Abstract
The most frequent and feared complication of paroxysmal nocturnal hemoglobinuria (PNH) is thrombosis. Recent research has demonstrated that the complement and coagulation systems are closely integrated with each influencing the activity of the other to the extent that thrombin itself has recently been shown to activate the alternative pathway of complement. This may explain some of the complexity of the thrombosis in PNH. In this review, the recent changes in our understanding of the pathophysiology of thrombosis in PNH, as well as the treatment of thrombosis, will be discussed. Mechanisms explored include platelet activation, toxicity of free hemoglobin, nitric oxide depletion, absence of other glycosylphosphatidylinositol-linked proteins such as urokinase-type plasminogen activator receptor and endothelial dysfunction. Complement inhibition with eculizumab has a dramatic effect in PNH and has a major impact in the prevention of thrombosis as well as its management in this disease.
Continuing Medical Education online
This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education through the joint sponsorship of Medscape, LLC and the American Society of Hematology.
Medscape, LLC is accredited by the ACCME to provide continuing medical education for physicians.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 70% minimum passing score and complete the evaluation at http://www.medscape.org/journal/blood; and (4) view/print certificate. For CME questions, see page 5105.
Disclosures
Anita Hill, Richard J. Kelly, and Peter Hillmen have previously received Honoraria and have been members of an advisory board of Alexion Pharmaceuticals, Inc; and Peter Hillmen has previously received research funding from Alexion Pharmaceuticals, Inc. The Associate Editor David Lillicrap and CME questions author Charles P. Vega, Associate Professor and Residency Director, Department of Family Medicine, University of California-Irvine, declare no competing financial interests.
Learning objectives
Upon completion of this activity, participants will be able to:
Assess the clinical presentation of paroxysmal nocturnal hemoglobinuria (PNH).
Analyze the risk of thrombosis among patients with PNH.
Evaluate proposed mechanisms of thrombosis in PNH.
Evaluate management options for thrombosis among patients with PNH.
Release date: June 20, 2013; Expiration date: June 20, 2014
Introduction
“We can safely say that PNH is the most vicious acquired thrombophilic state known in medicine”.1 This statement by Luzzatto et al reflects the fear of the complication of thrombosis by both patients with paroxysmal nocturnal hemoglobinuria (PNH) and their treating hematologists. The relationship between the “blood hemolytic system” and the coagulation system was described more than 6 decades ago by Crosby and Dameshek.2 These pioneers in the field of hematology, and especially in PNH research, demonstrate not only the fascination for this disease but in particular its relationship to the hemostasis system.
PNH is a condition in which uncontrolled complement activity leads to systemic complications, principally through intravascular hemolysis and platelet activation. It arises through a somatic mutation of the phosphatidylinositol glycan A (PIG-A) gene in bone marrow stem cells,3-5 resulting in disruption to glycosylphosphatidylinositol (GPI) biosynthesis6 and thereby a deficiency of all GPI-anchored proteins on the cell membrane.7-9 Among the deficient proteins are the complement regulatory proteins CD55 and CD59, resulting in increased complement sensitivity of PNH cells, intravascular hemolysis, promotion of inflammatory mediators, and systemic hemoglobin release.10
Incidence and sites of thrombosis
Thromboembolism is the most common cause of mortality in patients with PNH and accounts for approximately 40% to 67% of deaths of which the cause is known. Further, 29% to 44% of patients with PNH have been reported to have at least 1 thromboembolic event during the course of their disease, although the reason(s) a thrombotic event may suddenly occur remains an enigma.13-19 Analysis of data from pooled incidence cases found that in 19% of patients, visceral (hepatic, mesenteric, portal, splenic, inferior vena cava) thrombosis preceded the diagnosis of PNH. For the remaining patients, visceral thrombosis occurred at a median of 5 years (range, 0-24) after diagnosis.20
Poor survival is associated with the occurrence of thromboembolic complications (relative risk at 8 years, 10.2).19,21,22 Patients with thrombosis at presentation have only a 40% survival rate at 4 years.19 The relative risk of death is increased five- to 15.4-fold.17
This rate of thrombosis in PNH is likely to be underestimated, because a study using sensitive imaging techniques detected abnormalities suggestive of previous subclinical pulmonary thromboses in 6 of 10 patients with PNH (with no known prior thrombosis), even in patients with apparent recent disease onset. There was also evidence of subclinical myocardial damage in 2 of 10 patients. These subclinical thromboses are able to lead to long-term organ damage as reflected by compromised cardiac function in the majority of these patients.23
The theory that patients from different ethnic groups may have additional inherited prothrombotic traits was refuted by a study demonstrating no correlation between known inherited thrombophilias and thrombosis in PNH.24 However, Dragoni and colleagues have found a high rate of antiphospholipid antibodies in patients with PNH compared with healthy volunteers and patients with aplastic anemia, and they purported that it could be a contributory factor to thrombosis in PNH.25
Although studies have reported a strong correlation between a larger PNH neutrophil clone and the occurrence of thrombosis,14,16,26 thrombosis appears to also be markedly elevated in patients with smaller clones, as low as 10%, when compared with the normal population.26-28 In addition, Hugel and colleagues found no correlation between circulating platelet microparticles (discussed later) and level of PNH clone expression,29 which partly explains the unpredictability of thromboses seen in this condition.
Thrombosis in PNH may occur at any site. Common sites include the intraabdominal and cerebral veins, for reasons still unknown, making thrombosis a leading cause of morbidity as well as mortality. An explanation for the propensity of intraabdominal thrombosis in PNH is still needed. One theory proposed that as activated neutrophils lodge more readily in liver microvessels,30 the CD59-negative neutrophils found in PNH are more likely to localize here, interact with platelets (which are also more readily activated), and release serine proteases, hence concentrating procoagulant activity in this region.
Multiple sites are involved in more than one-fifth of cases. Hepatic vein thrombosis (Budd-Chiari syndrome) is recognized as one of the most common sites of thrombosis and affects 7.5% to 25% of patients with PNH28,31,32 and may lead to hepatic failure and thereby may be a common cause of mortality in PNH.33 The presentation may be with acute or chronic abdominal pain, but silent thrombosis is also seen. Interestingly, of patients with Budd-Chiari syndrome, the frequency of splanchic vein thrombosis at presentation is significantly higher in patients with PNH (47%) compared with the non-PNH patients (10%).28 Spontaneous Budd-Chiari syndrome should lead to a search for an underlying cause, with the recommendation that all cases should be considered for PNH screening.28
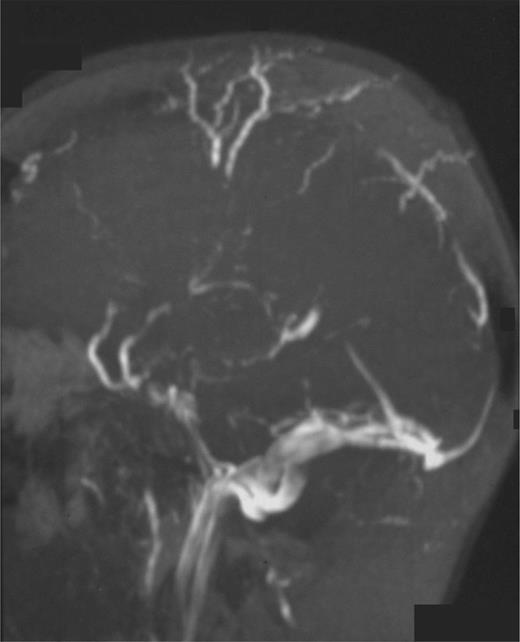
Superior sagittal sinus thrombosis (Figure 1) is the most frequent neurologic complication and results in death in more than one-third of cases.34 There is also a greater tendency for hemorrhagic infarction after this complication. Thromboses in the sagittal sinus, lateral sinus, cavernous sinus, and sigmoid sinus may all result in neurologic symptoms and signs such as severe headache, vomiting, seizures, altered level of consciousness, papilloedema, VI and VII cranial nerve palsies, central retinal vein thrombosis, and cerebellar or lower cranial nerve signs for sigmoid sinus thrombosis.35 Because the mortality rate from cerebrovascular events remains high, prompt diagnosis (frequently requiring sophisticated imaging techniques such as magnetic resonance imaging, magnetic resonance angiography, or magnetic resonance venography) and a high clinical suspicion is required. Magnetic resonance imaging techniques are preferred because contrast agents used with classic angiography could provoke exacerbation of hemolysis or nephrotoxicity.
Magnetic resonance angiography in a patient with PNH and superior sagittal sinus thrombosis with collateral vessel formation.
Magnetic resonance angiography in a patient with PNH and superior sagittal sinus thrombosis with collateral vessel formation.
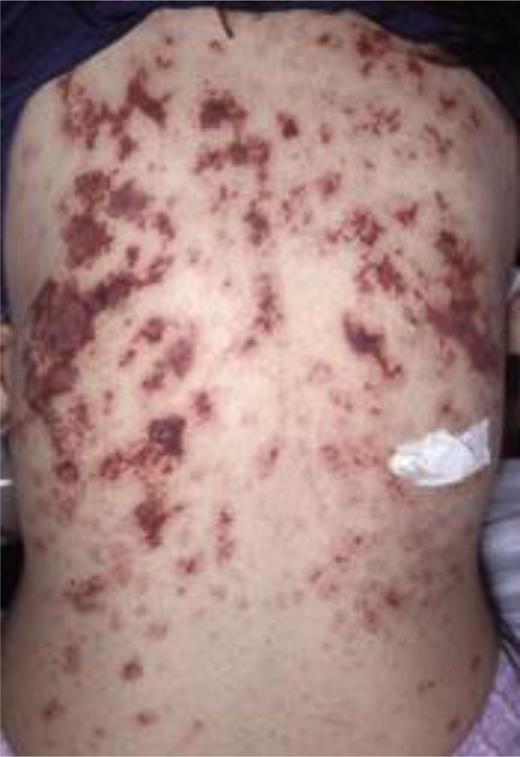
Painful discolored skin lesions result when dermal veins are affected. These lesions rarely ulcerate, but a separate condition resembling purpura fulminans can develop in PNH, affecting larger areas of skin with necrosis (Figure 2).36 Infarction of the bowel is also seen with symptoms and signs of intestinal obstruction. Mesenteric vein thrombosis results in pain that may be disproportionately exaggerated relative to the physical examination.20 Thrombosis may affect the small peripheral mesenteric veins and induce transient ischemia. As well as abdominal pain, there may be associated fever, obstruction, and rectal bleeding. Duodenal venous thrombosis is associated with papillary endothelial hyperplasia, ulceration, and a circumferential mass in the third portion of the duodenum.35 The authors have observed that patients have had small bowel resections secondary to stricture formation, which is likely to be ischemic in nature. Renal vein or artery thrombosis is uncommon but may result in a further cause of renal impairment in these patients.
Case of dermal vein thromboses in a patient known to have PNH. (Reproduced with permission from Watt SG, Winhoven S, Hay CR, Lucas GS. Purpura fulminans in PNH. Br J Haematol. 2007;137:271.)
Case of dermal vein thromboses in a patient known to have PNH. (Reproduced with permission from Watt SG, Winhoven S, Hay CR, Lucas GS. Purpura fulminans in PNH. Br J Haematol. 2007;137:271.)
Deep vein thrombosis of the lower limbs occurs more frequently in patients with PNH than in the general population and has been reported in approximately one-third of patients.15,37 Thromboses in the pulmonary vasculature are also recognized but not always in the form of emboli or in the presence of lower limb deep vein thromboses. In situ formation is likely and underappreciated.
Arterial thrombosis was also the first manifestation of PNH in approximately half of reports of stroke associated with PNH.38,39 It is important to recognize that arterial thromboses are also increased in patients with PNH26,38,40-43 frequently involving the cerebral and coronary arteries and supported by a recent analysis.44,45
Thrombosis is a well-recognized serious complication in pregnant patients with PNH. The management in this setting will not be covered in this manuscript.
When should we test for PNH in patients presenting with thrombosis?
Unlike the finding of most inherited thrombophilias, the finding of PNH in a patient presenting with thrombosis is likely to change specific management. It is therefore suggested that PNH testing be considered when investigating for thrombophilia.
Recommendations would be to consider testing for PNH by flow cytometry in those patients with unexplained thrombosis and those who:
are young,
have a thrombosis in an unusual site (eg, intraabdominal veins, cerebral veins, dermal veins),
have evidence of hemolysis, or
have any cytopenia.
A frequent question is whether a normal lactate dehydrogenase (LDH) level could exclude PNH and therefore negate the need for sending a sample for peripheral blood flow cytometric testing. There are, admittedly rare, situations in which the LDH may not be raised such as those with a predominant Type II (partially deficient of GPI-linked proteins) red cell population in which hemolysis may be minimal, in patients who are heavily red cell transfusion–dependent, and in some instances where the thrombosis has occurred in a patient with a small percentage of PNH cells. Screening by flow cytometry should therefore still be considered.
Proposed mechanisms of thrombosis in PNH
This is a complex area and one of continued research interest. There is an intrinsic relationship between the coagulation cascade and the complement system that is revealed by understanding some of the mechanisms thought to result in thrombosis in PNH. Platelet activation, complement-mediated hemolysis, impaired nitric oxide (NO) bioavailability, impairment of the fibrinolytic system, and inflammatory mediators are all proposed mechanisms and thought to be responsible for the increased thrombotic risk in patients with PNH. Multiple factors are likely to contribute to any one thrombotic event.
Activation of platelets
Platelet activation, known to initiate blood clotting, is likely to be the main culprit of the high incidence of thrombosis associated with PNH.21,46,47 Many mechanisms may result in platelet activation in patients with PNH. Although there is absence of CD55 on PNH platelets, platelets are capable of compensating for this with factor H present within α granules.48 The absence of CD59, however, renders platelets susceptible to attack by complement, with complement-mediated activation ensuing. Although complement activation of platelets theoretically may result in lysis or removal of platelets and thereby contributes, to a minor degree, to some of the thrombocytopenia49,50 the survival of platelets from PNH patients has been found to be normal.51,52 The phosphatidylserine externalization and production of microparticles is recognized in cells undergoing apoptosis. Phosphatidylserine becomes a determinant for phagocyte recognition of senescent or apoptotic cells to be cleared and may contribute to lowering of the platelet count.53-55 This has been further supported recently when studying the effect of the complement inhibitor, eculizumab. One study found that the platelet count rose,56 whereas another did not.57 The explanation for the latter study may lie in increased deposition of C3 on platelets, similar to that seen on PNH red cells for patients treated with eculizumab,58,59 and increased reticuloendothelial clearance in some patients. Therefore, rather than causing lysis of platelets, the complement attack of platelets results in morphologic changes and the release of vesiculated membrane attack complex (MAC). Platelet lysis is therefore minimized by this release from the cell surface of excess MAC by exovesiculation.29,48 The deposition of C5b-9 therefore leads to an increase of expression of activation-dependent proteins, and platelet stimulation is accompanied by the loss of membrane phospholipid asymmetry.47,60,61 These platelet vesicles or microparticles are very procoagulant in vitro and are present at significantly elevated levels in the blood of patients with PNH. The externalized phosphatidylserine on the microvesicles acts as a binding site for prothrombinase62 and tenase complexes.63 Therefore, absence of CD59 from platelets probably leads to thrombin generation, an increased sensitivity to aggregation by thrombin and increased thrombotic risk, both venous and arterial.29,47,64,65 The lack of correlation between thrombotic risk and platelet microparticles may be in part because platelet-derived microparticles also have anticoagulant activities inhibiting fibrin formation or some platelet activation, possibly caused by the presence of proteins C and S in the surface of the microvesicles downregulating the prothrombinase complexes and activated factor Va.21
Activated platelets also interact with neutrophils and can promote thrombus formation by release of neutrophil serine proteases and nucelosomes, synergistically activating Factor X further and thus triggering blood coagulation primarily through the extrinsic pathway.66 This mechanism may be more prevalent in PNH because of the readily activatible state of platelets. It also remains to be explored whether PNH neutrophils more readily release serine proteases and the interaction with tissue factor pathway inhibitor (TFPI) that is already thought to be altered in PNH (discussed later).
The activation of platelets may also in itself perpetuate or exacerbate events, in a feedback loop, in patients through continuing the activation of the alternative pathway of complement (through P-selectin) but also by initiating activation of the classical pathway of complement as a result of platelet-derived chondroitin sulfate.67-69
As well as the loss of CD59, further mechanisms by which platelets are activated in PNH (Table 1) are through the depletion of NO, the direct toxicity of cell-free hemoglobin, increased reactive oxygen species causing oxidative stress, the generation of thrombin, which itself further activates platelets,and as a consequence of endothelial dysfunction. It should be mentioned that one study determined that the platelets in PNH were hyporeactive and concluded that this may be caused by chronic hyperstimulation because of continual complement system attack.70 These findings need further research, in particular to assess whether they were simply a result of thrombocytopenia.
Causes of activated platelets in PNH
| 1. Complement-mediated through loss of CD59 and assembly of C5b-9 on the surface |
| 2. NO depletion |
| 3. Direct effects of free hemoglobin |
| 4. Increased levels of reactive oxygen species |
| 5. Endothelial dysfunction |
| 6. Thrombin activation |
| 1. Complement-mediated through loss of CD59 and assembly of C5b-9 on the surface |
| 2. NO depletion |
| 3. Direct effects of free hemoglobin |
| 4. Increased levels of reactive oxygen species |
| 5. Endothelial dysfunction |
| 6. Thrombin activation |
Although the complement activation of platelets does not appear to cause significant thrombocytopenia, it is well-recognized clinically that an unexpected drop in a patient’s platelet count may be an indicator that a thrombosis is occurring (silently in some cases) and requires further investigation. Lower resting platelet counts can be a consequence of overt, subclinical, and/or silent thromboses; hypersplenism consequent to a previous Budd-Chiari syndrome and/or liver disease; and increased sensitivity of megakaryocyte progenitors in PNH to complement.71 Therefore, bone marrow failure should not be considered as the only reason for thrombocytopenia in patients with PNH.
Intravascular hemolysis: depletion of NO, toxicity of free hemoglobin, and red cell microvesicles
Thrombotic events have been temporally associated with increased hemolysis,16,26,39,72 and intravascular hemolysis is also likely to be one of the principle contributors to thromboembolism in this disorder.73,74 The multiple mechanisms by which intravascular hemolysis may contribute to thrombosis are summarized in Table 2.
Consequences of intravascular hemolysis in the mechanisms of thrombosis in PNH
| 1. Release of free hemoglobin resulting in: |
| a. Platelet activation |
| b. Thrombophlebitis |
| c. NO depletion with consequences of: |
| i. Vasoconstriction |
| ii. Platelet activation and aggregation |
| iii. Increased expression of cellular adhesion molecules |
| iv. Secretion of procoagulant proteins |
| v. Ischemia-reperfusion injury |
| vi. Endothelial proliferation |
| vii. Increased inflammatory markers |
| viii. Factor XIII activation |
| ix. Increased levels of thrombin-antithrombin complexes and fibrin split products |
| d. Inhibition of ADAMTS13 |
| e. Direct endothelial dysfunction |
| f. Activation of endothelial cells and release of microparticles |
| g. Increased levels of tissue factor |
| h. Increased levels of reactive oxygen species |
| 2. Release of arginase resulting in NO depletion |
| 3. Release of procoagulant red cell microparticles |
| 1. Release of free hemoglobin resulting in: |
| a. Platelet activation |
| b. Thrombophlebitis |
| c. NO depletion with consequences of: |
| i. Vasoconstriction |
| ii. Platelet activation and aggregation |
| iii. Increased expression of cellular adhesion molecules |
| iv. Secretion of procoagulant proteins |
| v. Ischemia-reperfusion injury |
| vi. Endothelial proliferation |
| vii. Increased inflammatory markers |
| viii. Factor XIII activation |
| ix. Increased levels of thrombin-antithrombin complexes and fibrin split products |
| d. Inhibition of ADAMTS13 |
| e. Direct endothelial dysfunction |
| f. Activation of endothelial cells and release of microparticles |
| g. Increased levels of tissue factor |
| h. Increased levels of reactive oxygen species |
| 2. Release of arginase resulting in NO depletion |
| 3. Release of procoagulant red cell microparticles |
Proposed hemolysis-independent mechanisms of thrombosis in PNH
| 1. Platelet activation through: |
| a. Direct complement activity |
| b. Reactive oxygen species |
| c. Endothelial dysfunction |
| d. Thrombin activation |
| 2. Deficiency of u-PAR (although may only be significant in the presence of red cell microparticles) |
| 3. Deficiency of heparan sulfate |
| 4. Deficiency of TFPI |
| 5. Deficiency of PR3 |
| 6. Endothelial cell activation |
| 7. C5a mediated mechanisms |
| a. Increase in inflammatory cytokines |
| b. Downregulation of ADAMTS-13 |
| 8. Generation of tissue factor and PAI1 from PNH monocytes and neutrophils |
| 9. Decreased levels of protein S |
| 10. Protein C resistance because of increased factor VIII activity |
| 11. Thrombin activation of the complement system perpetuating mechanisms above |
| 1. Platelet activation through: |
| a. Direct complement activity |
| b. Reactive oxygen species |
| c. Endothelial dysfunction |
| d. Thrombin activation |
| 2. Deficiency of u-PAR (although may only be significant in the presence of red cell microparticles) |
| 3. Deficiency of heparan sulfate |
| 4. Deficiency of TFPI |
| 5. Deficiency of PR3 |
| 6. Endothelial cell activation |
| 7. C5a mediated mechanisms |
| a. Increase in inflammatory cytokines |
| b. Downregulation of ADAMTS-13 |
| 8. Generation of tissue factor and PAI1 from PNH monocytes and neutrophils |
| 9. Decreased levels of protein S |
| 10. Protein C resistance because of increased factor VIII activity |
| 11. Thrombin activation of the complement system perpetuating mechanisms above |
PAI1, plasminogen activator inhibitor 1.
Hemolysis, through factors such as toxicity of the free hemoglobin and NO depletion, has been implicated in the initiation of platelet activation and aggregation.75 The role of free hemoglobin has been demonstrated by the infusion of cross-linked hemoglobin in rats, which increases platelet aggregation and adhesion in vivo on prothrombotic surfaces such as an injured vessel wall.76 Further, administration of heme in healthy volunteers causes thrombophlebitis, demonstrating that heme can cause vascular inflammation followed by vascular obstruction in vivo.77 Interestingly, the addition of cell-free hemoglobin to human serum causes inhibition of the metalloprotease ADAMTS13, an enzyme critical in limiting platelet thrombus formation.78 The effects of free hemoglobin on endothelial function are discussed later. Further disintegration of heme releases toxic species of iron, which participate in biochemical reactions, such as the Fenton reaction, that generate free radicals and thus catalyze the formation of reactive oxygen species and result in loss of membrane lipid organization.79 Phosphatidylserine-positive red cells are more likely to adhere to endothelium as well as provide scaffolding for the tenase and prothrombinase complexes. Reactive oxygen species were higher and reduced glutathione lower when studied in patients with PNH, and the PNH cells themselves were at higher oxidative stress.80
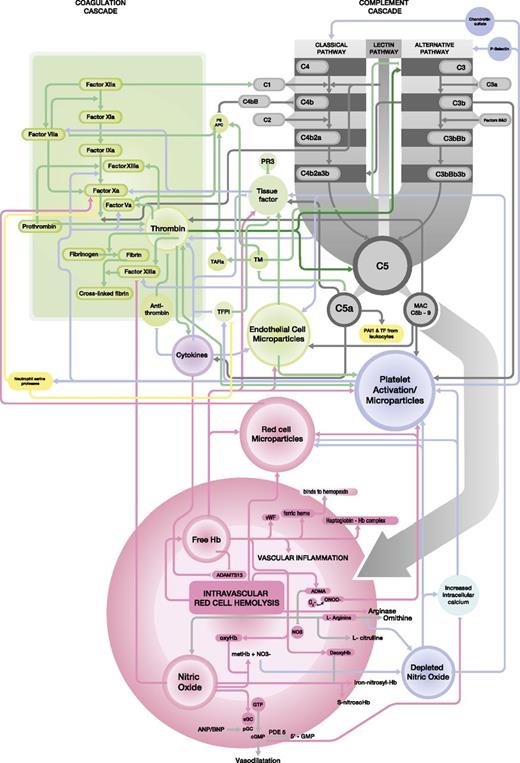
NO, a free radical, binds avidly to soluble guanylate cyclase resulting in increased intracellular cyclic guanosine monophosphate (cGMP)81 (Figure 3). CGMP activates cGMP-dependent kinases that decrease intracellular calcium concentration in smooth muscle, producing relaxation, vasodilatation, and increased regional blood flow, primarily by suppressing platelet aggregation, expression of cell adhesion molecules on endothelial cells, and secretion of procoagulant proteins.75,82-84 NO also limits platelet aggregation and ischemia-reperfusion injury,85 modulates endothelial proliferation,86 and has anti-inflammatory properties.87 Conversely, NO depletion is associated with platelet activation and an increase in soluble P-selectin expression,88,89 which in itself can further activate the complement system.67
Close integration between the complement cascade (grey) and the coagulation cascade (green). The relationships with red cell hemolysis, platelet activation, endothelial cells, and white blood cells are also demonstrated. Detailed information regarding each interaction is given the text.
Close integration between the complement cascade (grey) and the coagulation cascade (green). The relationships with red cell hemolysis, platelet activation, endothelial cells, and white blood cells are also demonstrated. Detailed information regarding each interaction is given the text.
NO also interacts with components of the coagulation cascade to downregulate clot formation. For example, NO has been shown to chemically modify and inhibit Factor XIII, which suggests that NO deficiency would enhance clot stability and reduce clot dissolution.90 Further, reduction of NO causes increases in fibrin split products and thrombin-antithrombin complexes, leading to significant fibrin deposition and thrombus formation in an animal model.91 Reduced NO production in l-arginine deficiency has been associated with increased thrombin-antithrombin complexes and fibrin split products, whereas reversal of NO deficiency with l-arginine causes a reduction in intravascular coagulopathy.92
The reaction of NO with oxyhemoglobin is fast and irreversible.93 Intravascular hemolysis results in the release of free hemoglobin into the plasma. The chronic nature of the hemolysis in PNH is such that even at baseline, in between paroxysms, there is sufficient release of free hemoglobin to saturate biochemical systems in place to remove it, resulting in NO depletion. The rate of NO depletion correlates with the severity of intravascular hemolysis of which LDH is a sensitive marker.94
In addition to hemoglobin decompartmentalization and NO scavenging, intravascular hemolysis also releases erythrocyte arginase, an enzyme that converts l-arginine, the substrate for NO synthesis, to ornithine, thereby further reducing the systemic availability of NO95 (Figure 3).
In addition, hemolytic rate (reticulocytosis) is associated with hemoglobin desaturation (ventilation/perfusion mismatch) and adhesion molecule expression (intracellular adhesion molecule 1 [ICAM-1], vascular cell adhesion molecule 1 [VCAM-1] and E-selectin96 ); it is possible that such a hypoxic state can induce hypoxia-inducing factor-1–dependent factors such as erythropoietin, vascular endothelial growth factor, and endothelin-1.73,97 Elevated VCAM-1 correlates strongly with the presence and extent of thrombosis.43
Circulating procoagulant microvesicles in association with red blood cells have also been described,98 although other investigators have found this source to be very low.21,29,99,100 The dissemination of prothrombotic seats could therefore occur because of the presence of high levels of these procoagulant microparticles, stemming from lysed red cells (also externalizing phosphatidylserine) in the blood flow.29
Deficiency or absence of other GPI-linked (or associated) proteins: u-PAR, heparan sulfate, TFPI, and proteinase-3
Urokinase-type plasminogen activator receptor (u-PAR; CD87) is a GPI-bound protein that is therefore absent from PNH monocytes and granulocytes. It binds pro-urokinase (uPA) to the cell surface, which converts plasminogen to plasmin and results in clot lysis. It is possible that the absence of u-PAR from the cell surface in PNH101 results in an increased tendency to thrombosis as a result of impaired fibrinolysis and reduced clot dissolution. Because of a lack of anchorage of u-PAR to the cell membrane, the increased plasma levels are thought to also contribute to the increased risk of venous thrombosis by competing with membrane-bound u-PAR.102,103 Sloand et al have proposed that the combination of reduced cell-bound u-PAR with increased levels of soluble u-PAR and the presence of red cell microvesicles is a contributory factor for thrombosis in patients with PNH.104 Other studies have not identified any fibrinolytic defects.21 In addition, although clot dissolution may be reduced by this mechanism, could this actually result in clot initiation? It may potentiate thrombosis but is unlikely to be a sole cause of thrombosis. Fibrinolytic defects, such as plasminogen deficiency, are not generally associated with thrombosis.105
Binding of antithrombin to endothelial cells is thought to be mediated by heparan sulfate, also a GPI-linked protein.106 It is reduced by inflammatory markers such as interleukin (IL)-1 and tumor necrosis factor. Its deficiency may partly contribute to the hypercoagulable state in PNH, although there have been no studies exploring this. Heparan sulfate–deficient mice have, however, been found to have the same amount of fibrin deposition as wild-type mice,107 raising the possibility that there is compensation for reduction in heparan sulfate by other glycosaminoglycans. Only complete deficiency appears to lead to thrombosis.106
TFPI is predominantly released by the endothelium (but is also present on the surface of monocytes, within platelets, and circulating in the plasma) and is anchored, most likely indirectly, through the GPI anchor. It is a potent anticoagulant protein that abrogates blood coagulation by inhibiting both factors Xa and the tissue factor–factor VIIa catalytic complex, making it the only physiologically active inhibitor of the initiation of blood coagulation. It has been suggested that defective expression or reduced activity (as TFPI is downregulated by inflammatory cytokines), potentially coexistent problems in PNH, may contribute to both arterial and venous thrombosis.108,109 However, TFPI is also found within platelets, and presumably expression through this route should be unaffected in patients with PNH and is an area for further study. Expression of TFPI on the surface of platelets after dual-agonist activation has been described.109 This raises the question of whether there is similar expression after complement activation in patients with PNH to counterbalance the reduced expression and downregulation.
Although proteinase-3 (PR3) is not itself a GPI-linked protein, it co-localizes with CD177 (NB1), which is a GPI-anchored protein. Deficiency of membrane-bound PR3 on granulocytes is therefore found in PNH. Membrane-bound PR3 modulates thrombus formation by cleaving the thrombin receptor and thereby decreasing thrombin-mediated platelet activation.110 The extent of platelet activation through this mechanism needs further study in PNH.
Endothelial dysfunction
Endothelial dysfunction occurs during any thrombotic event. Tissue factor, a key initiator of coagulation, is expressed in subendothelial mural cells and adventitial fibroblasts in and around the vessel wall and closely links the coagulation and complement cascades. The endothelium has also been implicated in the pathogenesis of thrombosis in hemolytic states.42,43 Free hemoglobin directly impairs endothelial function. Free hemoglobin and its breakdown oxidative product heme can directly activate endothelial cells and further promote inflammation and coagulation as well as increase tissue factor production and release of high molecular weight von Willebrand factor (VWF).
Microparticles circulating in PNH express endothelial markers: ICAM-1 (CD54), sVCAM-1, VWF, CD144 (VE-cadherin), and CD105 (endoglin), indicating chronic endothelial activation. CD144, similarly to CD105, is derived from a subportion of endothelial cell junctions. It has a very short half-life in the circulation; its presence in the circulation in PNH is therefore indicative of persistent endothelial damage associated with the chronic hemolysis of PNH. A similar association has been described in sickle-cell disease.111,112
As well as direct complement activation, similarly to platelets, the endothelial expression of cell adhesion molecules is also promoted by NO depletion because NO is known to suppress their expression.
Whether endothelial cells are affected by the PIG-A mutation is of considerable research interest. If they are found to be deficient of the complement regulatory proteins, CD55 and CD59, their dysfunction in PNH would be both primary and secondary contributors to thrombosis.
Other complement-mediated procoagulant mechanisms (independent of hemolysis)
Complement activation plays a major role in vascular inflammation. C5a may result in proinflammatory and prothrombotic processes through the generation of inflammatory cytokines such as IL-6, IL-8, and tumor necrosis factor-α. These will further activate the endothelium with the production of endothelial cell microparticles, potentially self-perpetuating the problem. IL-6 promotes thrombin formation. Complement activation on the surface of monocytes and neutrophils is also followed by the formation of the MAC. On these cells the MAC induces cell activation and also proliferation.113 Both the MAC formation and C5a may induce the expression of tissue factor as well as plasminogen activator inhibitor 1 by these leukocytes. One study demonstrated that complement activation (by antiphospholipid antibodies) and downstream signaling via C5a receptors in neutrophils leads to the induction of tissue factor (Figure 3).114 Il-6 may additionally deregulate the immune system and inhibit ADAMTS-13 or VWF cleaving protease activity.115
Much of protein S, the cofactor for activated protein C, circulates in complex with the complement protein C4b-binding protein, inhibiting its anticoagulant function. Decreased levels of both proteins C and S have been found in both sickle-cell disease and β-thalassemia and partly attributed to chronic consumption because of increased tissue factor expression, thrombin generation, and/or hepatic dysfunction.79 Because these contributing factors are readily seen in PNH, they may also be implicated in thrombosis in PNH. Patients with sickle-cell disease also appear to be more resistant to activated protein C, which may be a result of increased factor VIII coagulant activity as well as the reduced protein S. Patients with PNH may again be at risk because factor VIII levels (along with VWF) are raised because of the endothelial activation.
Thrombosis in PNH is also seen at the time of infection and may be partly caused by the increased hemolysis that usually coincides. There are also likely contributing hemolysis-independent mechanisms. The invading pathogens (or damaged host cells) are recognized by antigen-presenting cells, neutrophils, monocytes, macrophages, endothelial cells, and platelets, resulting in tissue factor exposure that is sustained by cytokines and chemokines. The pathogen can also further induce complement activation, promoting generation of more C5a and MAC. C5a feeds back to promote expression of tissue factor.12
Finally, the pathway turns full circle with the knowledge that a fourth pathway (separate to the classical, lectin, and alternative) has been described to activate the complement system in which thrombin itself cleaves and activates C3 and C5 (independent of C3).116 Therefore, thrombosis activates complement, perhaps leading to further thrombosis in PNH and then a vicious circle ensues. This might explain the observation that once a patient has their first thrombosis, this often heralds further thrombotic complications spiraling out of control, despite anticoagulation, until the patient eventually succumbs.
Management of thrombosis in PNH
Acute treatment of thrombosis
Thrombosis in a patient with PNH is a requirement for urgent intervention because of the high likelihood of mortality or significant disability and the rapid deterioration that frequently occurs. Attention needs to be given to the balance between bleeding (for example, because of the underlying bone marrow failure) and the highly thrombotic tendencies. Randomized controlled trials are lacking but experience has been gained in large PNH centers. The optimal management of acute thrombotic events requires immediate full anticoagulation (in the absence of major contraindications) beginning with heparin therapy (aiming for anti-Xa levels between 0.5 and 1.0 for those treated with low-molecular-weight heparin) and the commencement of the monoclonal antibody therapy, eculizumab. Continuing anticoagulation with the vitamin K antagonists is generally recommended in the long term if there are no contraindications (see discussion on secondary prophylaxis later). Recurrent thromboses and extension of existing thromboses are frequent complications in PNH. There is no published experience of the newer oral anticoagulants in PNH.
It has been reported that hemolysis may be exacerbated on the initiation of heparin because at low doses it activates the alternative complement pathway. However, at higher concentrations, it acts as an inhibitor.117 Nevertheless, the procoagulant alteration of the PNH red cells and platelets remains.118 The increasing use of eculizumab during the management of acute thrombosis in patients with PNH is proving effective and our group, as well as others, has had positive experience in this setting in a number of cases including in acute Budd-Chiari syndrome.119 In addition, the propagation of thrombosis, or the occurrence of further discrete thromboses in PNH after the initial clot, appears to be prevented by eculizumab. Indeed, development of any thrombosis in a patient with PNH is now considered one of the primary indicators to commence eculizumab therapy, and this should be done without delay. Eculizumab also halts the multiple sequential and/or simultaneous thromboses that are observed in PNH patients.
The management of Budd-Chiari syndrome in a patient with PNH, which may occur despite anticoagulant prophylaxis, is usually complex. As with other thrombotic events in this condition, immediate commencement of eculizumab is recommended. We have shown by our own series of patients in Leeds that urgent commencement of eculizumab can reduce mortality and long-term sequelae of Budd-Chiari syndrome.119 Anticoagulation alone would not be expected to restore hepatic blood flow. In a series of patients with Budd-Chiari syndrome, either extension of the thrombosis or a new thrombotic event occurred in 27% despite treatment with anticoagulation.28 Historically, there are reports on the use of thrombolytic therapy in this setting but this is less likely to be required with commencement of eculizumab therapy, particularly because hemorrhagic complications remain a concern. When portal hypertension is the predominant problem, a transjugular intrahepatic portosystemic shunt procedure is often helpful by decreasing portal pressure gradients, improving synthetic function, reducing transaminase levels, and controlling ascites.120 The main complication of a transjugular intrahepatic portosystemic shunt is the risk of the shunt thrombosing, but in our practice this is prevented with the combination of anticoagulation and eculizumab.
Allogeneic bone marrow transplant has been previously considered but, despite improvements in other indications, the associated morbidities and mortalities in hemolytic PNH remain unsatisfactory. Liver transplantation is contraindicated because of the risk of recurrent thrombosis found in all cases of patients with PNH who had Budd-Chiari syndrome and underwent liver transplantation, although most of the data are in the pre-eculizumab era.33 There is an increased risk of the development of hepatocellular carcinoma after a Budd-Chiari syndrome, and patients should be monitored for this complication, such as with regular blood tests for α-fetoprotein and/or liver ultrasound scans.
The previous high mortality from mesenteric vein thrombosis appeared to be associated with surgical intervention. Medical management, which would now include commencing eculizumab therapy if it is available, should be feasible when imaging demonstrates that the bowel infarction has not led to transmural necrosis and bowel perforation.121
Prevention of thrombosis
Preventing thrombosis in PNH is an important aim in the management of patients with PNH and would be expected to lead to reduced morbidity and mortality. In vitro, heparin and low-molecular-weight heparin therapy have been shown to inhibit the hemolysis in PNH.117,118 This is probably primarily a result of the potentiation of C1 inhibitor122 but may also be caused by inhibition of both the classic and alternative pathway of C3 convertases as well as interference with the assembly of the C5b-9 complex.123,124 Complement activation on PNH red blood cells induces procoagulant alteration of the red cell membranes,98 but inhibition of complement-mediated lysis of PNH red cells by heparin and low-molecular-weight heparin did not result in the inhibition of complement-induced procoagulant alteration of PNH red cells.118 This suggests the principle mechanism of heparin action in this setting is through the interaction with C5b-9 and not through the potentiation of C1 inhibitor. There have been reports of an increased incidence of heparin-induced thrombocytopenia and consequent thrombosis,125-127 thought to be explained by the increased platelet activation in PNH with induced release of platelet factor 4. If there is concern, theoretically, fondaparinux may be a safer formulation.
In patients who are not treated with eculizumab, consideration of primary prophylaxis should be given to reduce the risk of thrombosis if there is no contraindication, such as thrombocytopenia or other bleeding risk.26 However, there is a risk that anticoagulation in these patients may lead to complications and major hemorrhage.15,16,26,29 The risk of hemorrhage in patients with PNH may actually be higher because of the underlying bone marrow failure, which allows the PNH clone to expand. Given that eculizumab improves the management of established thrombosis in PNH, then the pros and cons of prophylactic anticoagulation needs discussion with the patient. In addition, thrombocytopenia is a relative contraindication to anticoagulation and this complication is not uncommon in patients with PNH. In addition, there are still clear cases of thromboses occurring while patients are therapeutically anticoagulated,14,28,33,37,39,127,128 which is less surprising when the proposed mechanisms are considered. After a thrombotic event, it appears that anticoagulation alone as secondary prevention is not sufficient.127,129
There are no studies of antiplatelet drugs, such as aspirin or clopidogrel, in PNH, but again, mechanistically, it is clear that they are unlikely to be of benefit and again there is a true risk of hemorrhage.
Considering that the mechanisms of thrombosis in PNH appear to lie with the role of platelet activation through direct complement activation as well as intravascular hemolysis and the release of free hemoglobin with all its consequent effects and mechanisms mediated through C5a, it might be anticipated that complement blockade should eliminate the risk of thrombosis, although there are no prospective trial data. Therefore, to explore the effect of long-term treatment with eculizumab on thrombosis risk more formally, the prespecified clinical outcome of thromboembolism on an intention-to-treat basis in a multinational phase III open-label extension study that enrolled patients from 3 independent eculizumab PNH clinical studies130-133 (N = 195) was evaluated. Eculizumab treatment resulted in a reduction in the thromboembolism event rate in each of the individual clinical studies (P < .001).37
Data for patients treated by the National PNH Centre, Leeds, UK, have recently been published supporting a continuing dramatic reduction in thrombosis rate and this is perhaps one of the important factors behind the significantly improved initial survival for patients treated with eculizumab.57 Further support from 2 studies found that eculizumab treatment of patients with PNH resulted in a rapid decrease in plasma tissue factor microparticles, thrombin generation, and inflammation as measured by D-dimers, thrombin-antithrombin complexes, plasmin-antiplasmin complexes, IL-6, and markers of endothelial cell activation (tissue-plasminogen activator, soluble VCAM, VWF, and TFPI).112,134 Therefore the clinical data and the supportive mechanistic evidence indicate that eculizumab has a major impact on the management of thrombosis in PNH.
An important question still to be addressed is whether anticoagulation can safely be discontinued in patients with PNH who have had a previous thrombosis and are receiving eculizumab. This has been achieved successfully and reported in 3 patients, although longer follow-up is required.129 Two findings support continuing anticoagulation after thrombosis: (1) The significantly lower levels of prothrombin fragment 1+2 (a direct marker of prothrombin activation to thrombin) when anticoagulated and the reduction in soluble u-PAR, which only occurs when concomitantly anticoagulated112,135 ; and (2) the report from 1 study demonstrating that the externalization of anionic phospholipids resulting in the procoagulant alteration of red cell membranes upon complement activation seems to occur before the step of C5b-7 insertion into the red cell membranes because C5-deficient serum also induced procoagulant alteration of PNH red cells.118 It seems prudent to advise continued anticoagulation in patients with a prior thrombosis who are receiving eculizumab, unless there are clear contraindications to anticoagulation or until more evidence of the safety of stopping anticoagulation therapy is generated. It is hoped that data collected from the Global PNH Registry will also aid the answer, and hematologists are strongly encouraged to enroll all PNH patients, regardless of clone size or therapy, into this registry (http://www.pnhregistry.org).
Conclusion
Thrombosis has been well-recognized as the leading cause of death in PNH. Preventing thrombosis in this disease and effectively treating thrombosis early on in its presentation are paramount. Appreciating the high frequency of thrombosis in PNH should lead one to thorough, and possibly multiple, investigations to exclude thrombosis. A patient presenting with thrombosis should be considered for screening for PNH if they fall into one of the 4 categories described.
The tendency toward thrombosis in patients with PNH is multifactorial in etiology, involving the absence of GPI-anchored complement inhibitors on the surfaces of circulating platelets, the high levels of intravascular free plasma hemoglobin with the consequent scavenging of NO, fibrinolytic defects, and the pro-inflammatory effects of C5a. The relative importance of each factor is not yet known but the integration between the 2 major host protection systems, coagulation and innate immunity, is obvious. The majority of the mechanisms relate to complement dysfunction and its consequences. Therefore eculizumab, which addresses these mechanisms, resulting in the reduction of thrombosis risk, has now become an important part of the management of this most feared complication. Because thrombosis is the leading cause of death, the impact of eculizumab on thrombosis largely explains the improved survival seen with eculizumab therapy.
Authorship
Contribution: A.H. wrote the review manuscript. R.J.K. and P.H. contributed to the discussions, reviewed and agreed the manuscript.
Conflict-of-interest disclosure: A.H., R.J.K., and P.H. have previously received Honoraria and have been members of an advisory board of Alexion Pharmaceuticals, Inc; and P.H. has previously received research funding from Alexion Pharmaceuticals, Inc.
Correspondence: Anita Hill, Department of Haematology, Level 3, Bexley Wing, St. James’s University Hospital, Leeds, UK; e-mail: anitahill@nhs.net.