Key Points
Blockade of PDGFRβ impairs lymphoma growth by depleting vascular mural cells.
Pericytes may represent a novel, antiangiogenic target for lymphoma therapy.
Abstract
Pericytes and vascular smooth muscle cells (VSMCs), which are recruited to developing blood vessels by platelet-derived growth factor BB, support endothelial cell survival and vascular stability. Here, we report that imatinib, a tyrosine kinase inhibitor of platelet-derived growth factor receptor β (PDGFRβ), impaired growth of lymphoma in both human xenograft and murine allograft models. Lymphoma cells themselves neither expressed PDGFRβ nor were growth inhibited by imatinib. Tumor growth inhibition was associated with decreased microvascular density and increased vascular leakage. In vivo, imatinib induced apoptosis of tumor-associated PDGFRβ+ pericytes and loss of perivascular integrity. In vitro, imatinib inhibited PDGFRβ+ VSMC proliferation and PDGF-BB signaling, whereas small interfering RNA knockdown of PDGFRβ in pericytes protected them against imatinib-mediated growth inhibition. Fluorescence-activated cell sorter analysis of tumor tissue revealed depletion of pericytes, endothelial cells, and their progenitors following imatinib treatment. Compared with imatinib, treatment with an anti-PDGFRβ monoclonal antibody partially inhibited lymphoma growth. Last, microarray analysis (Gene Expression Omnibus database accession number GSE30752) of PDGFRβ+ VSMCs following imatinib treatment showed down-regulation of genes implicated in vascular cell proliferation, survival, and assembly, including those representing multiple pathways downstream of PDGFRβ. Taken together, these data indicate that PDGFRβ+ pericytes may represent a novel, nonendothelial, antiangiogenic target for lymphoma therapy.
Introduction
Despite the fact that tumor cell–directed, multimodality treatment with chemotherapy, radiation, and biologic agents can induce remission in many subtypes of non-Hodgkin’s lymphoma (NHL), a significant proportion of patients continue to succumb to incurable disease.1-6 Recent studies have shown that stromal and vascular cell genetic signatures within the tumor microenvironment can predict disease behavior and clinical outcome in NHL subtypes.7,8 These findings highlight the importance of tumor stromal cells in the pathogenesis and potential therapy of lymphoma.
The tumor microenvironment supports the initiation and progression of cancerous growth, in part by building and sustaining the tumor’s vascular network.9-11 Emerging data on the proangiogenic properties of lymphoma cells and the mechanisms of vascular assembly suggest that angiogenesis is highly relevant to the biology and therapy of NHL.12 Drawing parallels from the extensive literature on solid malignancies, antiangiogenic lymphoma therapy has focused largely on vascular endothelial growth factor (VEGF), which can drive proliferation of both tumor and endothelial cells.12,13 However, small phase II clinical studies with the anti-VEGF monoclonal antibody bevacizumab have thus far shown equivocal efficacy in aggressive NHL,14,15 suggesting that non-VEGF angiogenic pathways may be highly relevant.
Platelet-derived growth factor-type BB (PDGF-BB) directs the recruitment of PDGF receptor (PDGFR)-expressing pericytes and their progenitors to neovessels, thereby promoting vascular maturation and stabilization.16,17 Genetic ablation of either PDGF-BB or PDGFRβ in developing mouse embryos leads to lethal microvascular leakage and hemorrhage.18-20 PDGF may also modulate the expression of other stromal angiogenic factors, such as basic fibroblast growth factor and VEGF.21,22 Pharmacologic intervention with receptor tyrosine kinase inhibitors that target PDGFRβ, such as imatinib mesylate or sunitinib malate, have shown efficacy in solid tumor models,22-27 partly by reducing pericyte density and attenuating angiogenesis. To date, however, specific targeting of PDGFRβ has not been extensively evaluated in lymphoid malignancies.
We previously characterized vascular assembly in human NHL subtypes28 and hypothesized that blood vessel stability depends on pericyte integrity. Here, we postulate that agents that selectively target pericytes will selectively disrupt tumor vascular integrity and attenuate lymphoma growth. To test this hypothesis, we treated both human diffuse large B-cell lymphoma in SCID mice and murine EL4 lymphoma in wild-type mice with either a pharmacologic PDGFRβ inhibitor, imatinib mesylate, or a PDGFRβ-specific monoclonal antibody. Our data indicate that both agents compromise tumor vascular integrity, mainly by targeting vascular mural cells, thereby attenuating lymphoma growth.
Materials and methods
Cell lines and reagents
All culture media and reagents, with the exception of fetal bovine serum (FBS; Hyclone, Logan, UT) and pericyte culture medium (ScienCell, Carlsbad, CA), were purchased from Mediatech Inc. (Manassas, VA). The human diffuse large B-cell lymphoma (DLBCL) cell line OCI-Ly7 was grown in 90% Iscove’s modified Dulbecco's medium and 10% FBS with penicillin/streptomycin (P/S), whereas DLBCL cell lines Karpas422 and Farage were grown in 90% RPMI 1640 and 10% FBS with P/S, l-glutamine, and N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid. The murine lymphoma cell line EL4 was grown in Dulbecco’s modified Eagle medium (DMEM) containing 10% horse serum with P/S. The rat vascular smooth muscle cell (VSMC) line A10 (ATCC) was grown in DMEM containing 10% FBS with P/S. The primary murine VSMCs (see supplemental Materials and Methods on the Blood website) were grown in DMEM containing 10% FBS with P/S, whereas the primary human brain pericytes were purchased from ScienCell and grown in its proprietary pericyte culture medium. All cell cultures were maintained at 37°C in a humidified incubator containing 5% CO2.
Cell growth inhibition assays
PDGFRβ+ VSMCs and DLBCL cell lines were grown at concentrations sufficient to keep untreated cells in exponential growth over the drug exposure period. Cell viability was measured using a fluorometric resazurin reduction method (CellTiter-Blue; Promega, Madison, WI) and trypan blue dye exclusion. Unless stated otherwise, the experiments were carried out in triplicate. The CompuSyn software package (Biosoft, Cambridge, UK) was used to plot dose-effect curves and determine the drug concentration that inhibits the growth of cells by 50% compared with control (GI50).
Mouse lymphoma models
All animal procedures were approved by the Institutional Animal Care and Use Committee of Weill Cornell Medical College. For human lymphoma xenograft experiments, 6- to 8-week-old SCID (National Cancer Institute, Bethesda, MD) mice were injected subcutaneously with low-passage 1 × 107 human Farage, OCI-Ly7, or Karpas422 cells. Tumor size was measured with electronic digital calipers (Fisher Scientific, Waltham, MA), and tumor volume (mm3) was calculated as length × width2 × 0.52. When tumor volumes reached 75 to 100 mm3, the mice were randomized to receive intraperitoneal (IP) injections of the following compounds for 2 to 3 weeks: imatinib (LC Laboratories, Woburn, MA) dissolved in phosphate-buffered saline (PBS) at 45 mg/kg twice daily, humanized anti-PDGFRβ antibody 2C5 (provided under a material transfer agreement with ImClone Systems, New York, NY) at 40 mg/kg 3 times weekly, PBS control, or human IgG control (Jackson ImmunoResarch Laboratory, West Grove, PA). For murine lymphoma allograft experiments, 6- to 8-week-old C57BL/6 mice (Jackson Laboratory, Bar Harbor, ME) were injected subcutaneously with low-passage 1.5 × 106 EL4 cells. When tumors reached 75 to 100 mm3, the mice were randomized to receive either imatinib or PBS by IP injections for 2 weeks. In some animals, tumor microvasculature was visualized by staining endothelial cells with Alexa fluor-647–conjugated isolectin GS-IB4 (Invitrogen) injected via lateral tail vein 20 minutes before death. All mice were killed by CO2 inhalation at the time of study completion.
Immunohistochemistry
For frozen specimens, 8- to 10-μm cryosections were fixed in ice-cold acetone/methanol (1:1 v:v) and then subjected to standard color- or fluorescence-based staining procedures as described.28 For confocal microscopy, 50-, 75-, or 10-μm cryosections were incubated with rat anti-mouse CD31 mAb (clone MEC13.3; BD Pharmingen, San Diego, CA), PDGFRβ mAb (clone APB5; eBioscience, San Diego, CA), phospho-PDGFR-β (sc-12908; Santa Cruz Biotechnology, Santa Cruz, CA), NG2 (Chemicon, Temecula, CA), CD68 (AbD Serotec, Raleigh, NC), Cy3-conjugated anti–α-SMA mAb (clone 1A4; Sigma-Aldrich, St. Louis, MO), or rabbit anti-cleaved caspase 3 mAb (clone 5A1E; Cell Signaling Technology, Danvers, MA) in various combinations overnight at 4°C, and then developed with Alexa Fluor 488–conjugated goat anti-rabbit or Alexa Fluor 555–conjugated goat anti-rat (Invitrogen). Apoptosis was detected on frozen tissue sections by terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL) assay (Trevigen, Gaithersburg, MD).
Image analysis
Stained sections were scanned and analyzed under a Zeiss Eclipse 80i microscope using a Qimaging Retiga EX camera. A Zeiss LSM510 laser scanning confocal microscope was used to assess coexpression of cellular markers in the same cell. Immunofluorescence signals were captured by an argon laser emitting at 488 nm and a helium-neon laser emitting at 546 nm, and collected using proprietary Zeiss software. To quantify staining markers, an average of 5 to 10 representative fields were photographed at 100× and 200× magnification, and ≥2 sections at different depths were analyzed for each tumor sample. The CD31+, NG2+, and α-smooth muscle actin (SMA)+ area densities, as well as the TUNEL+ apoptosis index, were defined as the percent of pixels covered by each individual staining marker and were scored using National Institutes of Health ImageJ as previously reported.28
FACS analysis
Mononuclear cells were prepared from blood, bone marrow, and tumor tissues. Tumor tissues were minced, digested with an enzyme cocktail containing 0.5% collagenase I (Worthington, 37°C, 1 hour), and filtered through a 30-μm strainer to isolate single-cell suspensions. For each flow reaction, 0.2 to 1.0 × 106 mononuclear cells were pretreated with Fc block (CD16/CD32; BD Pharmingen, San Diego, CA) and then labeled with the following antibodies: anti-human CD45-FITC, anti-mouse CD45-PerCP, anti-mouse VEGFR2-PE, anti-mouse CD31-APC, anti-mouse CD117-APC (all from BD Pharmingen), and anti-mouse CD140b-PE (eBioscience, San Diego, CA). Controls included unstained samples, samples stained with isotype-specific antibodies, and BD CompBeads. Analyses were performed in a Cytomics FC500 flow cytometer (Beckman Coulter, Brea, CA), and data were analyzed using FlowJo software (Treestar, Ashland, OR).
Immunoblotting
After a period of serum deprivation, PDGFRβ+ primary murine VSMCs, primary human brain pericytes, or A10 cells were pretreated with imatinib (LC Laboratories) for 90 minutes and then stimulated with 50 ng/mL of recombinant PDGF-BB (Peprotech, Rocky Hill, NJ and R&D Systems, Minneapolis, MN) for 10 minutes. Cell lysates were then subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotting with antibodies against PDGFRβ (Cell Applications, San Diego, CA), phospho-PDGFRβ (Santa Cruz Biotechnology), PDGFRα (Santa Cruz Biotechnology), phospho-PDGFRα (Abcam, Cambridge, MA), c-Kit (Santa Cruz Biotechnology), phospho-c-Kit (Abcam, Cambridge, MA), c-Abl (Santa Cruz Biotechnology), phospho-c-Abl (Cell Signaling Technology, Boston, MA), extracellular signal-regulated kinase (ERK)1/2, phospho-ERK1/2, v-akt murine thymoma viral oncogene homolog protein (AKT), and phospho-AKT (all from Cell Signaling Technology). Immunoblots were developed using the ECL kit (Perkin Elmer, Boston, MA).
PDGFRβ small interfering RNA knockdown
Primary human brain vascular pericytes (HBVPs) and human DLBCL cell lines (Karpas422 and Farage) were transfected with either a small interfering RNA (siRNA) targeting PDGFRβ (s10241; Invitrogen Life Technologies, Grand Island, NY) or a nontargeting siRNA (control) using Lipofectamine (Promega) or the Amaxa Nucleofector kit (Lonza, Basel, Switzerland), respectively. Transfection efficiency, assessed by cotransfection of a GFP-containing plasmid, was 80% to 95% for HBVPs and 55% to 65% for DLBCL cells. Viable cells were enumerated by hemocytometer at 24, 48, and 72 hours after transfection using trypan blue dye exclusion. PDGFRβ mRNA levels were determined using the ΔΔCT quantitative reverse transcription-polymerase chain reaction method using forward (GTTGGGCGAAGGTTACAAAA) and reverse (GGGTGGTCACTCCTCAGAAA) primers and RPL13A as an internal control and normalized to baseline expression. All experiments were repeated at least once with triplicate samples.
Biostatistical analysis
All data were considered continuous and were summarized using means and standard deviations. Differences between and among experimental groups were assessed using t test and analysis of variance, respectively. Nonparametric methods such as the Wilcoxon rank sum test and Kruskal–Wallis test were used when the underlying assumptions for t test and analysis of variance were violated. All P values are 2-sided, with significance evaluated at the .05 α level.
Results
Imatinib impaired growth of experimental lymphomas
Using an established xenograft model,29 human Farage, OCI-Ly7, and Karpas422 DLBCL cell lines were implanted in SCID mice, which were then treated twice daily with IP injections of either imatinib mesylate at 45 mg/kg or vehicle PBS for 2 to 3 weeks. Imatinib doses of up to 100 mg/kg twice daily (orally or IP) in mice result in mean peak and trough plasma levels of 4.6 to 6 and 1 to 1.5 μM, respectively, which are similar to those reported for humans receiving a midrange dose of 400 mg once daily.30-32
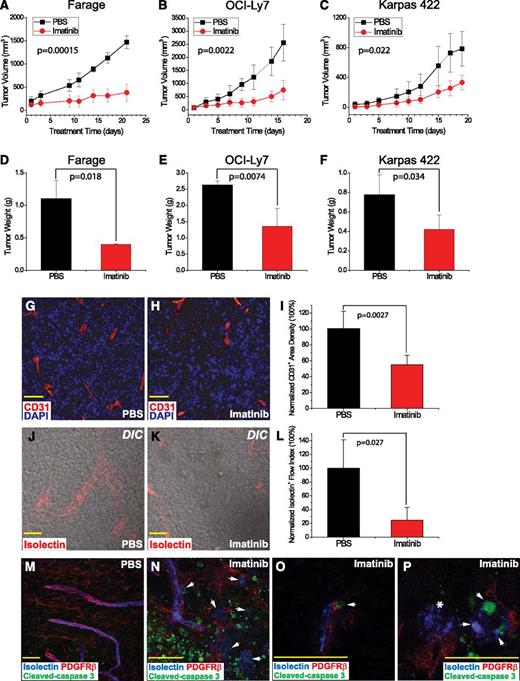
Compared with PBS, treatment with imatinib significantly impaired tumor growth, based on tumor volume (P = .00015, 0.0022, and 0.022, respectively), and tumor weight (P = .018, 0.0074, and 0.034, respectively) for Farage, OCI-Ly7, and Karpas422 xenografts at the time of harvest (Figure 1A-F). Histological analysis revealed an increased fraction of TUNEL-positive, apoptotic cells in the imatinib-treated lymphomas, in addition to central necrosis, which was present in all xenografts regardless of treatment (supplemental Figure 1).
Imatinib impaired growth of human lymphoma xenografts by disruption of tumor-associated microvasculature. (A-C) Tumor growth curves based on tumor volumes (mm3), comparing imatinib mesylate treatment (red) vs PBS control (black). (D-F) Tumor weight (g) at the time of tissue harvest, comparing imatinib (red) vs PBS (black). (G-I) Microvessels delineated by CD31 stain (red) in (G) PBS- and (H) imatinib-treated tumors, and (I) microvessel density normalized to PBS control. (J-L) Confocal DIC capture of functional vascular flow measured by isolectin (red) and normalized to PBS control. (M-P) Confocal analysis of pericyte marker staining in tumors. Blue marks functional vascular flow measured by isolectin, red outlines PDGFRβ+ pericytes, and green shows cleaved caspase 3 in apoptotic cells. (M) PBS-treated tumor. (N) Imatinib-treated tumor, which displays microvascular disintegration and flow leakage (white arrows), and patchy areas of tumor apoptosis/necrosis. (O) White arrows indicate apoptotic, yet structurally intact, PDGFRβ+ cells in close proximity to functional flow. (P) White arrows mark apoptosis of isolectin+ endothelial cells, and white asterisk (*) indicates leakage of infused intravascular isolectin. Scale bar, 50 μm.
Imatinib impaired growth of human lymphoma xenografts by disruption of tumor-associated microvasculature. (A-C) Tumor growth curves based on tumor volumes (mm3), comparing imatinib mesylate treatment (red) vs PBS control (black). (D-F) Tumor weight (g) at the time of tissue harvest, comparing imatinib (red) vs PBS (black). (G-I) Microvessels delineated by CD31 stain (red) in (G) PBS- and (H) imatinib-treated tumors, and (I) microvessel density normalized to PBS control. (J-L) Confocal DIC capture of functional vascular flow measured by isolectin (red) and normalized to PBS control. (M-P) Confocal analysis of pericyte marker staining in tumors. Blue marks functional vascular flow measured by isolectin, red outlines PDGFRβ+ pericytes, and green shows cleaved caspase 3 in apoptotic cells. (M) PBS-treated tumor. (N) Imatinib-treated tumor, which displays microvascular disintegration and flow leakage (white arrows), and patchy areas of tumor apoptosis/necrosis. (O) White arrows indicate apoptotic, yet structurally intact, PDGFRβ+ cells in close proximity to functional flow. (P) White arrows mark apoptosis of isolectin+ endothelial cells, and white asterisk (*) indicates leakage of infused intravascular isolectin. Scale bar, 50 μm.
Imatinib disrupted tumor-associated microvasculature
To determine whether apoptosis was associated with impaired angiogenesis, Farage tumors were stained with both anti–cleaved caspase 3, a marker for apoptosis, and anti-CD31 (PECAM), an endothelial marker. In addition, isolectin GS-IB4 was infused systemically to assess tumor vessel patency (Figure 1G-L). Compared with PBS treatment, administration of imatinib was strongly associated with loss of microvascular density and patency (Figure 1J-L). Furthermore, in imatinib-treated samples, differential interference contrast confocal microscopy revealed patchy areas of isolectin leakage associated with fragmented structures suggestive of disrupted microvessels (Figure 1K).
Anti-PDGFRβ staining of Farage tumors showed that PDGFRβ+ pericytes formed tubular structures surrounding blood vessels in PBS-treated tumors but were less organized and distinct in imatinib-treated tumors and nearly absent within areas of tumor cell death (Figure 1M-N). Confocal analysis revealed the presence of anti–cleaved caspase 3 staining within remaining PDGFRβ+ pericytes (Figure 1O), suggesting that the dropout of pericytes in imatinib-treated samples was associated with the activation of an apoptotic pathway. Endothelial apoptosis and disintegration was evident in areas of disrupted pericyte network (Figure 1P). These data suggested that imatinib treatment led to microvascular disruption and apoptotic cell death of PDGFRβ+ pericytes.
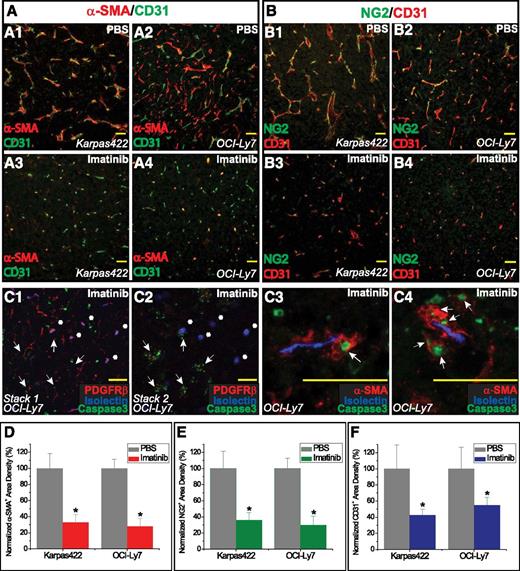
To determine whether pericyte loss was a general consequence of imatinib treatment of experimental lymphomas, we examined Karpas422 and OCI-Ly7 tumors. Anti–α-SMA and NG2 staining revealed shorter, narrower, and more flow-impaired microvessels surrounded by apoptotic pericytes (Figure 2A-C). Loss of pericytes was associated with an overall reduction in tumor vascularity (Figure 2D-F). In contrast, the vasculature of organs not involved with tumor, including lung, heart, liver, and kidney, in imatinib-treated mice showed normal histology and no increase in apoptosis (not shown).
Imatinib treatment of human DLBCL tumors was associated with pericytic dropout and disruption of microvasculature. (A1-A4) Analysis of α-SMA+ pericytes and CD31+ vessels in (A1,A3) Karpas422 and (A2,A4) OCI-Ly7 xenografts. (B1-B4) Analysis of NG2+ pericytes and CD31+ vessels in (B1,B3) Karpas422 and (B2,B4) OCI-Ly7 xenografts. (C) Confocal analysis of pericytes (red) in relation to intraluminal blood flow (isolectin, blue) in OCI-Ly7 tumors. (C1-C2) Two z-stack images focused at different depths within the same tissue; white arrows indicate disrupted microvessels with scanty PDGFRβ+ staining and abundant cleaved-caspase 3+, apoptotic nuclei; white asterisks (*) indicate microvessels with relatively intact blood flow and perivascular PDGFRβ+ staining. (C3-C4) White arrows indicate apoptotic α-SMA+ pericytes surrounding regions of functional blood flow. (D) Pericyte coverage was quantified as α-SMA+ area (red) and normalized to the PBS control. (E) Pericyte coverage was quantified as NG2+ area (green) and normalized to the PBS control. (F) Microvessel density was quantified as CD31+ staining area (blue) and normalized to the PBS control. *P < .05 compared with control. Scale bar, 50 μm.
Imatinib treatment of human DLBCL tumors was associated with pericytic dropout and disruption of microvasculature. (A1-A4) Analysis of α-SMA+ pericytes and CD31+ vessels in (A1,A3) Karpas422 and (A2,A4) OCI-Ly7 xenografts. (B1-B4) Analysis of NG2+ pericytes and CD31+ vessels in (B1,B3) Karpas422 and (B2,B4) OCI-Ly7 xenografts. (C) Confocal analysis of pericytes (red) in relation to intraluminal blood flow (isolectin, blue) in OCI-Ly7 tumors. (C1-C2) Two z-stack images focused at different depths within the same tissue; white arrows indicate disrupted microvessels with scanty PDGFRβ+ staining and abundant cleaved-caspase 3+, apoptotic nuclei; white asterisks (*) indicate microvessels with relatively intact blood flow and perivascular PDGFRβ+ staining. (C3-C4) White arrows indicate apoptotic α-SMA+ pericytes surrounding regions of functional blood flow. (D) Pericyte coverage was quantified as α-SMA+ area (red) and normalized to the PBS control. (E) Pericyte coverage was quantified as NG2+ area (green) and normalized to the PBS control. (F) Microvessel density was quantified as CD31+ staining area (blue) and normalized to the PBS control. *P < .05 compared with control. Scale bar, 50 μm.
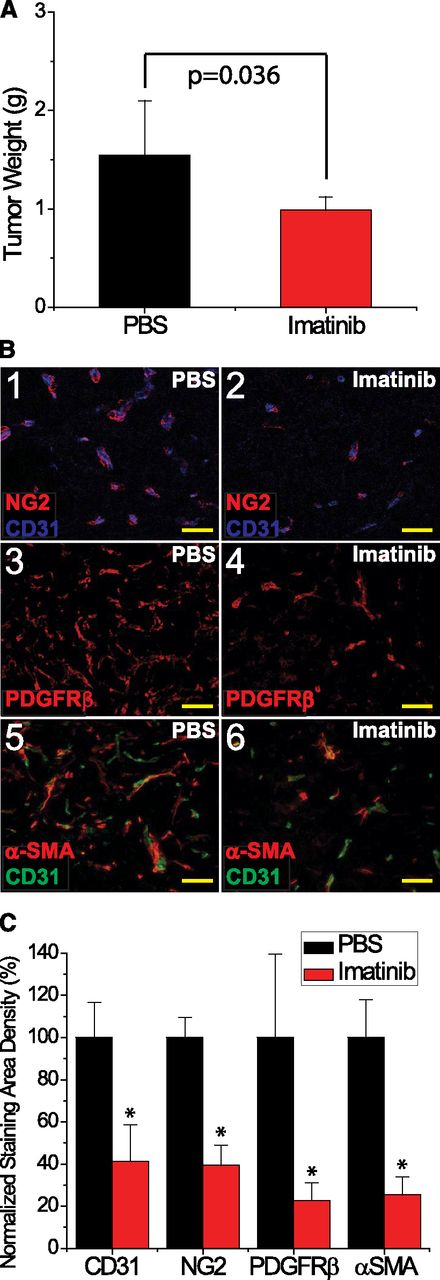
To determine whether immune deficits in the SCID mouse contributed to tumor susceptibility to imatinib, we studied a second model of lymphomagenesis. In the immunologically intact EL4 lymphoma-bearing C57Bl/6 mouse, imatinib treatment was associated with both impaired tumor growth, based on assessment of mass (Figure 3A; P = .036), and decreased pericyte coverage with reduced vascularization, based on immunohistologic staining using 3 separate mural cell markers and an endothelial cell marker CD31 (Figure 3B-C; P = .0007 for NG2, P = .0052 for PDGFRβ, P < .0001 for α-SMA, and P < .0001 for CD31). Thus, the effect of imatinib on tumor vascularity is not a reflection of immunologic deficiency.
Imatinib treatment of murine EL4 tumors impaired growth, decreased pericyte coverage, and reduced vascularization. (A) Tumor weight (g) at the time of tissue harvest, comparing imatinib (red) vs PBS (black). (B) Analysis of pericytes with (1-2) NG2, (3-4) PDGFRβ, and (5-6) α-SMA staining, and CD31+ vessels in EL4 tumors, in response to either imatinib or PBS. (C) Microvessel density was quantified as CD31+ staining density, and normalized to the PBS control. Pericyte coverage was quantified as NG2+, PDGFRβ+, or α-SMA+ area and normalized to the PBS control. *P < .05. Scale bar, 50 μm.
Imatinib treatment of murine EL4 tumors impaired growth, decreased pericyte coverage, and reduced vascularization. (A) Tumor weight (g) at the time of tissue harvest, comparing imatinib (red) vs PBS (black). (B) Analysis of pericytes with (1-2) NG2, (3-4) PDGFRβ, and (5-6) α-SMA staining, and CD31+ vessels in EL4 tumors, in response to either imatinib or PBS. (C) Microvessel density was quantified as CD31+ staining density, and normalized to the PBS control. Pericyte coverage was quantified as NG2+, PDGFRβ+, or α-SMA+ area and normalized to the PBS control. *P < .05. Scale bar, 50 μm.
Imatinib reduced lymphoma-associated vascular cells
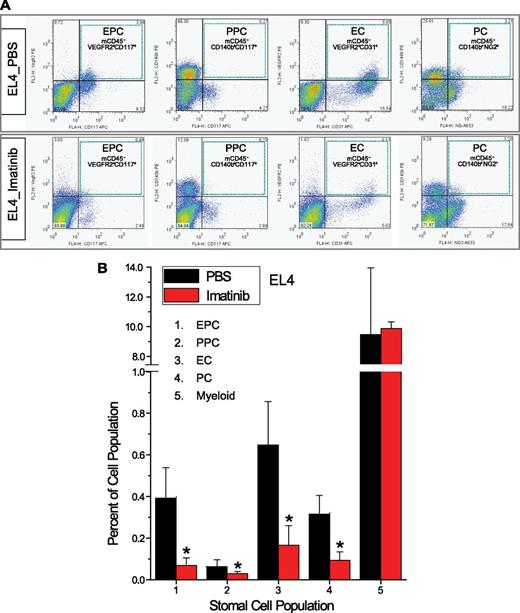
To quantify more precisely the impact of imatinib on tumor vasculature, fluorescence activated cell sorting (FACS) was carried out using single cell suspensions isolated from experimental lymphomas. In untreated human and murine experimental lymphomas, nontumor stromal cells represented up to 15% of the total cell population. In EL4 tumors propagated in wild-type mice, pericytes, defined as CD45−CD11b−CD31−PDGFRβ+, comprised 2.61 ± 0.25% of the total, whereas endothelial cells, defined as CD45−CD11b−CD31+, represented 0.42 ± 0.07%, and CD45+CD11b+ inflammatory cells represented 8.66 ± 2.05% (supplemental Figure 2). Of note, <5% of total endothelial cells and <1% of total inflammatory cells expressed PDGFRβ.
On imatinib treatment, populations of mature VEGFR2+CD31+ endothelial cells,33 mature PDGFRβ+ vascular stromal cells,25 and c-Kit+ endothelial and pericyte cell progenitors25,34,35 all declined in both murine (Figure 4A-B) and human lymphomas (supplemental Figure 3), whereas myeloid cells in both tumor types remained stable. In blood and bone marrow, on the other hand, these cell populations were not affected (not shown). Thus, recruitment, retention, and maturation of tumor endothelial and pericyte progenitors may require a microenvironment conditioned by intact PDGFRβ+ stromal cells.
FACS analysis of lymphoma vascular cell populations. (A) Quad-gate analysis of the EL4 tumor-associated cells. Murine EPCs (mCD45−VEGFR2+CD117+), mature ECs (mCD45−VEGFR2+CD31+), murine PPCs (mCD45−CD140b+CD117+), and mature PCs (mCD45−CD140b+NG2+) are shown. Blue-dotted squares outline individual quad-gates for EPCs, PPCs, ECs, and PCs. (B) Quantification of stromal cell populations in EL4 tumors treated with imatinib vs PBS. *P < .05 compared with control.
FACS analysis of lymphoma vascular cell populations. (A) Quad-gate analysis of the EL4 tumor-associated cells. Murine EPCs (mCD45−VEGFR2+CD117+), mature ECs (mCD45−VEGFR2+CD31+), murine PPCs (mCD45−CD140b+CD117+), and mature PCs (mCD45−CD140b+NG2+) are shown. Blue-dotted squares outline individual quad-gates for EPCs, PPCs, ECs, and PCs. (B) Quantification of stromal cell populations in EL4 tumors treated with imatinib vs PBS. *P < .05 compared with control.
Imatinib targeted tumor vasculature by inhibition of PDGFRβ signaling in mural cells
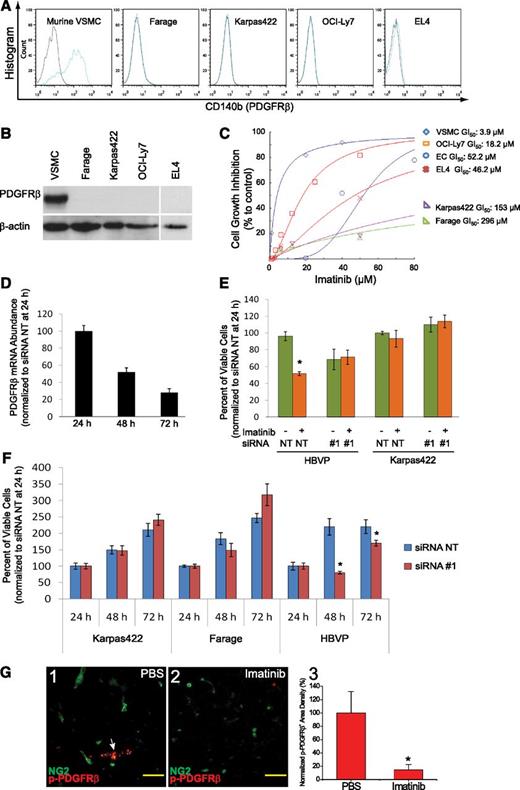
Primary murine VSMCs, unlike human DLBCL or murine EL4 cells, expressed PDGFRβ in a uniformly robust fashion in both FACS and immunoblot assays (Figure 5A-B), and were highly sensitive to imatinib (Figure 5C; GI50 of 3.9 μM). In contrast, primary microvascular endothelial cells, <1% of which expressed PDGFRβ, were resistant to imatinib (Figure 5C; GI50 of 52 μM). Similarly, Farage, Karpas422, OCI-Ly7, and EL4 lymphoma cell lines all failed to express PDGFRβ (Figure 5A-B) and displayed GI50 values (18-296 μM) that far exceeded therapeutic plasma levels of imatinib reported in mice (Figure 5C). The variable responses to imatinib in lymphoma cell lines may reflect the effect of imatinib on cellular survival pathways unrelated to PDGFRβ.
Imatinib inhibited PDGFRβ signaling in vascular mural cells. (A) FACS analysis of PDGFRβ expression in VSMC and lymphoma cells. (B) Immunoblot analysis of PDGFRβ protein expression in stromal and lymphoma cells. (C) Imatinib-mediated growth inhibition in VSMC, endothelial cells, and lymphoma cells. (D) Relative abundance of PDGFRβ mRNA at 24, 48, and 72 hours following transfection of siRNA #1 targeting PDGFRβ in HBVPs, and expressed as the percentage normalized to expression level at 24 hours after transfection. (E) Viable cell counts at 48 hours after treatment with imatinib (20 μM for HBVP, 50 μM for Karpas422), following transfection with siRNA for 24 hours in HBVPs and Karpas422 cells. All cell counts were normalized to values at 24 hours after siRNA transfection. (F) Viable cell counts at 24, 48, and 72 hours after transfection of siRNA nontargeting (NT) or PDGFRβ-directed siRNA #1 in HBVPs, Karpas422, and Farage DLBCL cell lines, and normalized to the values at 24 hours after transfection. Data are representative of triplicate experiments in C-F. (G) Immunostaining for phospho-PDGFRβ in NG2+ pericytes in Karpas422 tumors treated with either (1) PBS or (2) imatinib. White arrow indicates expression of phospho-PDGFRβ in NG2+ pericytes. (3) PDGFRβ signaling was quantified as phospho-PDGFRβ+ area and normalized to PBS control. *P < .05 compared with control. Scale bar, 50 μm.
Imatinib inhibited PDGFRβ signaling in vascular mural cells. (A) FACS analysis of PDGFRβ expression in VSMC and lymphoma cells. (B) Immunoblot analysis of PDGFRβ protein expression in stromal and lymphoma cells. (C) Imatinib-mediated growth inhibition in VSMC, endothelial cells, and lymphoma cells. (D) Relative abundance of PDGFRβ mRNA at 24, 48, and 72 hours following transfection of siRNA #1 targeting PDGFRβ in HBVPs, and expressed as the percentage normalized to expression level at 24 hours after transfection. (E) Viable cell counts at 48 hours after treatment with imatinib (20 μM for HBVP, 50 μM for Karpas422), following transfection with siRNA for 24 hours in HBVPs and Karpas422 cells. All cell counts were normalized to values at 24 hours after siRNA transfection. (F) Viable cell counts at 24, 48, and 72 hours after transfection of siRNA nontargeting (NT) or PDGFRβ-directed siRNA #1 in HBVPs, Karpas422, and Farage DLBCL cell lines, and normalized to the values at 24 hours after transfection. Data are representative of triplicate experiments in C-F. (G) Immunostaining for phospho-PDGFRβ in NG2+ pericytes in Karpas422 tumors treated with either (1) PBS or (2) imatinib. White arrow indicates expression of phospho-PDGFRβ in NG2+ pericytes. (3) PDGFRβ signaling was quantified as phospho-PDGFRβ+ area and normalized to PBS control. *P < .05 compared with control. Scale bar, 50 μm.
Next, we explored whether the sensitivity of vascular mural cells to imatinib was PDGFRβ dependent. First, we noted that PDGFRβ+ VSMCs adopted a narrow or rounded appearance and became cleaved caspase 3 positive following imatinib treatment (supplemental Figure 4). Second, PDGFRβ-specific siRNA reduced expression of PDGFRβ mRNA in HBVPs by 72% within 72 hours and rendered them resistant to imatinib-mediated cell death (Figure 5D-E). Karpas422 lymphoma cells, on the other hand, remained insensitive to imatinib after transfection with PDGFRβ siRNA (Figure 5E). Third, although knockdown of PDGFRβ had no effect on the growth of Karpas422 and Farage human lymphoma cell lines, it specifically impaired growth of cultured human brain vascular pericytes (Figure 5F). Finally, we noted that NG2-positive pericyte-like cells expressed anti–phospho-PDGFRβ reactive material in PBS-treated tumors but not in imatinib-treated tumors (Figure 5G). These data suggest that the therapeutic effect of imatinib in experimental lymphoma derives mainly from its effect on pericytes rather than endothelial cells or lymphoma cells themselves.
Imatinib regulated multiple signaling pathways downstream of PDGF-BB in vascular mural cells
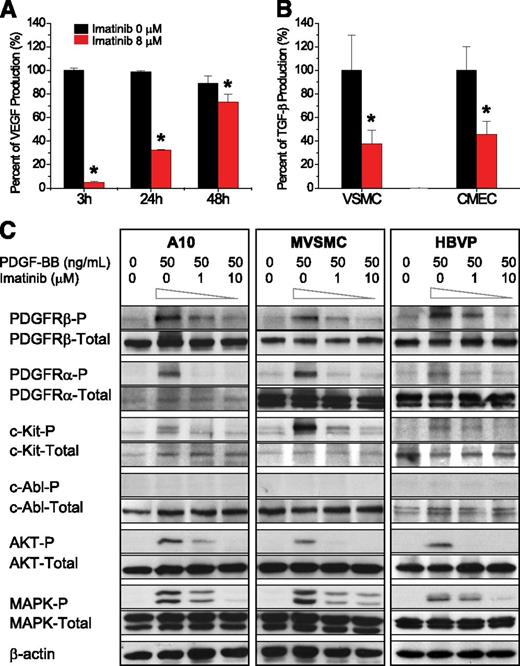
We used several approaches to examine the mechanism by which imatinib impaired PDGFRβ signaling in vascular mural cells. In imatinib-treated murine VSMCs, transcriptional profiling identified 36 and 276 genes displaying higher and lower expression, respectively (supplemental Table 2). On ingenuity pathway analysis, the regulated genes clustered into 11 networks, predominant among which were several related to PDGFRβ signaling and activation of downstream mediators, including phosphatidylinositol 3-kinase/AKT and mitogen-activated protein kinase (MAPK)/ERK1/2 (supplemental Table 3).36 In addition, we noted dramatic down-regulation of VEGF and TGF-β pathways essential for endothelial cell proliferation, survival, and vascular integrity (supplemental Figure 5A-B).37 Validity of these findings was confirmed for selected genes by real-time polymerase chain reaction conducted on cultured primary murine VSMCs harvested 24 and 48 hours after imatinib treatment and on FACS-sorted, CD45−PDGFRβ+ lymphoma-associated stromal cells 72 hours after imatinib treatment (supplemental Figure 5C, panels 1 and 2, respectively). Reduction in smooth muscle cell production of VEGF protein and smooth muscle and endothelial cell production of TGFβ were confirmed by enzyme-linked immunosorbent assay (Figure 6A-B). Together, these data indicate that imatinib induced decreases in expression of both PDGF pathway and non-PDGF pathway genes that govern vascular integrity.
Imatinib blocked angiogenic pathways in vascular mural cells. (A) Effect of imatinib on VEGF secretion from VSMCs at 3, 24, and 48 hours after treatment. (B) Effect of imatinib on TGF-β secretion from VSMCs and cardiac microvascular endothelial cells (CMECs) at 48 hours after treatment. (C) Effect of imatinib on PDGF-BB–induced phosphorylation of PDGFRα, PDGFRβ, c-Kit, c-Abl, AKT, and ERK1/2 by immunoblot of VSMCs and pericytes in culture.
Imatinib blocked angiogenic pathways in vascular mural cells. (A) Effect of imatinib on VEGF secretion from VSMCs at 3, 24, and 48 hours after treatment. (B) Effect of imatinib on TGF-β secretion from VSMCs and cardiac microvascular endothelial cells (CMECs) at 48 hours after treatment. (C) Effect of imatinib on PDGF-BB–induced phosphorylation of PDGFRα, PDGFRβ, c-Kit, c-Abl, AKT, and ERK1/2 by immunoblot of VSMCs and pericytes in culture.
To define the PDGFRβ-related signaling pathways impacted by imatinib in vascular mural cells, we conducted a series of phospho-immunoblot analyses (Figure 6C). At baseline, rat embryonic VSMCs (A10),38 primary murine VSMCs, and primary HBVPs all expressed PDGFRβ, PDGFRα, c-Kit, c-Abl, AKT, and MAPK. In a dose-dependent fashion, imatinib decreased PDGF-BB–mediated phosphorylation of cell surface receptors PDGFRβ, PDGFRα, and c-Kit, as well as intracellular signaling mediators, AKT and MAPK, whereas total expression of these proteins remained unchanged. Phosphorylation of c-Abl, on the other hand, was not detected in response to PDGF-BB, and therefore, was not impacted by imatinib in any of the mural cell types tested. These data reveal that multiple downstream signaling pathways, active in PDGFRβ+ mural cells, could be impacted by imatinib.
Antibody blockade of PDGFRβ disrupted lymphoma vascular integrity in vivo
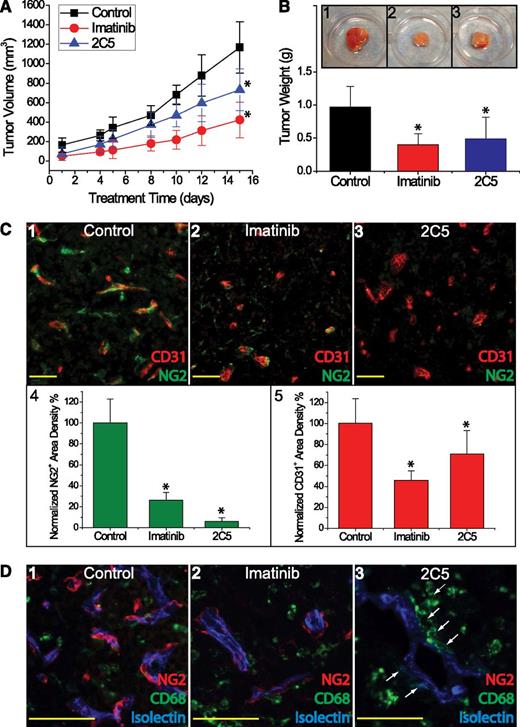
Finally, we asked whether selective blockade of PDGFRβ signaling could attenuate lymphoma growth in vivo. SCID mice bearing Farage and Karpas422 xenografts were treated with the monoclonal antibody 2C5, which blocks both human and murine PDGFRβ signaling. Compared with controls treated with either PBS or human IgG, 2C5 significantly inhibited Farage tumor growth, as assessed by tumor volume (P = .0045) and weight (P = .01) (Figure 7A-B). Immunohistochemical analysis revealed that 2C5 depleted pericytes to 6% of the control level, whereas imatinib reduced pericytes to 26% of control (Figure 7C). Microvascular density, as assessed by CD31 staining, decreased with 2C5 treatment compared with control (P = .04), although the degree of vascular attenuation with 2C5 treatment was less pronounced compared with imatinib (P = .065). Interestingly, although 2C5 did not significantly inhibit the growth of Karpas422 lymphoma, it did induce a significant reduction of pericyte and vascularization (P = .005), which mirrored the effects seen in Farage tumors (not shown).
Anti-PDGFRβ antibody 2C5 inhibited growth of Farage xenografts in SCID mice. (A) Tumor growth curves based on tumor volumes (mm3) for PBS or hIgG control (black squares), imatinib (red circles), and 2C5 (blue triangles) treatments. (B) Tumor weight (g) at the time of tissue harvest comparing 2C5 (blue) vs imatinib (red) vs control (black). Inset depicts gross morphology of the resected tumor xenografts treated with (1) control, (2) imatinib, and (3) 2C5. (C) Staining of endothelial cells (CD31, red) and pericytes (NG2, green) in Farage xenografts treated with (1) control, (2) imatinib, and (3) 2C5. (4) NG2+ pericytic density and (5) CD31+ endothelial density were normalized to their respective controls. (D) Confocal analysis of pericytes in Farage tumors treated with (1) control, (2) imatinib, and (3) 2C5. Blue marks functional vascular flow by isolectin staining; red outlines NG2+ pericytes; and green stains CD68+ myelomonocytic cells. White arrows indicate perivascular CD68+ cells in close association with microvessels. *P < .05 compared with control. Scale bar, 50 μm.
Anti-PDGFRβ antibody 2C5 inhibited growth of Farage xenografts in SCID mice. (A) Tumor growth curves based on tumor volumes (mm3) for PBS or hIgG control (black squares), imatinib (red circles), and 2C5 (blue triangles) treatments. (B) Tumor weight (g) at the time of tissue harvest comparing 2C5 (blue) vs imatinib (red) vs control (black). Inset depicts gross morphology of the resected tumor xenografts treated with (1) control, (2) imatinib, and (3) 2C5. (C) Staining of endothelial cells (CD31, red) and pericytes (NG2, green) in Farage xenografts treated with (1) control, (2) imatinib, and (3) 2C5. (4) NG2+ pericytic density and (5) CD31+ endothelial density were normalized to their respective controls. (D) Confocal analysis of pericytes in Farage tumors treated with (1) control, (2) imatinib, and (3) 2C5. Blue marks functional vascular flow by isolectin staining; red outlines NG2+ pericytes; and green stains CD68+ myelomonocytic cells. White arrows indicate perivascular CD68+ cells in close association with microvessels. *P < .05 compared with control. Scale bar, 50 μm.
Because hematopoietic cells have been reported to contribute to tumor angiogenesis, we next identified perivascular CD68+ hematopoietic myeloid cells, which intermingled with NG2+ pericytes, in Farage (Figure 7D) and Karpas 422 tumors (not shown) by immunostaining. Following imatinib treatment, CD68+ cells became diffusely scattered as vascular organization was disrupted (Figure 7D2). In contrast, tumors treated with 2C5 retained abundant perivascular CD68+ cells (Figure 7D3). These data suggest that both pericytes and myeloid cells may contribute to vascular stability, and that, when PDGFRβ+ pericytes are selectively depleted, CD68+ hematopoietic cells might serve as surrogate mural cells.
Discussion
Our study demonstrates for the first time that blockade of PDGFRβ impairs lymphoma growth by depleting vascular mural cells, disrupting tumor neovascular integrity, and reducing endothelial cell survival. Treatment with either a semiselective receptor tyrosine kinase inhibitor, imatinib mesylate, or a highly selective, PDGFRβ-specific monoclonal antibody impaired lymphoma growth dramatically without inducing systemic toxicity. Perivascular PDGFRβ+ pericyte-like stromal cells appear to be the major treatment target, providing proof of concept for future antilymphoma therapy that might combine both antitumor and antistromal cell strategies.
Here, imatinib mesylate impaired the growth of lymphoma in both human xenograft and murine allograft models. Inhibition of lymphoma growth correlated with loss of vascular integrity and perivascular apoptosis within tumor tissue. These data suggest that the tumor vasculature is a major target for imatinib. Indeed, treatment with imatinib had a direct antiproliferative effect on primary murine PDGFRβ+ VSMCs, but not endothelial cells, in vitro, and inhibited PDGFRβ activation in lymphoma microvascular pericytes in vivo. Because the lymphoma cell lines tested neither expressed PDGFRβ nor responded with significant growth inhibition when challenged with imatinib in vitro, it is unlikely that these cells represented its primary target in vivo. Similarly, imatinib had no effect on the survival of stromal inflammatory cells in vivo. On the other hand, both VSMC and pericytes expressed PDGFRβ and exhibited high sensitivity to imatinib. Moreover, knockdown of PDGFRβ expression in vascular pericytes protected them from imatinib-mediated growth inhibition, identifying PDGFRβ as a primary target in these cells.
Analysis of gene expression before and after imatinib provided insight into the potential mechanism of the drug’s action in vascular mural cells. A majority of the 312 differentially expressed genes (88%) were down-regulated and reflected overall antiproliferative and proapoptotic effects. The top scored networks included genes regulated directly by PDGF-BB and PDGFRβ, suggesting that disruption of PDGFRβ signaling was the primary action of imatinib. In addition, perturbation of proteolytic balance, manifested by increased expression of metalloproteinases, such as MMP3, MMP12, and MMP13, and decreased expression of proteinase inhibitors, such as TIMP1 and PAI-1, may have further destabilized the vascular integrity due to heightened turnover of extracellular matrix.39,40 In evaluating other potential effects of imatinib in VSMC or pericytes, we observed inhibition of PDGF-BB–dependent phosphorylation of PDGFRβ, PDGFRα, and c-Kit, possibly extending its usefulness to a broader range of stromal targets.
In addition to directly interfering with pericyte assembly and survival, imatinib appeared to deplete endothelial cells within the tumor stroma via an indirect pathway. Primary mature microvascular endothelial cells, the majority of which did not express PDGFRβ, were resistant to growth inhibition in the presence of therapeutic concentrations of imatinib. By down-regulating the expression and/or secretion of pericyte-derived proangiogenic growth factors such as VEGF and TGF-β, imatinib might, in an indirect manner, also induce secondary endothelial apoptosis and dropout. Sequential loss of tumor vessel pericytes, followed by endothelial cell dropout, in response to inhibition of PDGF-BB and PDGFRβ has been reported in solid tumor models as well.24,25,41 Because imatinib reduced vascular cell populations only in the tumor compartment, and not in the bone marrow or blood, we conclude that its therapeutic effect reflected suppression of local recruitment, proliferation, and differentiation of progenitors, through blockade of PDGFRβ and, possibly, c-Kit signaling.
Selective PDGFRβ blockade with the monoclonal antibody 2C5 had a highly significant, and more focused, antiangiogenic effect on lymphoma xenografts. Compared with imatinib, treatment with 2C5 was associated with statistically equivalent suppression of Farage tumor growth and even more dramatic suppression of pericyte numbers. Interestingly, 2C5 had a less dramatic effect on angiogenesis attenuation in Karpas422 lymphoma and a modest effect on tumor growth inhibition. The potential divergence of antiangiogenic and antitumor effects might reflect varying angiogenic adaptations by individual lymphomas. For example, the differential recruitment of perivascular myeloid CD68+ cells observed on 2C5, but not imatinib, treatment suggests that a PDGFRβ-independent, but imatinib-sensitive, pathway may partially compensate for the profound pericyte loss caused by PDGFRβ blockade in some tumors. By targeting a broader range of stromal targets, including PDGFRα+-activated fibroblasts and c-fms+ myelomonocytic cells,36,42 imatinib may potentially deliver more effective antivascular and antitumor effect by antagonizing multiple angiogenesis-dependent and angiogenesis-independent pathways.42,43 In addition, individual lymphomas may possess distinct resistance mechanisms; inhibition of PDGFRβ targeting may have minimal impact on tumors that overexpress VEGF,44 as high levels of VEGF may sustain endothelial proliferation and survival in the absence of pericytes. Finally, pericyte dysfunction and loss have been reported to lead to unexpectedly aggressive growth of some tumors with local invasion and distant metastasis both in a mouse model and in human patients.45,46
In conclusion, this study provides evidence that the pericyte, through its dependence on PDGFRβ signaling, represents a high-value, antiangiogenesis therapeutic target in experimental mouse lymphoma. This work also alludes to the possible role of additional imatinib-sensitive stromal cell types in sustaining tumor vasculature and growth. Finally, our data suggest that a multifocal attack, aimed at multiple stromal cell types within the tumor microenvironment, could enhance the therapeutic efficacy of treatment strategies directed toward lymphoma cells alone, thus achieving a more complete and durable response.
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Leona Cohen-Gould for assistance with confocal microscopy, members of the Weill Cornell Epigenetics Core Facility for assistance with gene expression microarray analyses, Jason McCormick for assistance with flow cytometry data analysis, Ralph Nachman for his critical review of the manuscript, and ImClone scientist, Juqun Shen, for supplying the anti-PDGFRβ monoclonal antibody 2C5.
This work was supported by the National Institutes of Health, National Heart, Lung, and Blood Institute, grants R01 HL090985 and R01 HL042493 to K.A.H., and an American Society of Clinical Oncology Junior Faculty Career Development award and grant K08 HL091517 to J.R.. L.C. is a Raymond and Beverly Sackler Scholar and a Scholar of the American Society of Hematology.
Authorship
Contribution: J.R. wrote the manuscript, designed the studies, coordinated the logistics, and performed the experiments and data analysis on the mouse xenograft studies and immunohistochemical staining; M.L., C.W., L.F., S.N.Y., and M.C. performed experiments; H.G. performed microarray data analysis; J.P.L. and A.M. reviewed the manuscript and coordinated logistics; and K.A.H. and L.C. contributed critically to the experimental design, coordination of logistics, interpretation of the data, and writing of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for L.F. is Department of Hematology, First Affiliated Hospital of Nanjing Medical University, Nanjing, China.
The current affiliation for H.G. is Department of Laboratory Medicine, University of California, San Francisco, CA.
Correspondence: Jia Ruan, Division of Hematology/Oncology, Department of Medicine, Weill Cornell Medical College, 1300 York Ave, New York, NY 10065; e-mail: jruan@med.cornell.edu; and Leandro Cerchietti, Division of Hematology/Oncology, Department of Medicine, Weill Cornell Medical College, 1300 York Ave, New York, NY 10065; e-mail: lec2010@med.cornell.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal