Key Points
Supraphysiologic Notch signals that maintain T-ALL self-renewal promote HSC differentiation at the expense of HSC self-renewal.
Abstract
The leukemia stem cell (LSC) hypothesis proposes that a subset of cells in the bulk leukemia population propagates the leukemia. We tested the LSC hypothesis in a mouse model of Notch-induced T-cell acute lymphoblastic leukemia (T-ALL) in which the tumor cells were largely CD4+CD8+ T cells. LSC activity was enriched but rare in the CD8+CD4−HSAhi immature single-positive T-cell subset. Although our murine T-ALL model relies on transduction of HSCs, we were unable to isolate Notch-activated HSCs to test for LSC activity. Further analysis showed that Notch activation in HSCs caused an initial expansion of hematopoietic and T-cell progenitors and loss of stem cell quiescence, which was followed by progressive loss of long-term HSCs and T-cell production over several weeks. Similar results were obtained in a conditional transgenic model in which Notch activation is induced in HSCs by Cre recombinase. We conclude that although supraphysiologic Notch signaling in HSCs promotes LSC activity in T-cell progenitors, it extinguishes self-renewal of LT-HSCs. These results provide further evidence for therapeutically targeting T-cell progenitors in T-ALL while also underscoring the need to tightly regulate Notch signaling to expand normal HSC populations for clinical applications.
Introduction
Somatic gain-of-function mutations in Notch1 occur frequently in human and murine T-cell acute lymphoblastic leukemia (T-ALL). Notch1 is a member of a family of heterodimeric receptors that are expressed at the cell membrane in an inactive state (for review, see Kopan and Ilagan1 ). The ectodomain of Notch1 consists of 36 epidermal growth factor (EGF)–like repeats, 3 Lin12/Notch repeats (LNR), and a juxtamembrane heterodimerization domain. The LNR and heterodimerization domains comprise a negative regulatory region (NRR) that keeps Notch inactive in the absence of ligand. Engagement of the EGF repeats by ligands expressed on neighboring cells leads to successive proteolytic cleavages of Notch at a juxtamembrane site termed S2 and an intramembranous site termed S3, which are carried out by ADAM-type metalloproteases and γ-secretase, respectively. These cleavages release the intracellular domain of Notch (ICN), allowing it to translocate to the nucleus, where it forms a transcriptional activation complex with the DNA binding factor CSL and coactivators of the Mastermind family. The duration of intracellular Notch 1 (ICN1) function is normally limited by a C-terminal PEST degron domain, which promotes ICN1 turnover. The unifying feature of the most common gain-of-function mutations in murine and human T-ALL is that they abolish NRR function and lead to ligand-independent generation of ICN1 whereas other common gain-of-function mutations result in deletion of the PEST degron.
Notch mutations are associated with multiple subtypes of T-ALL that range from the very immature early thymocyte precursor T-ALL to the mature cortical T-ALL at the double-positive (DP) stage of development,2 suggesting that Notch mutations drive transformation at multiple stages of T-cell differentiation. Results from several groups searching for leukemia stem cells (LSCs) using the leukemia-initiating cell (LIC) assay support this possibility. Specifically, multiple cell populations that immunophenotypically resemble normal stages of early T-cell development have enhanced capacity to serially transfer human3,4 and murine T-ALL5-7 to recipient mice.
Although Notch1 mutations predispose to oncogenic transformation, several groups have proposed that enhanced Notch1 signaling can be used to expand HSCs under carefully controlled conditions.8-10 For example, recent work from Delaney et al showed that ex vivo expansion of human cord blood cells with Notch ligands shortened the time to myeloid reconstitution.10 However, such studies have not rigorously shown that the expanded cells meet the criteria for long-term HSCs (LT-HSCs). Furthermore, multiple genetic studies have failed to identify a physiologic role for Notch signaling in the long-term maintenance or expansion of HSCs.11-13
In the current study, we initially sought to investigate the role of Notch signaling in HSCs on LSC generation. We used a retroviral murine bone marrow transplantation (BMT) model of Notch-induced T-ALL that faithfully models the human disease.14 We found that Notch-induced LIC activity was enriched within an immature T-cell population. In contrast, expression of Notch gain-of-function alleles, even those that were too weak to induce T-ALL, abolished LT-HSC activity by promoting T-cell differentiation at the expense of LT-HSC self-renewal. These data suggest that Notch signaling in HSCs must be limited to maintain HSC identity and self-renewal.
Methods
Mice
C57BL/6 mice (4-8 weeks old) were obtained from Taconic or NCI. Mx-Cre and Rag-1–deficient mice were obtained from The Jackson Laboratory. C57BL/6.Ly5.2 (B6-SJL, CD45.1+) were from the National Cancer Institute. Rosa26-LSL-ICN-GFP mice and Rosa26-LSL-YFP mice have been described.15 Cre expression in Mx-cretg/ Rosa26-LSL-ICN-GFP and Mx-cretg/Rosa26-LSL-YFP was induced with pI-pC (Sigma-Aldrich; 500 μg intraperitoneally every 2 days 10 times). All experiments were performed in accordance with National Institutes of Health guidelines for the care and use of animals under an animal protocol approved by the University of Pennsylvania Animal Care and Use Committee.
Constructs and retroviruses
The control GFP-activated MSCV-IRES-GFP construct (MigR1) has been described.16 High titer retroviral supernatant was produced with the use of transient transfection of 293T-cells and assessed for GFP titer by plating on 3T3 fibroblasts.17 Construction and cloning of the ΔEGFΔLNR and ΔEGFΔLNRΔPEST(ΔP) constructs were described previously.2,18,19
Antibodies
Antibodies from Pharmingen, Biolegend, or eBioscience were as follows: CD45.2 (104), CD45.1 (A20), CD150 (TC15-12F12.2), CD48 (HM48-1), Flt3 (A2F10), CD4 (RM4-5), CD8 (53-6.7), CD19 (1D3), Ter119, TCRb (H57-597), CD3e (14502C11), c-Kit (2B8), Sca-1/Ly-6A/E (E13-161.7), NK1.1 (PK136), B220 (RA3-B2), CD11b (M1/70), Gr-1 (RB6-8C5), and CD11c (HL3). Biotinylated antibodies were revealed with Streptavidin-Pacific Blue (Molecular Probes) or Streptavidin-PerCP (Pharmingen). Lineage+ cells were identified with anti–Gr-1, Ter119, B220, CD11b, CD19, CD11c, CD8, CD4, CD3ϵ, TCRb, and NK1.1.
Flow cytometry
Cells were stained on ice in PBS containing 2% fetal bovine serum, 10mM HEPES, and 0.02% NaN3 after blocking with rat and mouse IgG (Sigma-Aldrich) and 24G2 cell supernatant. Acquisition was performed on a FACS Calibur (Becton Dickinson) or LSRII (Becton Dickinson). Dead cells and doublets were excluded on the basis of forward scatter pulse width and side scatter pulse width characteristics and DAPI staining. Data were analyzed with FlowJo software (TreeStar). Cells were sorted with a FACSAria (Becton Dickinson).
BM transduction and transplantation
Retroviral transduction of BM cells and transfer into lethally irradiated recipients was performed as described.14,17 To summarize, BM cells were collected from 6- to 12-week-old mice 4 days after the intravenous administration of fluorouracil (5-FU; 250 mg/kg). The cells were cultured overnight in the presence of IL-3 (6 ng/mL), IL-6 (5-0 ng/mL), and SCF (100 ng/mL). The cells were then washed, resuspended in retroviral supernatant that had been normalized based on GFP-titer, placed in the same cytokine cocktail containing polybrene (4 μg/mL), and centrifuged at 1290g for 90 minutes. A second round of “spinoculation” was performed the next day. After washing with PBS, at least 5 × 105 cells were injected intravenously into lethally irradiated (900 rads) recipients. Mice were maintained on antibiotics in drinking water 2 weeks after BMT.
Quantitative real-time PCR
Total RNA was prepared with RNAEasy Micro Kit (QIAGEN). Random-primed total RNAs (2 μg) were reverse-transcribed with SuperScript II (Invitrogen). Mouse Deltex1 (Mm00492297_m1) and c-Myc (Mm00487803_m1) expression were validated using primer/probe sets from TaqMan Gene Expression Assays (Applied Biosystems). The 18s and Hes1 primer sets were previously described.11 Transcripts were amplified with either TaqMan Universal PCR Master Mix or Sybr Green PCR Master Mix (Applied Biosystems) on the ABI Prism 7900 sequence detection system (Applied Biosystems).
Competitive HSC transplantation
Sorted LT-HSCs (GFP/YFP+Lineage−c-Kit+Sca-1+CD150+CD48−) cells (500) from pI-pC–induced Mx-Cre+ × Rosa26-LSL-ICN-GFP or Rosa26-LSL-YFP were mixed with 200 000 competitor B6 BM and transplanted into lethally irradiated B6 recipients (900 rads). For secondary transplantation, BM GFP+ LSK cells were purified from recipients of MigR1 or ΔEGFΔLNRΔPEST-transduced Rag-1–deficient (B6) BM at 6 weeks after transplantation. Sorted LSK cells (500) were transplanted into lethally irradiated B6 recipients together with 200 000 competitor B6-SJL BM cells (CD45.1+). In limiting dilution analyses for LSC activity, decreasing numbers of sorted leukemia cells were injected into lethally irradiated syngeneic recipients. Mice were observed at least 6 months for T-ALL development. The progenitor frequency was calculated with L-Calc Version 1.1 software (StemCell Technologies).
Results
The immature single positive subset is enriched for LSC activity in Notch-induced T-ALL
We selected ΔEGFΔLNRΔP to study Notch gain-of-function in T-ALL.19 Like most mutated Notch1 proteins expressed in human and murine T-ALL, ΔEGFΔLNRΔP is targeted to the cell surface but bears an abnormality (in this case a deletion spanning the EGF and LNR domains) that abrogates NRR function, leading to ligand-independent generation of ICN1. The PEST deletion in ΔEGFΔLNRΔP (Δ2473-2555), a mutation originally identified in the human T-ALL cell line ALL-SIL,2 removes the degron motifs that target Notch for destruction and acts synergistically with the ΔEGFΔLNR mutation to increase ICN1 activity. This dual combination of mutations is seen in 15%-20% of human T-ALLs and most murine T-ALLs. In U2OS reporter assays, ΔEGFΔLNRΔP stimulates Notch signaling, but to a lesser degree than an activated Notch allele encoding the ICN1.19 ΔEGFΔLNRΔP was cloned into the MigR1 vector, which expresses ΔEGFΔLNRΔP and green fluorescent protein (GFP) from a bicistronic RNA containing an internal ribosomal entry site.14 The ΔEGFΔLNRΔP cDNA in MigR1 was packaged into ecotropic retroviruses and transduced into adult murine BM progenitors as described previously19 ; ΔEGFΔLNRΔP induces fully penetrant T-ALL that resembles human disease.19
To test the LSC hypothesis in our mouse model of Notch-induced T-ALL, we sorted BM from 12 leukemic mice into 3 GFP+ subpopulations; (1) double negative (DN) T cells (Thy-1+CD4−CD8−), (2) single positive (SP) T cells (CD4+CD8− or CD4−CD8+), and (3) double positive (DP) T cells (CD4+CD8+; supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). We then divided and transferred the sorted cells equally among 6 recipient mice. Absolute numbers of transferred cells per mouse were as follows: unsorted cells (1 500 000 cells), DN T cells (2500 cells), SP T cells (30 000 cells), and DP T cells (500 000 cells). As expected, all 6 mice receiving unsorted tumor cells developed T-ALL (supplemental Figure 1B). Half of the mice (3/6) receiving SP T cells developed T-ALL. None of the mice receiving DN or DP T cells developed T-ALL. These data suggested that LSC activity was markedly enriched in the SP compartment.
To further enrich for LSC activity within the SP compartment, SP BM cells from 16 leukemic mice were sorted into 4 GFP+ subpopulations: CD4+HSAlo, CD4+HSAhi, CD8+HSAlo, and CD8+HSAhi (supplemental Figure 1C). We then divided and transferred the sorted cells equally among 4 recipient mice. Absolute numbers of transferred cells were as follows: unsorted (3 000 000 cells), CD4+HSAlo (30 000 cells), CD4+HSAhi (1000 cells), CD8+HSAlo (300 cells), and CD8+HSAhi immature single positive (ISP; 600 000 cells). As expected, all 4 mice receiving unsorted BM cells developed T-ALL (supplemental Figure 1D). All 4 mice receiving CD8+HSAhi T cells developed T-ALL. None of the mice receiving CD4+HSAlo, CD4+HSAhi, or CD8+HSAlo cells developed T-ALL. Tumors initiated by CD8+HSAhi cells and unsorted cells had similar median latencies (18 and 31 days, respectively), suggesting that the predominant LSC activity is contained within the CD8+HSAhi subset. The CD8+HSAhi subset is “ISP-like” as they resemble the ISP stage of T-cell development, which are CD8+CD4−HSAhiTCRβ−.20 Transferred unsorted tumor cells and sorted ISP-like cells recapitulated the CD4/CD8 phenotype of the original tumor (data not shown). Retroviral integration analysis of tumors initiated by unsorted cells and the ISP-like subset showed that the leukemia-initiating clone in the unsorted tumor population was also found in the ISP-like population (data not shown). Together, these data suggest that the ISP-like compartment contained bona fide LSCs. These data are consistent with data from Li et al, who studied ICN1-initiated T-ALL.6
To determine the purity or frequency of LSC within this population, we transferred limiting numbers of tumor cells into lethally irradiated recipients (supplemental Table 1). This showed that the frequency of LSC activity within the ISP-like compartment was ∼ 1 in 953 cells. Transferring very large numbers of DP T cells (> 30 000) could generate T-ALL, but the frequency of leukemia-initiating cells within the DP T-cell compartment was very low (∼ 1 in 229 482 cells). These data suggest that even in the enriched ISP-like compartment, LSCs are not common.
Notch depletes HSC activity and numbers progressively over time
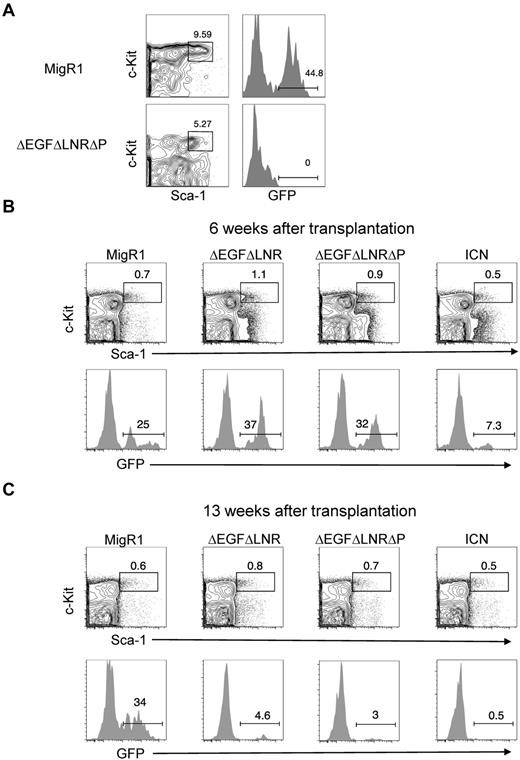
Given the natural “stemness” of hematopoietic stem cells/progenitor cells (HSPCs) to self-renew, we had originally hypothesized that these cells would contain the majority of the LSC activity in our retroviral model of Notch-induced T-ALL. However, we could not detect the HSPC subset (GFP+Lineage−Sca-1+Kit+) in the BM of leukemic mice and were therefore unable to purify these cells for LIC experiments (Figure 1A).
Weak, nonleukemogenic Notch alleles and strong, leukemogenic Notch alleles progressively deplete HSC activity and numbers over time. (A) Lethally irradiated mice were reconstituted with 5-FU–treated donor C57BL/6 BM cells transduced with MigR1 control or activated Notch1 (ΔEGFΔLNRΔP). BM cells at 6 weeks after transplantation were analyzed for donor-derived hematopoietic progenitor cells (GFP+Lineage−Sca-1+c-Kit+). Representative flow cytometry plots are shown. (B-C) Lethally irradiated CD45.1+ congenic mice were reconstituted with 5-FU–treated donor Rag1−/− CD45.2+ BM cells transduced with MigR1 control or activated Notch1 alleles (ΔEGFΔLNR, ΔEGFΔLNRΔP, and ICN). At 6 weeks (B) and 13 weeks (C) after transplantation, cells were gated on the HSC compartment (Lineage−Sca-1+c-Kit+Flt3−) and analyzed for donor reconstitution (GFP+). Experiments were performed twice.
Weak, nonleukemogenic Notch alleles and strong, leukemogenic Notch alleles progressively deplete HSC activity and numbers over time. (A) Lethally irradiated mice were reconstituted with 5-FU–treated donor C57BL/6 BM cells transduced with MigR1 control or activated Notch1 (ΔEGFΔLNRΔP). BM cells at 6 weeks after transplantation were analyzed for donor-derived hematopoietic progenitor cells (GFP+Lineage−Sca-1+c-Kit+). Representative flow cytometry plots are shown. (B-C) Lethally irradiated CD45.1+ congenic mice were reconstituted with 5-FU–treated donor Rag1−/− CD45.2+ BM cells transduced with MigR1 control or activated Notch1 alleles (ΔEGFΔLNR, ΔEGFΔLNRΔP, and ICN). At 6 weeks (B) and 13 weeks (C) after transplantation, cells were gated on the HSC compartment (Lineage−Sca-1+c-Kit+Flt3−) and analyzed for donor reconstitution (GFP+). Experiments were performed twice.
Our inability to detect HSPCs in the BM of leukemic mice suggested that Notch-activated HSPCs have a competitive disadvantage. We tested this possibility by reconstituting lethally irradiated CD45.1+ mice with Notch-transduced congenic CD45.2+ RAG1−/− HSPCs. As shown in previous experiments, the MigR1 retroviral vector transduces the most primitive LT-HSCs.11 We used RAG1-deficient donor cells because Notch-activation in this background fails to induce leukemia,21 which would otherwise confound our analysis of HSC numbers and activity. We initially tested 3 activated Notch constructs, ΔEGFΔLNR, ΔEGFΔLNRΔP, and ICN1. ΔEGFΔLNR has the weakest signal strength in transcriptional activation assays and fails to induce leukemia but retains the ability to promote ectopic T-cell development.19 ΔEGFΔLNRΔP has stronger signal strength and induces leukemia with 100% penetrance and relatively long latency.19 ICN1 has the strongest signal strength and induces leukemia with 100% penetrance and short latency.19
At 6 weeks after transplantation, MigR1, ΔEGFΔLNR, and ΔEGFΔLNRΔP reconstituted the HSC compartment (Flt3−Lineage−Sca-1+Kit+) to similar levels (∼ 25%-40%, Figure 1B). ICN1 reconstituted the HSC compartment to a lesser degree (∼ 7%). At 13 weeks after transplantation, MigR1 continued to reconstitute the HSC compartment (∼ 30%). However, all 3 activated Notch constructs failed to maintain reconstitution, failing to ∼ 0.5%-5% GFP+ cells (Figure 1C). We chose the ΔEGFΔLNRΔP construct to continue our study of the suppressive effects of Notch activation on HSCs.
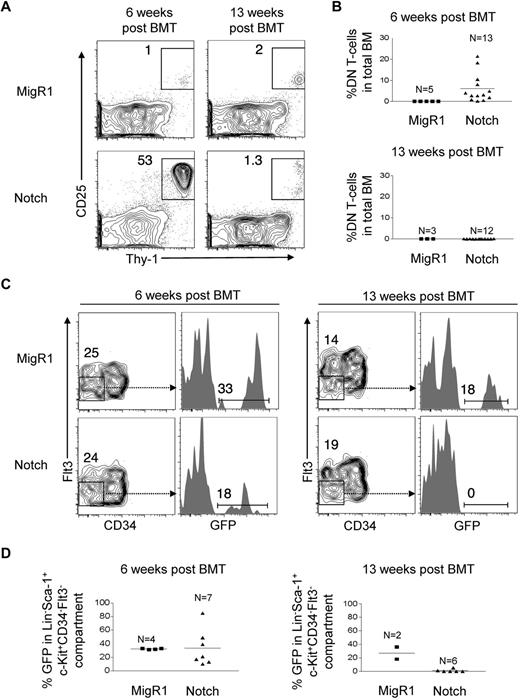
The conventional way to assess HSC activity is to measure myeloid or CD45+ cell reconstitution, but we could not use this method because Notch impairs differentiation along multiple lineages, in particular myeloid and B cells.17,22 As a surrogate measure of HSC activity, we thus chose to measure DN T cells (Lineage−Thy-1+CD25+), a cell type with a short lifespan whose replenishment is supported by Notch activity in HSCs.21 We measured DN T-cell production in the BMs of these mice at 6 weeks and 13 weeks after transplantation. We found that DN T-cell production by Notch-activated HSPCs was markedly increased at 6 weeks (∼ 750-fold elevated compared with MigR1 controls), but by 13 weeks decreased to levels similar to MigR1 controls (Figure 2A-B). We also measured the numbers of Lineage−c-Kit+Sca-1+CD34−Flt3−, a population that is highly enriched for LT-HSCs.23-26 We found that at 6 weeks after transplantation, Notch-activated HSPCs reconstituted the LT-HSC compartment to a similar degree as MigR1 controls, but that by 13 weeks, Notch-activated LT-HSCs were no longer detectable. These data suggest that Notch profoundly depletes both LT-HSC activity and numbers over time.
Notch progressively depletes HSC activity and numbers over time. (A) Lethally irradiated CD45.1+ congenic mice were reconstituted with 5-FU–treated donor Rag1−/−CD45.2+ BM cells transduced with MigR1 control or activated Notch1 (ΔEGFΔLNRΔP). BM DN T-cell production (Thy-1+CD25+) was measured at 6 weeks and 13 weeks after transplantation. Cells are gated on the CD45.2+GFP+Lineage− cells. (B) Scatter plot analysis of DN T-cell production by MigR1 controls and Notch mice at 6 and 13 weeks after transplantation. (C) The LT-HSC compartment (Lineage−Sca-1+c-Kit+CD34−Flt3−) was measured for donor reconstitution by GFP+ MigR1 control and Notch-activated HSCs at 6 and 13 weeks after transplantation. (D) Scatter plot analysis of donor-derived (GFP+) HSCs (Lineage−Sca-1+c-Kit+CD34−Flt3−) at 6 and 13 weeks after transplantation. Experiments were performed 3 times.
Notch progressively depletes HSC activity and numbers over time. (A) Lethally irradiated CD45.1+ congenic mice were reconstituted with 5-FU–treated donor Rag1−/−CD45.2+ BM cells transduced with MigR1 control or activated Notch1 (ΔEGFΔLNRΔP). BM DN T-cell production (Thy-1+CD25+) was measured at 6 weeks and 13 weeks after transplantation. Cells are gated on the CD45.2+GFP+Lineage− cells. (B) Scatter plot analysis of DN T-cell production by MigR1 controls and Notch mice at 6 and 13 weeks after transplantation. (C) The LT-HSC compartment (Lineage−Sca-1+c-Kit+CD34−Flt3−) was measured for donor reconstitution by GFP+ MigR1 control and Notch-activated HSCs at 6 and 13 weeks after transplantation. (D) Scatter plot analysis of donor-derived (GFP+) HSCs (Lineage−Sca-1+c-Kit+CD34−Flt3−) at 6 and 13 weeks after transplantation. Experiments were performed 3 times.
Previous work showed that retroviral activation of Notch1 using a protocol similar to ours promoted the expansion of a Lin−Sca-1+subset27 ; thus, the observed severe contraction of HSC numbers was unexpected. The Lin−Sca-1+subset contains both Lin−Sca-1+c-Kit+ (LSK) and Lin−Sca-1+c-Kit− cells. In our studies, we failed to observe an expansion of the LSK subset, which contains the HSPC population, and instead saw a relative expansion of the Lin−Sca-1+c-Kit− subset (supplemental Figure 2). Because Notch potently induces the T-cell fate, we analyzed the Lin−Sca-1+c-Kit− cells for expression of the T-lineage markers, CD25 and Thy-1, and found that they were expressed by nearly 100% of these cells (supplemental Figure 2), indicating that this population consists mainly of DN T-cells.
Notch simulates HSPC proliferation
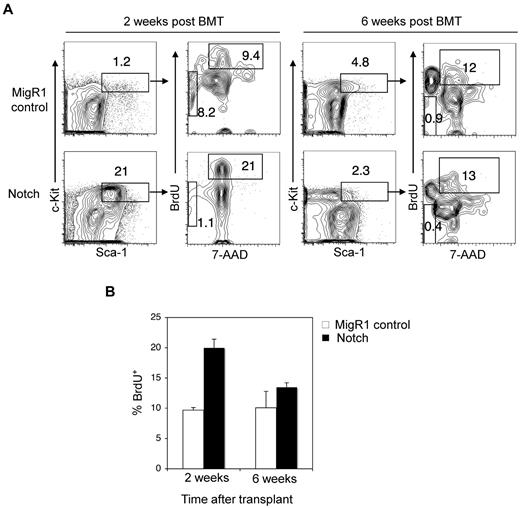
In contrast to our results showing that Notch activation depletes the LSK subset, the authors of previous studies have shown that activated Notch alleles expand LSK cells.27-32 One explanation is that the previous authors may have been describing the short-term effects of Notch stimulation. To explore this possibility, we measured stem cell engraftment at early time points after transplantation of Notch-activated HSPCs. At 2 weeks after transplantation, we found that Notch-activated HSPCs homed to the BM and expanded robustly (∼ 18-fold) over competitor HSPCs in repopulating the BM (Figure 3A). To measure cell proliferation, mice were injected with BrdU 16 hours before the harvest of BM. BrdU incorporation into Notch stimulated HSPCs was ∼ 1.6-fold greater than MigR1 controls (Figure 3A-B). Notch activation did not increase apoptosis in LSK cells. We then explored whether Notch induces HSC senescence through p16. HSC aging results in impaired repopulating ability in transplantation assays, a defect that is reversed by loss of p16INK4A.33
HSPCs are transiently driven into cell cycle by Notch signaling. (A) Lethally irradiated mice were reconstituted with 5-FU–treated donor Rag1−/− BM cells transduced with MigR1 control or activated Notch1. At 2 weeks and 6 weeks after transplantation, the mice were injected with BrdU and then killed 16 hours later. (A) The donor-derived BM HSPC compartment (GFP+Lineage−Sca-1+c-Kit+) was stained with 7-AAD and intracellular antibody against BrdU. Cells entering cell cycle are BrDU+ with 2N DNA content by 7-AAD stain. Apoptotic cells are BrdU− and found in the sub2N region of the 7-AAD stain. (B) Bar graph analysis of BrdU+ MigR1 control and Notch HSPCs entering cell cycle at 2 and 6 weeks after transplantation. Three mice per condition.
HSPCs are transiently driven into cell cycle by Notch signaling. (A) Lethally irradiated mice were reconstituted with 5-FU–treated donor Rag1−/− BM cells transduced with MigR1 control or activated Notch1. At 2 weeks and 6 weeks after transplantation, the mice were injected with BrdU and then killed 16 hours later. (A) The donor-derived BM HSPC compartment (GFP+Lineage−Sca-1+c-Kit+) was stained with 7-AAD and intracellular antibody against BrdU. Cells entering cell cycle are BrDU+ with 2N DNA content by 7-AAD stain. Apoptotic cells are BrdU− and found in the sub2N region of the 7-AAD stain. (B) Bar graph analysis of BrdU+ MigR1 control and Notch HSPCs entering cell cycle at 2 and 6 weeks after transplantation. Three mice per condition.
We transduced activated Notch1 alleles (ΔEGFΔLNR and L1601P) into p16−/− BM progenitors and transferred these cells to lethally irradiated syngeneic recipients. L1601P is one of the most common NRR mutations found in human T-ALL2 and (like ΔEGFΔLNR) induces ectopic T-cell development but not T-ALL when expressed in murine HSCs in vivo.22 At 16 weeks after transplantation, we analyzed donor-reconstitution of the long-term stem cell compartment (Lineage−c-Kit+Sca-1+CD150+Flt3−), the short-term stem cell compartment (Lineage−c-Kit+Sca-1+CD150−Flt3−), and multipotential progenitors (Lineage−c-Kit+Sca-1+CD150−Flt3+). We found that the weak L1601P allele significantly impaired reconstitution of all 3 hematopoietic precursor subsets and that loss of p16 did not reverse these defects (supplemental Figure 3A-B), which are therefore p16-independent. These data suggest that although Notch activation transiently expands HSPCs, over the long-term, HSPC numbers decrease in the face of increased Notch signaling because of depletion of the LT-HSC pool.
Noncompetitive transplantation of Notch-activated HSPCs fails to rescue lethally irradiated mice
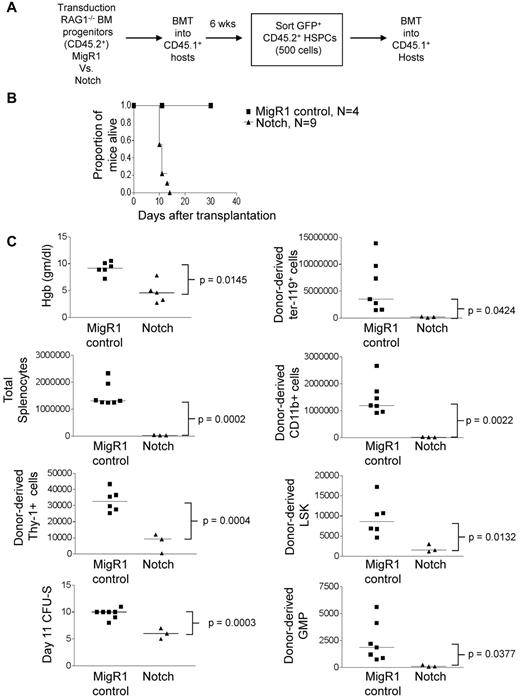
Although Notch-activated HSPCs were outcompeted by wild-type HSPCs, it was possible that they would have been fully functional in the absence of competition for limited hematopoietic niches. To address this possibility, we tested the ability of Notch-transduced HSPCs to rescue lethally irradiated mice under noncompetitive conditions. We reconstituted lethally irradiated CD45.1+ congenic mice with MigR1 or ΔEGFΔLNRΔP-activated CD45.2+ RAG1−/− hematopoietic progenitor cells. After 6 weeks, Lin−c-Kit+Sca-1+CD45.2+GFP+ cells were isolated by flow sorting and transferred to lethally irradiated CD45.1+ congenic mice at 500 cells per mouse (Figure 4A). To confirm that Notch was activated in these cells, we compared the expression of Notch targets dtx1, hes1, and myc (supplemental Figure 4). Notch induced dtx1 and hes1 ∼ 50- to 60-fold in HSPCs but failed to induce myc, suggesting that this target is regulated by Notch in a tissue-specific and/or developmental-specific manner (eg, T cells and breast cancers34-36 ). Strikingly, all mice that received Notch-transduced HSPCs quickly became moribund and were killed between 11 and 14 days after transplantation (Figure 4B). In contrast, mice that received MigR1-transduced HSPCs survived for > 4 months (Figure 4B and data not shown). Recipients of Notch-activated HSPCs had lower hemoglobin levels, fewer total splenocytes and CFU-SDay11 units, and fewer donor-derived Ter-119+ erythroid progenitors, splenic CD11b+ myeloid cells, Thy-1+ T-lineage cells, HSPCs, and granulocyte-macrophage progenitors (Lineage−c-Kit+Sca-1−CD34+CD16/32hi cells; Figure 4C). These data suggest that the transplanted Notch-activated HSPCs cannot maintain short-term hematopoiesis, even in the absence of competition.
Noncompetitive transplantation of Notch-activated HSPCs leads to hematopoietic failure. (A) Lethally irradiated CD45.1+ congenic mice were reconstituted with 5-FU–treated donor CD45.2+Rag1−/− BM cells transduced with MigR1 control or activated Notch1. At 6 weeks after transplantation, CD45.2+GFP+Lineage−Sca-1+c-Kit+ cells (HSPCs) were sorted and transplanted into CD45.1+ hosts at 500 cells per mouse. (B) Kaplan-Meier graph showing the proportion of mice alive after transplantation. (C) Scatter plot analysis of moribund mice at 11 days after transplantation showing peripheral blood hemoglobin concentration; absolute numbers of total donor-derived (CD45.2+GFP+) splenocytes, splenic ter119+ erythroid cells, splenic CD11b+ myeloid cells, Thy-1+ T cells, splenic Lineage−Sca-1+c-Kit+ HSPCs, and splenic Lineage−Sca-1−c-Kit+CD34+CD16/62hi granulocyte-macrophage progenitors (GMPs); and day 11 CFU-S colonies. The experiment was performed twice; 4 mice for MigR1 and 9 mice for activated Notch1.
Noncompetitive transplantation of Notch-activated HSPCs leads to hematopoietic failure. (A) Lethally irradiated CD45.1+ congenic mice were reconstituted with 5-FU–treated donor CD45.2+Rag1−/− BM cells transduced with MigR1 control or activated Notch1. At 6 weeks after transplantation, CD45.2+GFP+Lineage−Sca-1+c-Kit+ cells (HSPCs) were sorted and transplanted into CD45.1+ hosts at 500 cells per mouse. (B) Kaplan-Meier graph showing the proportion of mice alive after transplantation. (C) Scatter plot analysis of moribund mice at 11 days after transplantation showing peripheral blood hemoglobin concentration; absolute numbers of total donor-derived (CD45.2+GFP+) splenocytes, splenic ter119+ erythroid cells, splenic CD11b+ myeloid cells, Thy-1+ T cells, splenic Lineage−Sca-1+c-Kit+ HSPCs, and splenic Lineage−Sca-1−c-Kit+CD34+CD16/62hi granulocyte-macrophage progenitors (GMPs); and day 11 CFU-S colonies. The experiment was performed twice; 4 mice for MigR1 and 9 mice for activated Notch1.
Competitive transplantation of Notch-activated HSPCs into lethally irradiated mice leads to progressive loss of donor-derived HSC activity and numbers over time
Although the results from the noncompetitive transplantation experiment were dramatic, the observed lethality during the immediate posttransplantation period hindered our efforts to address whether Notch-activated HSPCs were capable of long-term self-renewal. To overcome this problem, we used a competitive transplantation approach. Lethally irradiated CD45.1+ congenic mice were reconstituted with MigR1 or ΔEGFΔLNRΔP transduced CD45.2+ RAG1−/− hematopoietic progenitor cells in addition to 200 000 syngeneic CD45.1+ BM cells. We measured DN T-cell production in the BMs of these mice at 2 weeks and 16 weeks after transplantation. We found that DN T-cell production by Notch-activated HSPCs was initially robust at 2 weeks at ∼ 240-fold greater levels than MigR1 controls. However, at 16 weeks, DN T-cell production decreased to undetectable levels (Figure 5A-B). In contrast to MigR1 controls, peripheral myeloid reconstitution by Notch-activated HSPCs was not detected from 4 to 16 weeks after transplantation (supplemental Figure 5). We also measured LT-HSCs numbers using the Lin−c-Kit+Sca-1+CD34−Flt3− markers (Figure 5C-D). We found that Notch-activated HSPCs reconstituted the LT-HSC compartment to a similar level as MigR1 controls at 2 weeks after transplantation. However, by 16 weeks, these LT-HSCs were no longer detectable. These data show that Notch profoundly depletes both LT-HSC activity and numbers over time.
Competitive transplantation of Notch-activated HSPCs leads to progressive exhaustion of HSC activity and numbers. A similar experiment was performed as in Figure 4A but with the addition of 200 000 whole CD45.1+ BM cells to prevent mortality from hematopoietic failure. (A) BM DN T-cell production (Thy-1+CD25+) in MigR1 control and Notch mice was measured in the BM at 2 and 16 weeks after transplantation. (B) Scatter plot analysis of DN T-cell production by MigR1 controls and Notch mice at 2 and 16 weeks after transplantation. (C) The LT-HSC compartment (Lineage−Sca-1+c-Kit+CD34−Flt3−) was measured for donor reconstitution by GFP+ MigR1 control and Notch-activated LT-HSCs at 2 and 16 weeks after transplantation. (D) Scatter plot analysis of donor-derived (GFP+) LT-HSCs (Lineage−Sca-1+c-Kit+CD34−Flt3−) at 2 and 16 weeks after transplantation. Experiment was performed 3 times. MigR1, 6 mice at 2 weeks; 5 mice at 16 weeks. Notch, 3 mice at 2 weeks; 6 mice at 16 weeks.
Competitive transplantation of Notch-activated HSPCs leads to progressive exhaustion of HSC activity and numbers. A similar experiment was performed as in Figure 4A but with the addition of 200 000 whole CD45.1+ BM cells to prevent mortality from hematopoietic failure. (A) BM DN T-cell production (Thy-1+CD25+) in MigR1 control and Notch mice was measured in the BM at 2 and 16 weeks after transplantation. (B) Scatter plot analysis of DN T-cell production by MigR1 controls and Notch mice at 2 and 16 weeks after transplantation. (C) The LT-HSC compartment (Lineage−Sca-1+c-Kit+CD34−Flt3−) was measured for donor reconstitution by GFP+ MigR1 control and Notch-activated LT-HSCs at 2 and 16 weeks after transplantation. (D) Scatter plot analysis of donor-derived (GFP+) LT-HSCs (Lineage−Sca-1+c-Kit+CD34−Flt3−) at 2 and 16 weeks after transplantation. Experiment was performed 3 times. MigR1, 6 mice at 2 weeks; 5 mice at 16 weeks. Notch, 3 mice at 2 weeks; 6 mice at 16 weeks.
Nonretroviral-mediated Notch activation depletes LT-HSC activity and numbers
Even with use of the MigR1 control, it is difficult to completely rule out the possibility that retroviruses carrying Notch alleles cannot transduce LT-HSCs. To address this alternative possibility, we studied Rosa26-LSL-ICN-GFP mice, which are genetically engineered to conditionally express a form of ICN1 and GFP from the Rosa26 locus.15 The Rosa26-LSL-YFP control mouse has a conditional YFP gene “knocked in” to the same locus. We mated both strains of mice with Mx-Cre transgenic mice to generate mice that express ICN1 and GFP (“Notch”) or YFP (“YFP control”) in HSCs after injection of pI-pC. Given the leukemogenic potential of activated Notch, we were concerned that T-ALL would develop in the Notch mice, but of 40 mice analyzed, none developed T-ALL during the 16-week experimental period. Because only high doses of Notch1 induce T-ALL,22 we assume that the absence of leukemia reflects low level expression of ICN1 from the weak Rosa26 promoter.
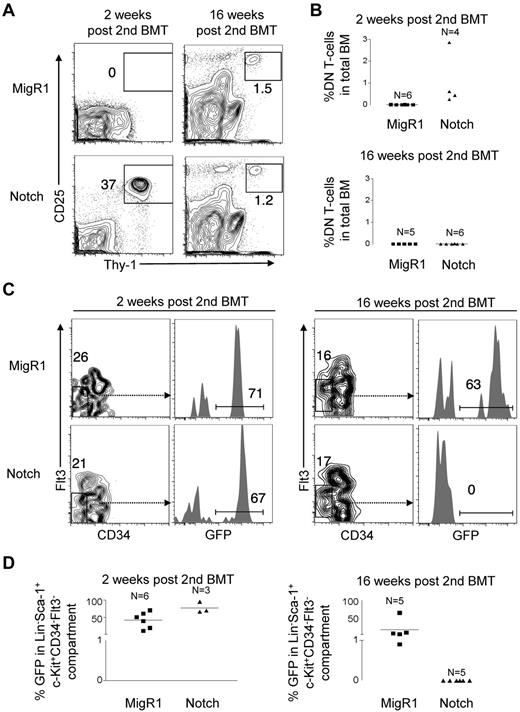
We first measured BM DP T-cell production in these mice as a surrogate of HSC activity. DP T-cell induction by Notch was initially robust at 4 weeks after injection; ∼ 56-fold greater compared with YFP controls (Figure 6A-B). However, at 16 weeks after BMT, DP production fell ∼ 60-fold to barely detectable levels (Figure 6A-B). Mature GFP+ B cells and granulocytes were undetectable in the peripheral blood of Notch mice (data not shown). There was no difference in complete blood counts (white blood counts, hemoglobin levels, and platelet counts) between the Notch mice and the YFP control mice (data not shown). Therefore, LT-HSCs that did not activate Notch provided adequate long-term hematopoietic support. We also measured LT-HSCs numbers using the “SLAM” markers Lin−c-Kit+Sca-1+CD150+CD48−, which enriches LT-HSCs to at least 40%-50% purity.37 We found that Notch-activated LT-HSCs were only slightly reduced in number relative to YFP control LT-HSCs at 4 weeks. However, by 16 weeks Notch-activated LT-HSCs fell ∼ 7.1-fold to barely detectable levels and were ∼ 21-fold reduced compared with YFP control LT-HSCs (Figure 6C-D). These data confirm the results seen with retroviral activation of Notch in LT-HSC, and indicate that even “subleukemogenic doses” of Notch activation profoundly deplete both LT-HSC activity and numbers over time.
Notch progressively depletes LT-HSC activity and numbers over time in nontransplanted Rosa26-Flox-STOP-Flox-Notch-GFP mice. Mx-cretgRosa26-Flox-STOP-Flox-YFP control mice (“YFP control”) and Mx-cretgRosa26-Flox-STOP-Flox-ICN-GFP (“Notch”) mice were injected with pI-pC to activate YFP control and ICN-GFP, respectively in HSCs. (A) BM DP T-cell production (CD4+CD8+) by the HSC compartment was measured at 4 and 16 weeks after injection in YFP control and Notch mice. (B) Scatter plot analysis of BM DP T-cell production by YFP control and Notch mice at 4 and 16 weeks after injection. (C) The LT-HSC compartment (Lineage−Sca-1+c-Kit+CD150+CD48−) was measured for the donor-derived cells (YFP+ or GFP+) in YFP control and Notch mice at 4 and 16 weeks after injection. (D) Scatter plot analysis of donor-derived (YFP+ or GFP+) LT-HSCs (Lineage−Sca-1+c-Kit+CD150+CD48−) in YFP control and Notch mice at 4 and 16 weeks after injection. One experiment was performed; 3 mice per condition at 4 weeks and 5 mice per condition at 6 weeks.
Notch progressively depletes LT-HSC activity and numbers over time in nontransplanted Rosa26-Flox-STOP-Flox-Notch-GFP mice. Mx-cretgRosa26-Flox-STOP-Flox-YFP control mice (“YFP control”) and Mx-cretgRosa26-Flox-STOP-Flox-ICN-GFP (“Notch”) mice were injected with pI-pC to activate YFP control and ICN-GFP, respectively in HSCs. (A) BM DP T-cell production (CD4+CD8+) by the HSC compartment was measured at 4 and 16 weeks after injection in YFP control and Notch mice. (B) Scatter plot analysis of BM DP T-cell production by YFP control and Notch mice at 4 and 16 weeks after injection. (C) The LT-HSC compartment (Lineage−Sca-1+c-Kit+CD150+CD48−) was measured for the donor-derived cells (YFP+ or GFP+) in YFP control and Notch mice at 4 and 16 weeks after injection. (D) Scatter plot analysis of donor-derived (YFP+ or GFP+) LT-HSCs (Lineage−Sca-1+c-Kit+CD150+CD48−) in YFP control and Notch mice at 4 and 16 weeks after injection. One experiment was performed; 3 mice per condition at 4 weeks and 5 mice per condition at 6 weeks.
Competitive transplantation of Notch-activated virus-free LT-HSCs into lethally irradiated mice leads to progressive loss of HSC activity and numbers over time
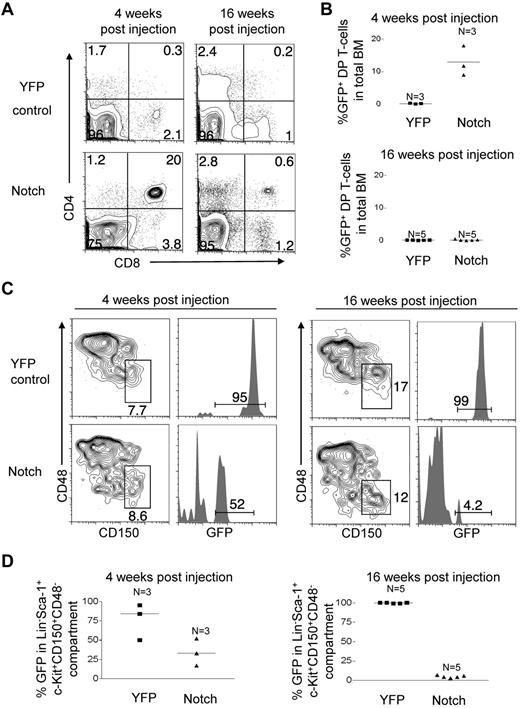
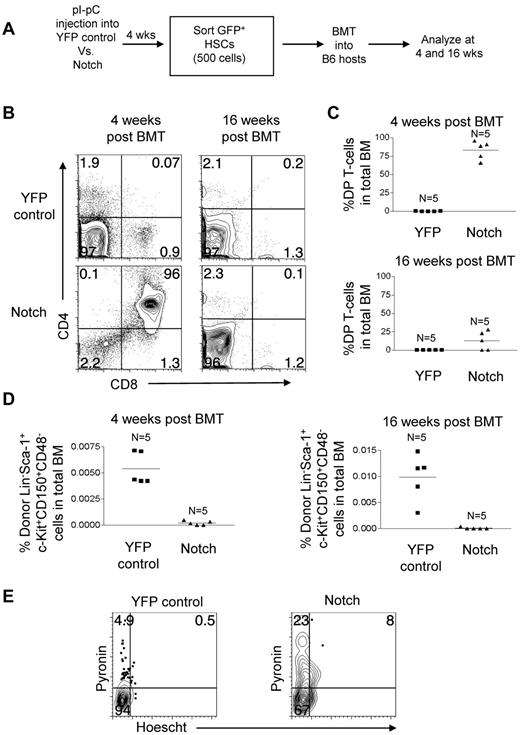
To directly test the fitness of the Notch-activated virus-free LT-HSCs, we measured their ability to reconstitute long-term in a competitive setting. YFP control mice and Notch mice were injected with pI-pC. At 4 weeks, 500 LT-HSCs (GFP/YFP+Lineage−c-Kit+Sca-1+CD150+CD48−) in the BM were isolated by flow sorting and transferred to lethally irradiated mice with 200 000 syngeneic whole BM cells per mouse (Figure 7A). The ratio of donor to competitor LT-HSCs was approximately 25 to 1. At 4 weeks after transplantation, Notch-activated LT-HSCs generated ∼ 1000-fold more DP T cells than YFP control LT-HSCs (Figure 7B-C). However, at 16 weeks after transplantation, DP T-cell production by Notch-activated LT-HSCs decreased dramatically by ∼ 6.5-fold (Figure 7B-C). At 4 weeks after transplantation, Notch-activated LT-HSCs numbers were ∼ 28-fold reduced compared with YFP-control LT-HSCs, and by 16 weeks after transplantation Notch-activated LT-HSCs numbers fell even further compared with YFP-control LT-HSCs (Figure 7D). These data show that despite having a ∼ 25-fold advantage in numbers compared with coinjected wild-type LT-HSCs, the LT-HSCs with activated Notch are markedly impaired in repopulating ability.
Competitive transplantation of Notch-activated LT-HSCs from Rosa26-Flox-STOP-Flox-ICN-GFP mice leads to cell autonomous exhaustion of HSC activity and numbers. (A) Mx-cretgRosa26-Flox-STOP-Flox-YFP (“YFP control”) mice and Mx-cretgRosa26-Flox-STOP-Flox-ICN-GFP (“Notch”) mice were injected with pI-pC to activate YFP and ICN-GFP, respectively in HSCs. Then, 4 weeks later YFP+ or GFP+ Lineage−Sca-1+c-Kit+CD150+CD48− BM cells (HSCs) were sorted and transplanted into syngeneic hosts at 500 cells per mouse along with 200 000 whole syngeneic BM cells. (B) BM DP T-cell production (CD4+CD8+) in YFP control and Notch mice was measured in the bone marrow at 4 and 16 weeks after transplantation. (C) Scatter plot analysis of DP T-cell production by YFP control and Notch mice at 4 and 16 weeks after transplantation. (D) The LT-HSC compartment (Lineage−Sca-1+c-Kit+CD150+CD48−) was measured for donor reconstitution by YFP+ control and Notch HSCs at 4 and 16 weeks after transplantation. Scatter plot analysis of donor-derived (YFP+ or Notch) LT-HSCs (Lineage−Sca-1+c-Kit+CD150+CD48−) at 4 and 16 weeks after transplantation. (E) Cell-cycle analysis was performed in sorted YFP control and Notch LT-HSCs (YFP+ or GFP+ Lineage−Sca-1+c-Kit+CD150+CD48− BM cells). Propidium iodide+ cells were excluded. Pyronin+/Hoechst 33 342+ cells (top left quadrant) are in G1. Pyronin−/Hoescht− cells (bottom left quadrant) are in G0. Pyronin+/Hoechst 33 342+ cells (top right quadrant) are in S/G2/M.
Competitive transplantation of Notch-activated LT-HSCs from Rosa26-Flox-STOP-Flox-ICN-GFP mice leads to cell autonomous exhaustion of HSC activity and numbers. (A) Mx-cretgRosa26-Flox-STOP-Flox-YFP (“YFP control”) mice and Mx-cretgRosa26-Flox-STOP-Flox-ICN-GFP (“Notch”) mice were injected with pI-pC to activate YFP and ICN-GFP, respectively in HSCs. Then, 4 weeks later YFP+ or GFP+ Lineage−Sca-1+c-Kit+CD150+CD48− BM cells (HSCs) were sorted and transplanted into syngeneic hosts at 500 cells per mouse along with 200 000 whole syngeneic BM cells. (B) BM DP T-cell production (CD4+CD8+) in YFP control and Notch mice was measured in the bone marrow at 4 and 16 weeks after transplantation. (C) Scatter plot analysis of DP T-cell production by YFP control and Notch mice at 4 and 16 weeks after transplantation. (D) The LT-HSC compartment (Lineage−Sca-1+c-Kit+CD150+CD48−) was measured for donor reconstitution by YFP+ control and Notch HSCs at 4 and 16 weeks after transplantation. Scatter plot analysis of donor-derived (YFP+ or Notch) LT-HSCs (Lineage−Sca-1+c-Kit+CD150+CD48−) at 4 and 16 weeks after transplantation. (E) Cell-cycle analysis was performed in sorted YFP control and Notch LT-HSCs (YFP+ or GFP+ Lineage−Sca-1+c-Kit+CD150+CD48− BM cells). Propidium iodide+ cells were excluded. Pyronin+/Hoechst 33 342+ cells (top left quadrant) are in G1. Pyronin−/Hoescht− cells (bottom left quadrant) are in G0. Pyronin+/Hoechst 33 342+ cells (top right quadrant) are in S/G2/M.
Notch stimulates loss of quiescence and proliferation of LT-HSCs
Our results from the retroviral transduction studies showed that Notch stimulated proliferation of HSPCs (Figure 3). This observation led us to hypothesize that the loss of HSC activity in Notch mice was related to loss of quiescence. To test this hypothesis in the nonretroviral model, LT-HSCs (GFP+/YFP+Lin−c-Kit+Sca-1+CD150+CD48−) were sorted from the BM of pI-pC–treated YFP control mice and Notch mice. These cells were then stained with pyronin and Hoechst 33 342 (Figure 7E). Pyronin− cells are in G0 phase of the cell cycle and pyronin+ cells are in G1/S/G2/M of the cell cycle. As expected, few LT-HSC in the YFP control mice were cycling (∼ 5.4% pyronin+). In contrast, ∼ 6-fold more Notch-activated LT-HSCs were cycling (∼ 31% pyronin+). Furthermore, of the cycling LT-HSCs, a greater percentage of the Notch-activated LT-HSCs were in S/G2/M phase relative to G1 (26% vs 9.3% in YFP controls). These data suggest that Notch induces loss of stem cell quiescence and stimulates cell cycle entry, which ultimately leads to T-lineage differentiation at the expense of self-renewal.
Discussion
Although the original goal of our studies was to characterize the LIC in Notch-induced T-ALL, in the course of these studies we discovered that supraphysiologic Notch signaling in LT-HSCs leads to HSC exhaustion. Using both retroviral and transgenic models of Notch activation in LT-HSCs, we found that both leukemic and nonleukemic Notch1 gain-of-function alleles promoted ectopic T-cell development at the expense of LT-HSC self-renewal. At early time points, increased Notch activity expanded HSPCs; however, at later time points, HSPC number and function decreased dramatically. We failed to observe defects in homing or survival, but observed loss of stem cell quiescence simultaneously with T-cell development. The increased production of T-lineage cells was transient, presumably because the progenitors of these cells, the LT-HSCs, failed to self-renew. An unlikely explanation for our observations is silencing of the GFP marker. We observed functional defects in immature T-cell generation irrespective of GFP expression. Silencing would also not explain the hematopoietic defects (eg, anemia) in the noncompetitive transfers in Figure 4. Rather, the most likely explanation is that supraphysiologic signaling enforces these transduced cells to differentiate into T cells at the expense of both differentiation to other lineages and self-renewing divisions (supplemental Figure 6). Consistent with this explanation, our previous reports showed that that transduction of HSPCs with Notch1 alleles induced these cells to differentiate to the T-cell lineage at the expense of the myeloid and B-cell lineages.17,22 In supplemental Figure 5, we showed impaired differentiation of HSPCs transduced with ΔEGFΔLNRΔP to the myeloid lineage consistent with our previous reports. Similarly, a recent report from Klinakis et al showed that ectopic Notch1 activation in HSPCs in Ef1α1-lsl-Notch1ICMx1-cre+ mice suppressed myeloid-specific genes and drove T-cell differentiation at the expense of myeloid differentiation.38 Supraphysiologic Notch signaling may induce loss of quiescence and reprogram LT-HSC into T-cells, resulting in loss of LT-HSCs.
Our results and those of other groups also show that the LSC in mouse models of T-ALL resides in an immature T-cell compartment.5-7 In all of these mouse models, Notch1 is activated, but the surface phenotype of the LSC varies between models. It is possible that the surface phenotype of the LSC may depend not only on Notch activation, but also on other contributing factors in the genetic background. Notably, the LSC in most of these studies appears to be rare, representing less than 1% of cells, and has a different phenotype than the bulk of the tumor cells.7,39 These findings are consistent with experiments using primary human T-ALLs.3,4 Furthermore, unlike HSCs that have been purified to ∼ 50% homogeneity,7 to our knowledge, no group has been able to purify T-ALL LSCs to a similar extent, which represents a major obstacle to studying the genetic and epigenetic determinants of self-renewal. Nevertheless, our data clearly show that oncogenic Notch signals do not expand HSC populations. Thus, the pathways downstream of Notch that drive self-renewal in T-ALL39,40 are inoperative in HSCs. For example, myc, a critical regulator of Notch-induced T-cell transformation and self-renewal of T-ALL cell lines, was not induced in Notch-expressing HSPCs (supplemental Figure 4).
There has been a longstanding debate over the effects of supraphysiologic Notch signaling on hematopoietic stem and progenitor cells. Although in some studies authors suggested a positive effect,27-32,41-44 other studies suggested a negative effect.45,46 Our findings show that Notch stimulates murine HSPCs over the short term but over longer periods depletes this population. Thus, the positive effects of supraphysiologic Notch signaling observed in earlier studies may have been produced through effects on differentiated multipotential precursors rather than LT-HSCs, which are rare and difficult to study via use of the earlier 4-color flow cytometric techniques. For example, an earlier report showing that supraphysiologic Notch signaling expands Sca-1+/Lineage− cells was interpreted as evidence showing that Notch signaling expands HSCs.27 However, our data suggest that these cells are likely to be immature T cells, not HSCs (supplemental Figure 2). The observed outgrowth of Sca-1+Lineage− cells seen previously27 likely reflects induction of the T-cell lineage by Notch rather than HSC expansion. We have used the latest in vivo flow cytometric methods to separate the effects of supraphysiologic Notch signaling on LT-HSC and more differentiated multipotential precursors. In so doing, we may have resolved some of the longstanding debate surrounding the effects of supraphysiologic Notch signaling on hematopoietic stem and precursor cells and highlighted differing requirements for Notch in LT-HSC and leukemic stem cell self-renewal.
Because of the deleterious effects of excessive Notch signaling on HSCs, Notch signaling must be tightly regulated. The cosubmitted manuscript from Maeda and coworkers underscores one level of regulatory control, restraint of Notch signaling by LRF.47 In LRF-deficient mice, Notch ligand is overexpressed, resulting in up-regulation of Notch1 signaling to supraphysiologic levels and depletion of LT-HSCs. Thus, both our study and those of the Maeda group link the loss of HSC self-renewal to supraphysiologic Notch signaling.
In contrast to our present work, several other authors have examined the role of physiologic signaling of Notch in LT-HSCs. Studies in which they used transgenic mice carrying a dominant-negative form of Mastermind-like protein (ie, DN-MAML), Rbpj-deficient mice, Jagged1-deficient mice, Notch1-deficient mice, and/or Notch2-deficient mice have shown that Notch is dispensable for maintenance of adult HSCs under physiologic and homeostatic conditions.11-13,48 A recently established database (www.immgen.org) shows low levels of Notch receptors and targets (Hes and Hey family members) in purified HSCs, consistent with a dispensable physiologic role. In contrast, Notch signaling, in particular Notch2, may influence the kinetics of optimal LT-HSC recovery after injury.48 Although our current study has no bearing on physiologic roles of Notch signaling to better shed light on these studies, they nonetheless show that the supraphysiologic levels of Notch signaling that drive leukemic stem cell self-renewal antagonize hematopoietic stem cell self–renewal.
Our results caution translational research efforts that stimulate Notch signaling to expand stem cells for clinical applications49 or to treat myeloid tumors where Notch may function as a tumor suppressor.38 Hematopoietic stem cell transplantation (HSCT) is a potentially curative strategy in many different hematologic disorders. However, stem cells for HSCT frequently are limiting. To increase stem cells, Varnum-Finney et al8 and Delaney et al9 stimulated HSPCs ex vivo with plate-bound Delta1-Ig before transplantation into mice. This method of Notch activation differs from our methods with regard to duration and dose. In their study, low levels of Notch ligands promoted short-term reconstitution. Greater levels of ligand induced apoptosis.9 Although these groups have not yet shown that their methods expand LT-HSCs, it is clear that they expand short-term progenitors. Indeed, their methods enhanced generation of HSCs with short-term repopulating ability and increased myeloid reconstitution in human HSCT recipients.10
The patients in this study did not attain long-term multilineage engraftment with these cells. However, it should be noted that HSCT was performed with T cell–depleted units, which have limited potential to engraft. One may be tempted to advocate for stronger levels of Notch signaling to attain long-term multilineage engraftment. In contrast, our findings suggest the opposite. Raising Notch signaling to stronger levels may deplete LT-HSCs. Recent work from Butler et al suggests that a better solution is to expose HSCs to Notch ligands and angiocrine factors presented by endothelial cells; however, it is not yet evident whether the Notch ligands are acting directly on the HSCs or are important to maintain the HSC niche.50 Similarly, Itch-deficient mice exhibit increased LT-HSC numbers and function.51 Although Itch deficiency increases Notch signaling, it also dysregulates other pathways that may influence HSCs.51 When we take these studies together, we note there seems to be a fine balance between Notch-driven expansion, Notch-driven differentiation, and Notch-driven exhaustion, one that has yet to be fully understood and requires further study. The successful use of Notch to expand LT-HSCs will likely require careful titration of the dose and duration of Notch signals9 as well as other supportive factors. Thus, although it is clear that high doses and constitutive activation of Notch are detrimental, it remains to be seen whether ligand-based methods of Notch stimulation, such as advocated by the Varnum-Finney et al8 and Delaney et al,9 can be optimized to support LT-HSC self-renewal.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Hong Sai, Candace Romany, and M. Eden Childs for excellent technical assistance; Ben Stanger for providing reagents; and Hudan Liu, Kosta Pajcini, and Ivan Maillard for critically reviewing the manuscript. They are grateful to the following cores at the University of Pennsylvania: mouse husbandry (ULAR), the Abramson Cancer Center Flow Cytometry Core, and the AFCRI Cores.
This study was supported by grants from the National Institutes of Health (AIO47833, CA119070) and the Leukemia & Lymphoma Society SCOR Program to W.S.P. and J.C.A. M.Y.C. was supported by a career development award from the National Cancer Institute (5K08 CA120544-02).
National Institutes of Health
Authorship
Contribution: M.Y.C., O.S., and L.X. performed research and collected data; M.Y.C., J.C.A., and W.S.P. designed the research, interpreted data, and wrote the manuscript; and M.Y.C. also analyzed data, performed statistical analysis, and generated all figures.
Conflict-of-interest disclosure: W.S.P. owns individual stock in Pfizer and Amgen. The remaining authors declare no competing financial interests.
Correspondence: Mark Y. Chiang, Rm 2043, Taubman BSRB, 109 Zina Pitcher Pl, Ann Arbor, MI 48109-2200; e-mail: markchia@umich.edu; or Warren S. Pear, 654 BRB II/III, 421 Curie Blvd, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA 19104; e-mail: wpear@mail.med.upenn.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal