sequential chemotherapy/radiotherapy gives comparable results. Logistic difficulties of arranging timely radiotherapy make it unlikely that concurrent chemoradiotherapy can be used routinely outside clinical trials. Furthermore, sequential chemotherapy/radiotherapy usually means that radiotherapy is given in CR or very good partial remission, so that radiotherapy is better tolerated. Sequential chemotherapy/radiotherapy is therefore preferred. When used together with chemotherapy, the necessary dose of radiotherapy has not been critically examined. Because long-term toxicity of radiotherapy is rarely reported, it seems reasonable to employ a conventional dose of at least 50 Gy in combination with chemotherapy. For patients intolerant of chemotherapy because of serious medical comorbidities, radiotherapy alone may be acceptable if PET/CT clearly shows localized nasal disease.
Selected studies on the treatment of adult patients with NK/T-cell lymphomas
| Disease . | No. . | Treatment . | . | . | . | . | Adverse effects . | |||
|---|---|---|---|---|---|---|---|---|---|---|
| ORR . | CR . | OS . | PFS . | Mucositis . | Leucopenia . | TRM . | Reference . | |||
| Relapsed/refractory | 19 | AspaMetDex | 78% | 61% | 2 y: 40% | 2 y: 40% | NR | Grade 3/4: 44% | 0% | 16 |
| Newly diagnosed, relapsed/refractory, any stage | 87 | SMILE ± sandwiched RT (50 Gy) | 81% | 66% | 5 y 50% | 4 y DFS: 64% | NR | Grade 3/4: 67% | 5.7% | 24 |
| Newly diagnosed, stage I/II nasal | 18 | RT (median 50 Gy) | 78% | 78% | 5 y: 30% | 5 y: 30.5% | NR | NR | NR | 51 |
| Newly diagnosed, stage I/II nasal | 31 | RT (median 50 Gy) | 100% | 97% | 5 y: 66% | 5 y: 61% | NR | NR | NR | 52 |
| Newly diagnosed, stage I/II nasal | 17 | CHOP + RT (45 Gy) | 58% | 58% | 3 y: 59% | NR | NR | NR | NR | 54 |
| Newly diagnosed, stage I/II nasal | 27 | Concurrent RT (50 Gy) + 2/3DeVIC | 81% | 77% | 2 y: 78% | 2 y: 67% | Grade 3/4: 30% | Grade 3/4: 93% | 0% | 56 |
| Newly diagnosed, stage I/II nasal | 30 | Concurrent RT (40 Gy) + cisplatin + VIPD | 83% | 80% | 3 y: 86% | 3 y: 85% | Grade 1/2: 11% | Grade 3/4: 14% | 6.6% | 57 |
| Newly diagnosed, stage I/II nasal | 26 | LVP + sandwich RT (56 Gy) | 89% | 81% | 2 y: 89% | 2 y: 81% | Grade 3: 23% | Grade 3: 3% | 0% | 59 |
| Newly diagnosed, stage I/II nasal | 27 | GELOX + sandwiched RT (56 Gy) | 96% | 74% | 2 y: 86% | 2 y: 86% | Grade 3: 15% | Grade 3/4: 33.3% | 0% | 60 |
| Newly diagnosed, stage IV, or relapsed/refractory | 38 | SMILE | 79% | 45% | 1 y: 55% | 1 y: 53% | NR | Grade 3/4: 100% | 5.3% | 62 |
| Disease . | No. . | Treatment . | . | . | . | . | Adverse effects . | |||
|---|---|---|---|---|---|---|---|---|---|---|
| ORR . | CR . | OS . | PFS . | Mucositis . | Leucopenia . | TRM . | Reference . | |||
| Relapsed/refractory | 19 | AspaMetDex | 78% | 61% | 2 y: 40% | 2 y: 40% | NR | Grade 3/4: 44% | 0% | 16 |
| Newly diagnosed, relapsed/refractory, any stage | 87 | SMILE ± sandwiched RT (50 Gy) | 81% | 66% | 5 y 50% | 4 y DFS: 64% | NR | Grade 3/4: 67% | 5.7% | 24 |
| Newly diagnosed, stage I/II nasal | 18 | RT (median 50 Gy) | 78% | 78% | 5 y: 30% | 5 y: 30.5% | NR | NR | NR | 51 |
| Newly diagnosed, stage I/II nasal | 31 | RT (median 50 Gy) | 100% | 97% | 5 y: 66% | 5 y: 61% | NR | NR | NR | 52 |
| Newly diagnosed, stage I/II nasal | 17 | CHOP + RT (45 Gy) | 58% | 58% | 3 y: 59% | NR | NR | NR | NR | 54 |
| Newly diagnosed, stage I/II nasal | 27 | Concurrent RT (50 Gy) + 2/3DeVIC | 81% | 77% | 2 y: 78% | 2 y: 67% | Grade 3/4: 30% | Grade 3/4: 93% | 0% | 56 |
| Newly diagnosed, stage I/II nasal | 30 | Concurrent RT (40 Gy) + cisplatin + VIPD | 83% | 80% | 3 y: 86% | 3 y: 85% | Grade 1/2: 11% | Grade 3/4: 14% | 6.6% | 57 |
| Newly diagnosed, stage I/II nasal | 26 | LVP + sandwich RT (56 Gy) | 89% | 81% | 2 y: 89% | 2 y: 81% | Grade 3: 23% | Grade 3: 3% | 0% | 59 |
| Newly diagnosed, stage I/II nasal | 27 | GELOX + sandwiched RT (56 Gy) | 96% | 74% | 2 y: 86% | 2 y: 86% | Grade 3: 15% | Grade 3/4: 33.3% | 0% | 60 |
| Newly diagnosed, stage IV, or relapsed/refractory | 38 | SMILE | 79% | 45% | 1 y: 55% | 1 y: 53% | NR | Grade 3/4: 100% | 5.3% | 62 |
For a list of the regimens, please refer to Table 1.
DFS, disease-free survival; No., number of patients; NR, not reported; RT, radiotherapy.
Treatment of localized disease outside the nasal region
Reports of localized NK/T-cell lymphoma outside the nasal areas are scanty. Studies examining nonnasal lymphomas were performed before PET/CT was available, so it was unclear if they were genuinely nonnasal without nasal primaries. However, taken at face value, nonnasal lymphomas had poor outcomes with CHOP, radiotherapy, or their combination.18 In a retrospective study of 13 patients with localized nonnasal NK/T-cell lymphoma (skin/soft tissue, n = 10; gastrointestinal, n = 3), CHOP-based chemotherapy with sandwiched radiotherapy or surgery resulted in an ORR of 69% and CR in 54%.64 However, CR was brief and 57% of patients relapsed and died. The 3-year OS was only 50%. In another retrospective analysis of 20 patients with skin NK/T-cell lymphoma, radiotherapy (n = 5) gave a higher CR rate than chemotherapy (n = 15) (80% vs 40%). Radiotherapy with or without chemotherapy also resulted in better OS than chemotherapy.65 In another retrospective study where PET/CT was used to exclude occult nasal primaries, 11 patients with nonnasal lymphomas treated with SMILE had an ORR of 72.7%, with CR achieved in 54.5%.24
Current data indicate that localized nonnasal lymphomas might not fare as well as nasal lymphomas. Nonnasal lymphomas of any stage therefore should be treated with systemic chemotherapy with involved-field radiotherapy if applicable.
Treatment of advanced-stage and relapsed/refractory NK/T-cell lymphoma
Combination chemotherapy remains the mainstay of treatment. In a phase 2 study of the SMILE regimen, 38 patients (newly diagnosed stage IV disease, n = 14; first relapse, n = 14; primary refractory disease, n = 4) were treated.66 The ORRs for newly diagnosed stage IV, relapsed, and primary refractory diseases after 2 cycles of SMILE were 80%, 93%, and 25% respectively, and the corresponding CR rates were 40%, 64%, and 0%. With a median follow-up of 24 months, the 1-year OS and PFS were 55% and 53%. Patients with relapsed disease had the best outcome (OS: 79%; PFS: 71%), followed by newly diagnosed stage IV disease (OS: 45%; PFS: 45%) and primary refractory disease (OS: 25%; PFS: 25%). Grade 3/4 neutropenia occurred in all patients, with serious infections in 61% of them. There were 2 therapy-related mortalities.
In another prospective study that examined relapsed/refractory patients treated with SMILE outside a trial setting, the ORR was 77%, with CR achieved in 66%.24 The estimated 5-year OS was 52.3%; the 4-year disease-free survival was 68.2%. Interestingly, these survivals were comparable to those of newly diagnosed patients. However, 72.7% of patients developed grade 3/4 neutropenia, and treatment-related mortality was 7%.
A multicenter phase 2 study examined another l-asparaginase–based regimen in relapsed/refractory NK/T-cell lymphoma.16 Nineteen patients were treated with 3 cycles of AspaMetDex (l-asparaginase, methotrexate, and dexamethasone) (Table 1). The ORR was 78%, with CR achieved in 61%. With a median follow-up of 26 months, the estimated 2-year OS and PFS were both around 40%. Grade 3/4 neutropenia developed in 44.4% of patients.
Current data therefore indicate that advanced-stage and relapsed/refractory NK/T-cell lymphomas should be treated with l-asparaginase–containing regimens that incorporate non–MDR-dependent drugs.
Treatment of aggressive NK-cell leukemia
Few data were available on the treatment of aggressive NK-cell leukemia. In a retrospective series of 22 patients, 13 patients were treated with anthracycline-containing chemotherapy and only 3 achieved CR.25 The median survival was merely 58 days. However, occasional survivals had been reported in patients treated with l-asparaginase–containing regimens, followed by allogeneic HSCT in patients who showed a response.67
Follow-up of NK/T-cell lymphoma patients in remission
Because of chronic infection due to tumor obstruction or radiotherapy-induced mucosal damage, linings of the nasal cavity and paranasal sinuses are often thickened, making it difficult to monitor by CT or MRI for disease recurrence. PET/CT gives a functional and anatomical assessment of lymphoma status and is the recommended follow-up investigation.23 Another biopsy specimen should be obtained in PET/CT-positive cases, as infection may also result in 18-fluorodeoxyglucose uptake. Plasma EBV DNA is a sensitive surrogate biomarker of lymphoma load.23,47 Patients in apparent remission, but with EBV DNA either persistently detectable or rising from a previous unquantifiable level, will invariably relapse, indicating that they will require additional therapy.23 Serial plasma EBV DNA quantification is therefore important in the follow-up of NK/T-cell lymphoma patients in remission. It should be performed on a long-term basis, as the lymphoma is known to relapse after even decades of remission.68
Management of relapsed NK/T-cell lymphomas
For patients relapsing after radiotherapy or combined radiotherapy and anthracycline-containing chemotherapy, the use of an l-asparaginase–containing regimen is highly efficacious.16,24,66 The optimal salvage treatment of patients relapsing after receiving l-asparaginase containing regimens as primary treatment is undefined. Recent evidence suggests that gemcitabine-containing regimens may be effective in these patients,69 which will require further validation. As with other aggressive lymphomas, relapsed patients achieving a remission again should be considered for high-dose chemotherapy with hematopoietic stem cell support.
Prognostic indicators
Three prognostic models have been found relevant. The international prognostic index (IPI) designed for diffuse large B-cell lymphoma has been shown to predict outcome.18,70 In a retrospective study of 67 patients, the 10-year OS was significantly longer in patients with low IPI (≤1) as compared with those with intermediate and high IPI (≥2), irrespective of the treatment modality (primary radiotherapy, CHOP, or CHOP-like regimens).64 The Korean prognostic score, based on a retrospective analysis of 262 patients, identified 4 prognostic factors: B symptoms, stage, lactate dehydrogenase, and regional lymphadenopathy.71 A retrospective analysis of 172 patients with NK/T-cell lymphoma and aggressive NK-cell leukemia identified nonnasal disease, stage, performance status, and numbers of extranodal involvement to be significant prognosis factors.72
These prognostic models have intrinsic problems. They used only presentation parameters and were not validated prospectively. Only CT scan was used. Patients were treated heterogeneously with relatively ineffective anthracycline-containing regimens. In a recent study of 87 patients treated with SMILE, only IPI but not the Korean prognostic score was significant, suggesting that when new regimens are used, prognostic scores must be reappraised.24 Furthermore, biological parameters such as circulating EBV DNA47,48,73 and interim PET/CT findings should also be considered in the construction of future prognostic models.
Role of HSCT in the management of NK/T-cell lymphoma
The use of HSCT has been explored in NK/T-cell lymphomas.49,74 However, most studies contained small number of patients, with heterogeneous indications, inclusion criteria, and HSCT protocols, so that results are difficult to interpret. Furthermore, the optimal timing, conditioning regimen, source of HSC, and whether a graft-versus-lymphoma effect is present in the allogeneic setting are still undefined.
Autologous HSCT
A retrospective analysis of 58 published cases of autologous HSCT had shown that patients transplanted in remission had significantly superior outcome compared with those in refractory disease or nonremission.74 In another study, 47 patients undergoing autologous HSCT were examined.75 With a median follow-up of 117 months, the estimated 5-year OS was 56%, which was not different from that of a historical-matched control group (48%, P = .13). The transplant-related mortality (TRM) was 9%. Disease status pre-HSCT was also the most significant prognostic factor.
Although data of autologous HSCT are not plentiful, some deductions are possible. Early-stage nasal lymphomas treated with combined chemotherapy and radiotherapy have results comparable with those of autologous HSCT.74 Therefore, frontline HSCT is not warranted in these patients. Patients not achieving a remission fare poorly with HSCT, and novel treatment modalities are preferred. For patients with poor-risk or advance-stage lymphoma in remission, whether autologous HSCT improves outcome as compared with newer regimens such as SMILE remains unclear. Autologous HSCT is best tested in clinical trials.
Allogeneic HSCT
A retrospective study analyzed 28 patients with NK-cell malignancies receiving myeloablative (n = 23) and reduced-intensity conditioning (RIC) (n = 5) HSCT.74 Bone marrow (n = 8) and mobilized peripheral blood HSC (n = 20) were obtained from 26 sibling donors and 3 unrelated donors. With a median follow-up of 34 months, the 2-year OS and PFS were 40% and 34%. Grade 3/4 acute graft-versus-host disease developed in 14% of patients. The overall TRMs for the myeloablative and RIC groups were 30% and 20%, respectively. In another retrospective analysis, 12 patients with NK/T-cell lymphoma undergoing myeloablative (75%) or RIC allogeneic HSCT were studied.76 The majority (67%) of patients had stage IV disease. HSC sources were cord blood (n = 5) and bone marrow from related donors (n = 2) and siblings (peripheral blood hematopoietic stem cells, n = 3; bone marrow, n = 2). With a median follow up of 16 months, the 3-year OS and PFS were 55% and 53%. TRM was 8%.
With the limited data available, patients with advanced or relapsed lymphoma in remission can be offered allogeneic HSCT, preferably in a trial setting.
Future perspectives
In the last 2 decades, after NK/T-cell lymphomas have been recognized as distinct clinicopathologic entities, treatment outcome has improved considerably. Our current recommendations, incorporating our experience and that in the literature, are presented schematically in Figure 3. Continued efforts should be devoted to improving chemotherapeutic regimens, identifying better prognostic scores, and defining the role of HSCT (Figure 3). Clinicians should be ready to translate findings from basic and genomic studies into targeted therapy, an approach that holds much promise in the treatment of other types of aggressive lymphomas.
Acknowledgments
Authorship
Contribution: E.T. and Y.-L.K. conceived, wrote, and approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Y.-L. Kwong, Department of Medicine, Professorial Block, Queen Mary Hospital, Pokfulam Road, Hong Kong; e-mail: ylkwong@hkucc.hku.hk.