Key Points
CpG(A)-siRNA oligonucleotides allow for targeting genes specifically in human TLR9+ immune cells and blood cancer cells.
Tumoricidal and immunostimulatory properties of CpG(A)-STAT3 siRNA provide a novel therapeutic opportunity for hematologic malignancies.
Abstract
STAT3 operates in both cancer cells and tumor-associated immune cells to promote cancer progression. As a transcription factor, it is a highly desirable but difficult target for pharmacologic inhibition. We have recently shown that the TLR9 agonists CpG oligonucleotides can be used for targeted siRNA delivery to mouse immune cells. In the present study, we demonstrate that a similar strategy allows for targeted gene silencing in both normal and malignant human TLR9+ hematopoietic cells in vivo. We have developed new human cell-specific CpG(A)-STAT3 siRNA conjugates capable of inducing TLR9-dependent gene silencing and activation of primary immune cells such as myeloid dendritic cells, plasmacytoid dendritic cells, and B cells in vitro. TLR9 is also expressed by several human hematologic malignancies, including B-cell lymphoma, multiple myeloma, and acute myeloid leukemia. We further demonstrate that oncogenic proteins such as STAT3 or BCL-XL are effectively knocked down by specific CpG(A)-siRNAs in TLR9+ hematologic tumor cells in vivo. Targeting survival signaling using CpG(A)-siRNAs inhibits the growth of several xenotransplanted multiple myeloma and acute myeloid leukemia tumors. CpG(A)-STAT3 siRNA is immunostimulatory and nontoxic for normal human leukocytes in vitro. The results of the present study show the potential of using tumoricidal/immunostimulatory CpG-siRNA oligonucleotides as a novel 2-pronged therapeutic strategy for hematologic malignancies.
Introduction
The proliferation and survival of the majority of hematologic cancers depends on constitutive activity of STAT transcription factors.1,2 The first evidence linking STAT activity with human blood cancer was derived from studies on multiple myeloma (MM). Permanent activity of STAT3 observed in myeloma cells was critical for their survival because of up-regulation of antiapoptotic BCL-XL protein.3 Later reports identified constitutive activation of STAT3 not only in myeloma but also in other hematologic malignancies, with the highest frequency in B-cell lymphoma (BCL) and acute myeloid leukemia (AML) patient blasts.1,4,5 The presence of activated STAT3 in leukemic blasts was associated with decreased disease-free survival of AML patients.4 As a point of convergence for downstream signaling from cytokine and growth factor receptors, STAT3 plays a critical role in mediating cross-talk within the tumor microenvironment, which promotes tumor immune tolerance, vascularization, and metastasis.6 Because STAT3 operates in both cancer cells and nonmalignant tumor-associated cells, it represents a highly desirable target for cancer therapy.6 These important findings instigated numerous attempts to develop STAT3 inhibitors; however, pharmacologic inhibition of a protein lacking enzymatic activity is challenging.4,7 An additional complication is the close structural similarity between oncogenic STAT3 and functionally distinct STAT1, a transcriptional factor critical for generation of antitumor immunity by IFNs.8,9 The tyrosine kinase inhibitors upstream from STAT3, such as JAK, SRC, c-KIT, and FLT3 in leukemia, gained attention as promising blood cancer therapeutics.4 However, the effect of small-molecule drugs, including FLT3 inhibitors, in most clinical trials was short-lived.10,11 Other conventional treatment regimens for hematologic malignancies are limited by the high toxicity to normal tissues, development of drug resistance, and low disease-free survival rates.12
The emergence of therapeutic strategies based on RNA interference (RNAi) created a unique opportunity to silence any disease-related target gene.13,14 The major obstacle in the clinical application of RNAi is targeted siRNA delivery into the cells of interest15,16 and the sensitivity of the immune system to stimulation by nucleic acids.17 However, immune cells may themselves be essential therapeutic targets in cancer therapy.6,18,19 We have demonstrated recently that ligands for intracellular receptors, such as TLR9, can be used as targeting moieties for cell-specific siRNA delivery.20 Chemically synthesized CpG-siRNA molecules, generated by linking siRNA to a CpG oligodeoxyribonucleotide (ODN), targeted and silenced genes specifically in mouse TLR9+ immune cells including dendritic cells (DCs), macrophages, and B cells in vitro and in vivo.20,21 We demonstrated that CpG-Stat3 siRNA treatment disrupted immunosuppressive signaling network in several solid-tumor models, resulting in a potent antitumor immunity in mice.20,22
In contrast to the mouse system, expression of human TLR9 in the steady state is mostly limited to DCs, although it can become up-regulated under inflammatory conditions.23,24 TLR9 is commonly expressed in many hematologic malignancies, including AML, MM, and BCL.25-28 Activation of TLR9 was shown either to enhance antigen-presenting functions or to induce apoptosis of primary malignant B cells.27,29 TLR9 agonists have been tested in numerous clinical trials as anticancer reagents for the treatment of hematologic malignancies including AML, MM, and BCL.29,30 They were proven to be safe and well-tolerated by patients and did not seem to induce adverse effects such as tumor cell proliferation and survival, which have been reported in some in vitro studies.25,27,29,31 However, the TLR9 agonists used as single agents or even combined with vaccinations failed to overcome strongly immunosuppressive tumor environment in cancer patients.29,32 We have shown previously that STAT3 is an important negative feedback regulator that restricts the immunostimulatory effects of several TLRs, including TLR9.33 The elimination of STAT3 combined with TLR9 triggering was shown to induce potent immunostimulatory effects in mice.20 These results underscore the need for combining TLR9 agonists with treatment strategies disrupting immunosuppressive effects of the tumor microenvironment. As demonstrated recently by Brody et al in a phase 1/2 study, local radiotherapy combined with intratumoral (IT) injections of CpG-ODN succeeded in activating tumor-specific memory T cells in lymphoma patients.30
Tumor cells of hematopoietic origin pose special problems for nonviral siRNA delivery because of their low transfection efficiency.34 In the present study, we demonstrate that TLR9-mediated siRNA delivery using type-A CpG-ODN linked to siRNA molecules induces targeted gene silencing in normal human immune cells and in malignant hematopoietic cells. CpG(A)-siRNA conjugates can overcome the limitations of small-molecule drugs by expanding the list of therapeutic targets in TLR9+ hematologic malignancies to crucial yet currently nondruggable molecules. Our TLR9 cell-specific siRNA delivery approach is particularly suited for targeting STAT3, which functions both in cancer cells and in tumor-associated immune cells.
Methods
Cell culture
Human RPMI 8226, KMS-11, U266 myeloma, and mouse A20 lymphoma cells were purchased from ATCC. The human KG1a, MonoMac1, MonoMac6, MOLM13, MV4-11, THP1, and TF1 leukemic cell lines were obtained from Dr D. Tenen (Beth Israel Deaconess Medical Center, Boston, MA). The primary leukemic samples were from R.B. (City of Hope [CoH]) with written, informed consent of all patients. Sample acquisition was approved by the CoH institutional review board in accordance with the Declaration of Helsinki. Healthy PBMCs were derived from anonymous donors by the Clinical Immunobiology Correlative Studies Laboratory (CICSL) at CoH or were purchased from CTL. PBMC-derived monocytes were cultured for 5 days in 12-well cell-culture plates using RPMI 1640 medium containing 10% FBS together with GM-CSF (100 ng/mL) and IL-4 (50 ng/mL) or Flt3-L (100 ng/mL) to generate myeloid (CD1c+) or plasmacytoid (CD303+) DCs, respectively.
Oligonucleotide design and synthesis
The sequences of mouse cell-specific CpG1668(B)-siRNAs have been described previously.20 To generate human cell-specific CpG-siRNAs, the D19 ODNs were linked to antisense (AS) strands of siRNAs using 5 units of the C3 carbon chain (CH2)3 (Glen Research). The resulting constructs were hybridized to complementary siRNA sense (SS) strands to generate CpG-siRNA conjugates (deoxyribonucleotides are underlined and asterisks indicate phosphothioation sites): Human STAT3 siRNA (SS): 5′ GGAAGCUGCAGAAAGAUACGACUGA 3′; CpG(A)-STAT3 siRNA(AS): 5′ G*G*TGCATCGATGCAGG*G*G*G*G-linker-UCAGUCGUAUCUUUCUGCAGCUUCCGU 3′; human BCL-XL siRNA (SS): 5′ GAGCUAUCAGGAACAGCUAUGGGAG 3′; CpG(A)-BCL-XL siRNA(AS): 5′ G*G*TGCATCGATGCAGG*G*G*G*G-linker-CUCCCAUAGCUGUUCCUGAUAGCUCCC 3′; Luciferase (Luc) siRNA (SS): 5′ GGUUCCUGGAACAAUUGCUUUUACA 3′; CpG(A)-Luc siRNA(AS): 5′ G*G*TGCATCGATGCAGG*G*G*G*G-linker-UGUAAAAGCAAUUGUUCCAGGAACCAG 3′; control RNA (SS): 5′ UCCAAGUAGAUUCGACGGCGAAGTG 3′; CpG(A)-control RNA(AS): 5′ G*G*TGCATCGATGCAGG*G*G*G*G-linker-CACUUCGCCGUCGAAUCUACUUGGAUU 3′.
For uptake studies, CpG(A)-STAT3 siRNAs were labeled using fluorescein (FITC) or Cy3. Conjugates were added to culture media with reduced 5% FBS to limit degradation by nucleases.
qPCR and protein assays
For quantitative PCR (qPCR), total RNA was extracted from cultured or primary cells using the RNeasy Plus kit (QIAGEN). After cDNA synthesis using iScript kit (Bio-Rad), samples were analyzed using probes from the Universal Probe Library and specific primer pairs for human STAT3: 5′-CTGCCTAGATCGGCTAGAAAAC-3′, 5′-CCCTTTGTAGGAAACTTTTTGC-3′, UPL #25; TLR9: 5′-TGTGAAGCATCCTTCCCTGTA-3′, 5′-GAGAGACAGCGGGTGCAG-3′, UPL #56; BCL-XL: 5′-GCTGAGTTACCGGCATCC-3′, 5′-TTCTGAAGGGAGAGAAAGAGATTC-3′, UPL #10; IL-12/p35: 5′-CACTCCCAAAACCTGCTGAG-3′, 5′-CAATCTCTTCAGAAGTGCAAGG-3′, UPL #50; and IL-12/p40: 5′-CCCTGACATTCTGCGTTCA-3′, 5′-AGGTCTTGTCCGTGAAGACTCTA-3′, UPL #37. TBP, GAPDH, and 18S were detected using Roche's Reference Gene Assays. ProbeFinder Version 2.45 software (Roche) was used to design probe-primer sets. Sequence-specific amplification was analyzed on the CFX96 Real-Time PCR Detection System (Bio-Rad). The data were normalized to the GAPDH, TBP, or 18S expression and the relative expression levels were calculated using the 2−ΔΔCt method. Western blot analysis to detect TLR9, STAT3, BCL-XL, and β-actin expression was performed as described previously.19 Concentrations of cytokines in cell-culture supernatants from cultured primary PBMCs or DCs were measured at the CICSL core using Bio-Plex arrays and the Luminex system (Bio-Rad).
Flow cytometry
Single-cell suspensions of cultured cells or tumor tissues freshly prepared by mechanic tissue disruption and collagenase D/DNase I treatment as described previously19 were stained with 7-amino-actinomycin D and/or fluorochrome-coupled annexin V (BD Biosciences). For extracellular staining of immune markers, cultured myeloid DC (mDC) or plasmacytoid DC (pDC) cells were stained with different combinations of fluorochrome-labeled antibodies to CD11c, CD303, HLA-DR, or CD86 (eBiosciences). Fluorescence data were collected on an Accuri C6 flow cytometer (BD Biosciences) and analyzed using CFlow Version 1.0 or FlowJo Version 7.6.5 software (TreeStar).
Confocal microscopy
For confocal microscopy, cells cultured in 24-well plates on number 1.5 coverslips were fixed using 2% paraformaldehyde, washed, stained with anti-EEA1 (Santa Cruz Biotechnology) primary and Alexa Fluor 555–coupled secondary antibodies (Life Technologies), mounted in Vectashield Hard-set mounting medium with DAPI (Vector Laboratories), and analyzed by confocal microscopy using a Zeiss LSM510 META NLO Axiovert 200M inverted microscope equipped with a C-Apochromat 40×/1.2 water immersed objective and a Hamamatsu R6357 PMT detector. We used Zeiss LSM Version 4.2 software, SP1 for image acquisition, and Zeiss LSM Image Browser Version 4.2.0.121 for postacquisition analysis and preparation for publication. All fixed cell samples were stored at +4°C and scanned at ambient temperature.
RACE assay
The 5′-rapid amplification of cDNA ends (RACE) assay was performed using the GeneRacer Kit (Life Technologies) using a modified manufacturer's protocol. Briefly, 150 ng of total RNA isolated from cultured pDCs or MV4-11 tumors was ligated to 250 ng of RNA adapter (Life Technologies), reverse transcribed using the iScript kit (Bio-Rad), and amplified by touchdown PCR with adapter- and gene-specific primers. PCR products were used as template in nested PCR with forward adapter-specific (Life Technologies) and reverse sequence–specific (5′-CAGGCACCAGGAGGCACTTGTCT-3′) primers. Nested PCR products were separated on 1% agarose gel and the major products of predicted length were purified using the QIAquick gel extraction kit (QIAGEN). Purified products were sequenced using Hitachi AB Model 3730 DNA Analyzer at the CoH DNA Sequencing Core. Derived sequences were aligned with genomic sequences of STAT3 using Vector NTI Version 10 software (Life Technologies).
In vivo experiments
Mouse care and experimental procedures were performed under pathogen-free conditions in accordance with established institutional guidance and approved protocols from the institutional animal care and use committees of the CoH. For subcutaneous (SC) tumor challenge, we injected 1-5 × 106 of KMS-11, MonoMac6, MV4-11, or A20 tumor cells into immunodeficient 7- to 8-week-old NOD/SCID/IL-2RγKO (NSG) mice or into Balb/C mice. After tumors reached average size of approximately 5-8 mm, mice received daily IT injections of 100 μg (5 mg/kg) of the indicated CpG-siRNAs or PBS. In A20 cells, established tumors were irradiated locally with single collimated 20-Gy dose from a Cs137 source using a MARK I irradiator (J. L. Shepherd). Tumor growth was monitored every other day with caliper measurements.
Immunofluorescence staining
Flash-frozen tumor specimens were fixed in paraformaldehyde, permeabilized with methanol, and stained with antibodies specific to neutrophils (7/4; Cedarlane Labs) and active-caspase 3 (Cell Signaling Technology), then detected with fluorochrome-coupled secondary antibodies (Invitrogen). After staining the nuclei using Hoechst 33342 (Life Technologies), slides were mounted in Vectashield (Vector Laboratories) and analyzed by fluorescence microscopy. We present the representative results from 2 independent experiments using samples isolated from 4 mice.
Statistical analysis
Unpaired t test was used to calculate the 2-tailed P value to estimate statistical significance of differences between 2 treatment groups in the whole study. One- or 2-way ANOVA plus Bonferroni posttest were applied to assess the statistical significance of differences between multiple treatment groups or in tumor growth kinetics between treatment groups, respectively. Statistically significant P values are indicated in the figures with asterisks. Data were analyzed using Prism Version 4.0 software (GraphPad).
Results
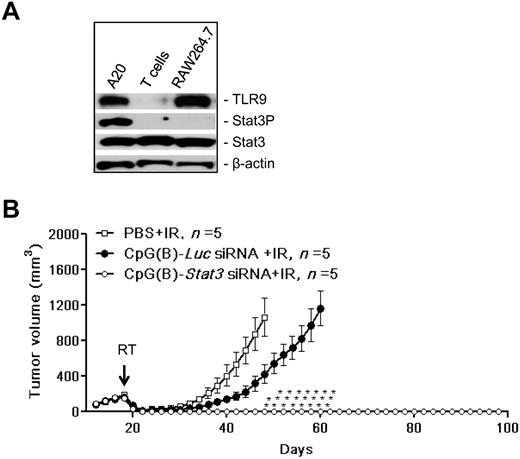
CpG-Stat3 siRNA amplifies the effects of local radiation therapy against BCL
We have shown previously that TLR9 triggering with simultaneous elimination of the Stat3 immune checkpoint control generates tumor antigen–specific immune responses in mice.20 Pioneering studies by others demonstrated that combining TLR9 agonists with local tumor irradiation led to synergistic immunotherapeutic effects in solid and blood cancers such as BCL.29,30,35 Therefore, in the present study, we assessed whether CpG(B)-Stat3 siRNA molecules can further enhance the effect of local radiotherapy by reducing the survival/radioresistance of TLR9+ tumors and by augmenting the immunostimulatory effects of TLR9 triggering on both tumor and immune cells. For our proof-of-principle experiments in Balb/C mice, we selected TLR9+ A20 BCL cells, which showed high levels of constitutively active Stat3 (Figure 1A). As shown in Figure 1B, IT injections of CpG(B)-Stat3 siRNA combined with local irradiation of A20 lymphomas resulted in complete tumor rejection in all treated mice. Although the treatment with control CpG(B)-Luc siRNA delayed A20 growth, it did not prevent later tumor relapse. Moreover, radiotherapy combined with CpG(B)-Stat3 siRNA generated long-term protective immunity against the primary tumor. None of these mice (0 of 5) developed tumors when rechallenged with the same number of A20 tumor cells injected in the opposite flank. These results prompted us to develop the CpG-siRNA strategy for clinical translation as a novel approach to immunotherapy of human TLR9+ blood malignancies.
Therapeutic effect of local radiation therapy combined with CpG-Stat3 siRNA treatment on TLR9+ BCL in mice. (A) Stat3 is constitutively activated in TLR9+ A20 BCL cells. Shown are results from the Western blot analysis of TLR9, activated and total Stat3 compared with β-actin used as a loading control; TLR9+ RAW264.7 macrophages and TLR9− CD4 T cells were used for reference. (B) Mice with established SC growing A20 BCL were injected IT using CpG(B)-Stat3 siRNA, control CpG(B)-Luc siRNA, or PBS only twice daily (on days 16 and 17) before radiotherapy and then 6 hours after radiotherapy and 2 more times every other day (on days 20 and 22). Local tumor irradiation at a single 20-Gy dose on day 18 is indicated by an arrow. Shown is the A20 tumor growth kinetics in the experiment using 5 mice per each treatment group. Data are shown as means ± SEM. Statistically significant differences between groups treated with CpG(B)-Stat3 siRNA and controls treated with CpG(B)-Luc siRNA are indicated with asterisks.
Therapeutic effect of local radiation therapy combined with CpG-Stat3 siRNA treatment on TLR9+ BCL in mice. (A) Stat3 is constitutively activated in TLR9+ A20 BCL cells. Shown are results from the Western blot analysis of TLR9, activated and total Stat3 compared with β-actin used as a loading control; TLR9+ RAW264.7 macrophages and TLR9− CD4 T cells were used for reference. (B) Mice with established SC growing A20 BCL were injected IT using CpG(B)-Stat3 siRNA, control CpG(B)-Luc siRNA, or PBS only twice daily (on days 16 and 17) before radiotherapy and then 6 hours after radiotherapy and 2 more times every other day (on days 20 and 22). Local tumor irradiation at a single 20-Gy dose on day 18 is indicated by an arrow. Shown is the A20 tumor growth kinetics in the experiment using 5 mice per each treatment group. Data are shown as means ± SEM. Statistically significant differences between groups treated with CpG(B)-Stat3 siRNA and controls treated with CpG(B)-Luc siRNA are indicated with asterisks.
CpG(A)-siRNA–mediated target gene silencing in human immune cells
We adapted CpG-siRNA conjugates for targeted gene silencing in normal and malignant human hematopoietic cells, which are a challenge for nucleic acid delivery. For the design of the human cell–specific CpG-siRNA (Figure 2A), we used a 25/27mer Dicer-substrate STAT3 siRNA selected from more than 20 sequences based on the high silencing efficacy (> 95%).36 The 25/27mer siRNA sequence was selected to enable intracellular uncoupling of both parts of the molecule by Dicer endonuclease.20 We conjugated STAT3 siRNA to both type-B (CpG7909) and type-A (D19) CpG-ODNs. CpG(B)/CpG7909 was shown previously to activate B-cell populations and pDCs, whereas CpG(A)/D19 is known for a broad target spectrum, including human pDCs, mDCs, normal B cells, and B cell–derived malignant cells.37-39 Our initial studies suggested that CpG(A)-STAT3 siRNA may avoid induction of IL-6 and IL-10 cytokines, which are known to activate STAT3 as a negative feedback regulator of TLR9 signaling in immune cells. As expected, both IL-6 and IL-10 were secreted by human PBMCs treated using type-B but not type-A CpG oligonucleotides and conjugates thereof (supplemental Figure 1A-B, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). CpG(A)-STAT3 siRNA also had only a minimal effect on IFNα production, which is known as a potential trigger of immunotoxicity in vivo.17 In contrast, IFNα was highly elevated in PBMCs treated with unconjugated type-A ODN (supplemental Figure 1C). Based on these results, the CpG(A)-siRNA design was selected for further studies. First, we compared uptake of CpG(A)-STAT3 siRNA and unconjugated STAT3 siRNA by primary human immune cells using flow cytometry. Both molecules were labeled with Cy3 fluorochrome on the 5′ end of the SS strand to follow the localization of the siRNA part of the molecule. As shown in Figure 2B, CpG(A)-STAT3 siRNACy3 was rapidly internalized by several populations of primary human immune cells in the absence of any transfection reagents. Within 30-60 minutes of incubation, CpG(A)-STAT3 siRNACy3 was already internalized by the majority of CD14+ monocytes, cultured CD1c+ mDCs, CD303+ pDCs, and, to a lesser extent, by CD19+ B cells (Figure 2B left column). The maximum conjugate uptake was observed in concentrations ranging from 100-250nM (Figure 2B middle column). In contrast, neither naive T cells (CD3+) nor natural killer (NK) cells (CD56+) internalized the CpG(A)-STAT3 siRNACy3. As expected, the internalization of unconjugated siRNACy3 was negligible even after 18 hours of incubation at the highest concentration (Figure 2B right column). We also confirmed that CpG(A)-STAT3 siRNAs in doses of up to 500nM did not affect the viability or levels of mitochondrial enzymes in primary human PBMCs (supplemental Figure 2). These data suggest that the CpG part of the conjugate facilitates the delivery of siRNA into several human immune cell populations without detectable signs of toxicity.
Design of the CpG(A)-STAT3 siRNA conjugate for targeting STAT3 in human TLR9+ cells. (A) Structure and sequence of the CpG(A)-STAT3 siRNA: CpG(A) ODN (D19 sequence) was conjugated to the STAT3 siRNA guide (AS) strand through a flexible carbon chain linker; deoxyribonucleotides are underlined; asterisks indicate phosphothioation sites; shown is a position of the fluorochrome (Cy3) at the 5′ end of the STAT3 siRNA passenger strand. (B) Targeted delivery of STAT3 siRNA into various populations of primary human immune cells in vitro. Human PBMCs were incubated in the presence of fluorescently labeled CpG(A)-STAT3 siRNACy3 or unconjugated STAT3 siRNACy3 in various concentrations for the times indicated without any transfection reagents. Percentages of Cy3+CD14+ monocytes, CD303+ (BDCA2+) pDCs, CD1c+ (BDCA1+) mDCs, CD19+ B cells, CD56+ NK cells, and CD3+ T cells were assessed by flow cytometry. Similar results were obtained from 3 independent experiments. (C) CpG(A)-STAT3 siRNA treatment leads to STAT3 gene silencing in various immune cell populations. Monocytes, mDCs, pDCs, and B cells were incubated with 500nM CpG-siRNAs targeting STAT3 or Luc (as negative control) for 18 hours. STAT3 expression was measured using qPCR. Shown are results normalized to TBP gene expression levels from 1 of 3 independent experiments; STAT3 expression level in control CpG-Luc siRNA-treated samples was set as 100%. Data are shown as means ± SEM (n = 3). Statistically significant differences are indicated with asterisks. ***P = .0005; **P = .0034; *P = .03. (D) TLR9 is expressed in target immune cell populations sensitive to CpG(A)-siRNA–mediated gene silencing. TLR9 expression was measured by qPCR in enriched populations of monocytes, T cells, and B cells or in cultured mDCs and pDCs. The results are representative of 2 independent experiments performed in triplicate. Data are shown as means ± SEM. (E) STAT3 silencing by CpG(A)-siRNA conjugates depends on TLR9 targeting and activation. STAT3 expression was assessed by qPCR in cultured pDCs treated for 18 hours using CpG(A) alone, CpG(A) linked to nonsilencing control RNA, nontargeting GpC(A)-STAT3 siRNA, or CpG(A)-STAT3 siRNA. Shown are results from 1 of 2 independent experiments performed in triplicate. Data are shown as means ± SEM.
Design of the CpG(A)-STAT3 siRNA conjugate for targeting STAT3 in human TLR9+ cells. (A) Structure and sequence of the CpG(A)-STAT3 siRNA: CpG(A) ODN (D19 sequence) was conjugated to the STAT3 siRNA guide (AS) strand through a flexible carbon chain linker; deoxyribonucleotides are underlined; asterisks indicate phosphothioation sites; shown is a position of the fluorochrome (Cy3) at the 5′ end of the STAT3 siRNA passenger strand. (B) Targeted delivery of STAT3 siRNA into various populations of primary human immune cells in vitro. Human PBMCs were incubated in the presence of fluorescently labeled CpG(A)-STAT3 siRNACy3 or unconjugated STAT3 siRNACy3 in various concentrations for the times indicated without any transfection reagents. Percentages of Cy3+CD14+ monocytes, CD303+ (BDCA2+) pDCs, CD1c+ (BDCA1+) mDCs, CD19+ B cells, CD56+ NK cells, and CD3+ T cells were assessed by flow cytometry. Similar results were obtained from 3 independent experiments. (C) CpG(A)-STAT3 siRNA treatment leads to STAT3 gene silencing in various immune cell populations. Monocytes, mDCs, pDCs, and B cells were incubated with 500nM CpG-siRNAs targeting STAT3 or Luc (as negative control) for 18 hours. STAT3 expression was measured using qPCR. Shown are results normalized to TBP gene expression levels from 1 of 3 independent experiments; STAT3 expression level in control CpG-Luc siRNA-treated samples was set as 100%. Data are shown as means ± SEM (n = 3). Statistically significant differences are indicated with asterisks. ***P = .0005; **P = .0034; *P = .03. (D) TLR9 is expressed in target immune cell populations sensitive to CpG(A)-siRNA–mediated gene silencing. TLR9 expression was measured by qPCR in enriched populations of monocytes, T cells, and B cells or in cultured mDCs and pDCs. The results are representative of 2 independent experiments performed in triplicate. Data are shown as means ± SEM. (E) STAT3 silencing by CpG(A)-siRNA conjugates depends on TLR9 targeting and activation. STAT3 expression was assessed by qPCR in cultured pDCs treated for 18 hours using CpG(A) alone, CpG(A) linked to nonsilencing control RNA, nontargeting GpC(A)-STAT3 siRNA, or CpG(A)-STAT3 siRNA. Shown are results from 1 of 2 independent experiments performed in triplicate. Data are shown as means ± SEM.
We also examined whether CpG(A)-STAT3 siRNA uptake resulted in target gene silencing in primary and cultured immune cell populations. Fresh, magnetically separated monocytes and B cells or cultured mDCs and pDCs were incubated for 18 hours with 500nM CpG(A)-siRNA conjugates targeting STAT3 or luciferase genes, the latter being used as a negative control. As shown in Figure 2C, STAT3 expression was significantly reduced by approximately 60% in both cultured mDCs and pDCs. The STAT3 knockdown was less pronounced in primary B cells, likely because of weaker conjugate uptake, and was undetectable in monocytes. CpG-siRNA–mediated gene silencing in mouse immune cells requires TLR9 expression.20 Because TLR9 is less ubiquitously expressed in humans than in rodents, we examined whether the lack of TLR9 limited the silencing effect of CpG(A)-STAT3 siRNA. We assessed the expression of TLR9 mRNA in studied immune cell populations using qPCR. As expected, TLR9 was expressed at high levels in quiescent human pDCs and B cells and moderately in mDCs, whereas it was almost undetectable in monocytes and T cells (Figure 2D). TLR9 expression is known to be sensitive to changing environmental conditions and was shown to be augmented by certain proinflammatory cytokines.24 Similarly, monocyte differentiation into pDCs or mDCs in the presence of Flt3 ligand or GM-CSF/IL-4, respectively, up-regulated TLR9 (Figure 2D and supplemental Figure 3). To assess role of TLR9 triggering for induction of RNAi, we conjugated STAT3 siRNA to GpC-ODN with sequence identical to D19-ODN except for the inversion of a CpG motif. The GpC-ODN lacks the ability to trigger TLR9 activation, although it is still internalized by target cells.37 The GpC(A)-STAT3 siRNA failed to silence STAT3 in human pDCs as measured by qPCR (Figure 2E). Therefore, TLR9 activation is necessary for CpG(A)-siRNA–induced gene silencing in target immune cells.
Immunostimulatory properties of CpG(A)-STAT3 siRNA
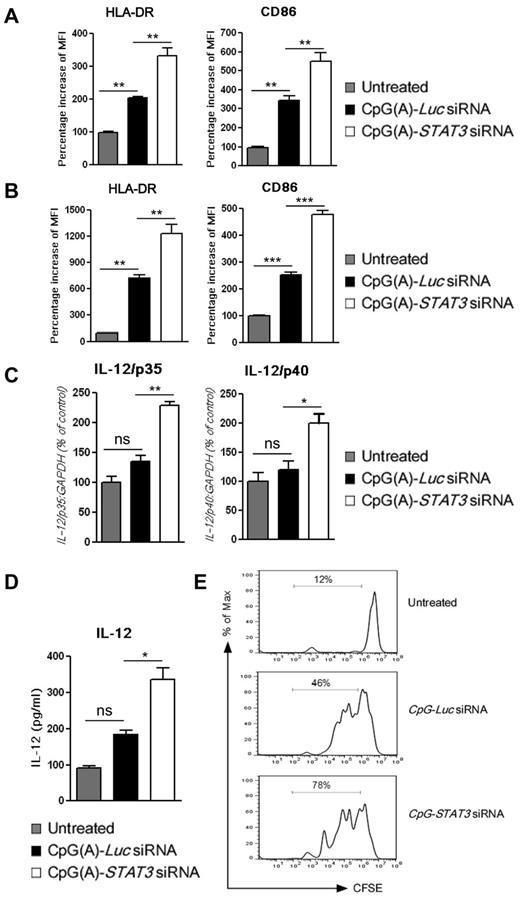
CpG(A)-STAT3 siRNA, which triggers TLR9 signaling while suppressing the inhibitory effect of STAT3, should have improved immunoadjuvant properties on human immune cells, as was observed previously in mice.20 We evaluated immunostimulatory effects of CpG(A)-STAT3 siRNA in monocyte-derived, cultured mDCs and pDCs. Immature mDCs (Figure 3A) or pDCs (Figure 3B) were incubated for 48 hours with 500nM CpG(A)-STAT3 siRNA or control CpG(A)-Luc siRNA and analyzed for immune activation marker expression by flow cytometry. Although both CpG(A)-siRNAs up-regulated surface expression of HLA-DR complex and the costimulatory molecule CD86, the effect of TLR9 triggering was strongly enhanced by STAT3 gene silencing in the tested DC populations. As shown by flow cytometric analysis, the presence of RNA part of the conjugate did not contribute to the immunostimulatory effect of CpG-ODN (supplemental Figure 4). Both CpG(A) alone and nonsilencing control conjugate, CpG(A)-conRNA, up-regulated HLA-DR and CD86 in pDCs to similar levels, whereas conjugate that does not activate TLR9 (GpC-STAT3 siRNA) failed to show any effect. The pDC activation was correlated with increased expression of mRNAs for both p35 and p40 subunits of IL-12, a critical activator of NK- and T cell–mediated immunity (Figure 3C). We also detected significantly increased secretion of IL-12 in supernatants from pDCs cultured in the presence of CpG(A)-STAT3 siRNA, but not in CpG(A)-Luc siRNA-treated controls (Figure 3D). Finally, we verified that CpG(A)-STAT3 siRNA-induced pDC activation augmented their ability to induce T-cell proliferation in comparison to controls (Figure 3E). Together with lack of conjugate immunotoxicity (supplemental Figure 2), these results suggest that CpG(A)-STAT3 siRNA is a potent nontoxic immunoadjuvant in the human system.
CpG-STAT3 siRNA stimulates the immune activity of human dendritic cells in vitro. Cultured mDCs (A) or pDCs (B) were incubated for 48 hours in the presence of 500nM CpG-siRNAs targeting STAT3 or Luc (as a negative control). The surface expression of HLA-DR and CD86 immune activation markers on both DC populations was assessed by flow cytometry. (C-D) IL-12 expression in pDCs treated as described for panels A and B was evaluated in total RNA samples (C) or in cell-culture supernatants (D) using qPCR or Luminex assays, respectively. Presented are results averaged from 3 independent experiments and analyzed for statistical significance. Data are shown as means ± SEM (n = 3). ns indicates not significant. (E) STAT3 blocking in cultured human pDCs augments their immunostimulatory effect on T cells. Allogeneic CD3+ T cells were labeled using CFSE and incubated for 5 days at a 1:2 ratio with pDCs pretreated as indicated. T-cell expansion was assessed by CFSE dilution using flow cytometry.
CpG-STAT3 siRNA stimulates the immune activity of human dendritic cells in vitro. Cultured mDCs (A) or pDCs (B) were incubated for 48 hours in the presence of 500nM CpG-siRNAs targeting STAT3 or Luc (as a negative control). The surface expression of HLA-DR and CD86 immune activation markers on both DC populations was assessed by flow cytometry. (C-D) IL-12 expression in pDCs treated as described for panels A and B was evaluated in total RNA samples (C) or in cell-culture supernatants (D) using qPCR or Luminex assays, respectively. Presented are results averaged from 3 independent experiments and analyzed for statistical significance. Data are shown as means ± SEM (n = 3). ns indicates not significant. (E) STAT3 blocking in cultured human pDCs augments their immunostimulatory effect on T cells. Allogeneic CD3+ T cells were labeled using CFSE and incubated for 5 days at a 1:2 ratio with pDCs pretreated as indicated. T-cell expansion was assessed by CFSE dilution using flow cytometry.
TLR9-mediated siRNA delivery into human MM and AML cells
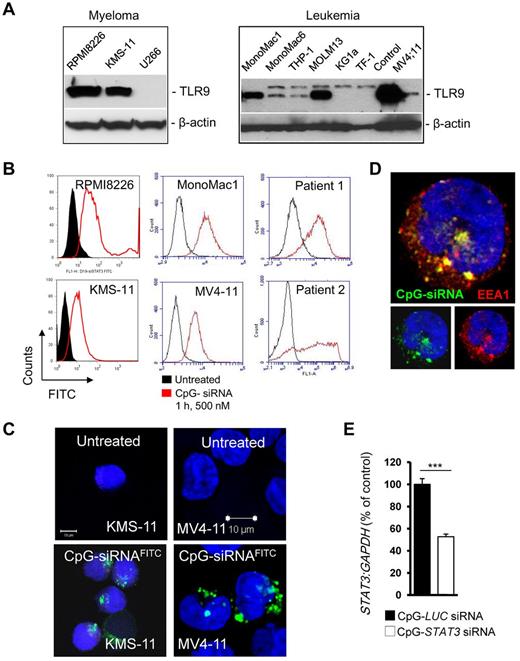
Previous studies demonstrated frequent expression of TLR9 mRNA in various malignancies of hematopoietic origin such as myeloma and B-cell lymphoma.28,31 We analyzed protein expression of TLR9 in 3 MM and 7 AML cell lines using Western blotting. TLR9 protein was present at moderate to high levels in 2 of 3 tested MM cells and in 5 of 7 AML cells, confirming earlier reports (Figure 4A). We also examined the ability of TLR9+ tumor cells to internalize CpG(A)-STAT3 siRNA using flow cytometry and confocal microscopy. After 1 hour of incubation with 500nM CpG(A)-STAT3 siRNAFITC, the majority of TLR9+ MM and AML cells, including primary leukemic blasts derived from AML patients, were FITC+ as detected by flow cytometry (Figure 4B). We used confocal microscopy to verify the intracellular localization of CpG(A)-STAT3 siRNAFITC in KMS-11 myeloma and MV4-11 leukemia cells (Figure 4C). After internalization, CpG(A)-STAT3 siRNAFITC was accumulated within early endosomes, as indicated by near complete colocalization with an early endosomal marker, EEA1 (Figure 4D). Finally, we determined the gene-silencing effect of CpG(A)-STAT3 siRNA in KMS-11 cells. As measured by qPCR, STAT3 mRNA expression was reduced by approximately half within 18 hours after incubation of KMS-11 cells with 500nM CpG(A)-STAT3 siRNA compared with a negative control (Figure 4E). The STAT3-silencing effect of CpG(A)-siRNA in rapidly dividing KMS-11 cancer cells was transient and decreased after prolonged cell culture (data not shown), which is likely an effect of limited stability of naked CpG-siRNA oligonucleotides in serum-containing media.20 Based on these results, we conclude that human TLR9+ hematopoietic tumor cells are appropriate targets for CpG-siRNA conjugates.
CpG-siRNA uptake and gene silencing in TLR9+ MM and AML cells. (A) The majority of tested MM (left) and AML (right) cells expressed high levels of TLR9 protein. Representative results from at least 2 Western blotting analyses are shown; positions of TLR9 and β-actin for loading control are indicated. (B) CpG-siRNA is quickly internalized by established MM and AML cells as well as by patient AML blasts in vitro. Cells were incubated with 500nM FITC-labeled CpG-STAT3 siRNA for 1 hour. The percentage of FITC+ cells was analyzed by flow cytometry. (C-D) CpG-STAT3 siRNA accumulated in early endosomes shortly after intracellular uptake. KMS-11 myeloma cells (C) and MV4-11 leukemia cells (C-D) were treated with 500nM CpG-STAT3 siRNAFITC for 1 hour. The intracellular localization of the conjugate was assessed by confocal microscopy. Green indicates CpG-STAT3 siRNAFITC; red, EEA1 (early endosome marker); and blue, nuclear staining with DAPI. (E) Target gene silencing in cultured KMS-11 cells incubated with 500nM CpG-STAT3 siRNA for 18 hours. Shown are results averaged from 4 independent experiments analyzed by qPCR and normalized to GAPDH.
CpG-siRNA uptake and gene silencing in TLR9+ MM and AML cells. (A) The majority of tested MM (left) and AML (right) cells expressed high levels of TLR9 protein. Representative results from at least 2 Western blotting analyses are shown; positions of TLR9 and β-actin for loading control are indicated. (B) CpG-siRNA is quickly internalized by established MM and AML cells as well as by patient AML blasts in vitro. Cells were incubated with 500nM FITC-labeled CpG-STAT3 siRNA for 1 hour. The percentage of FITC+ cells was analyzed by flow cytometry. (C-D) CpG-STAT3 siRNA accumulated in early endosomes shortly after intracellular uptake. KMS-11 myeloma cells (C) and MV4-11 leukemia cells (C-D) were treated with 500nM CpG-STAT3 siRNAFITC for 1 hour. The intracellular localization of the conjugate was assessed by confocal microscopy. Green indicates CpG-STAT3 siRNAFITC; red, EEA1 (early endosome marker); and blue, nuclear staining with DAPI. (E) Target gene silencing in cultured KMS-11 cells incubated with 500nM CpG-STAT3 siRNA for 18 hours. Shown are results averaged from 4 independent experiments analyzed by qPCR and normalized to GAPDH.
In vivo gene silencing using CpG(A)-siRNA conjugates
We evaluated the feasibility of using CpG(A)-siRNA for targeting survival signaling in TLR9+ tumors in vivo. First, we assessed the percentage of tumor cells penetrated by IT-injected conjugate. MV4-11 leukemia cells were injected SC into the immunodeficient NSG mice. The established MV4-11 tumors, which reached a size of approximately 100 mm3, were injected IT with a single 20- or 100-μg dose of fluorescently labeled CpG(A)-STAT3 siRNACy3. The percentages of viable Cy3+ cells were analyzed using flow cytometry in tumors harvested 3 hours after injection. The IT uptake of the CpG(A)-siRNA conjugate was dose dependent and reached 76% of viable cells within MV4-11 tumors at the 100-μg dose (Figure 5A). To verify whether local CpG(A)-siRNA delivery could reduce target protein expression in the whole TLR9+ tumor, we tested silencing of STAT3 and BCL-XL proteins, which are known for their survival-promoting role in blood cancers.1,4 Four daily injections of CpG(A)-siRNAs targeting BCL-XL (Figure 5B) or STAT3 (Figure 5C) consistently reduced protein levels of both targets by average 65% or 61%, respectively. As confirmed by qPCR, STAT3 mRNA expression was similarly reduced in CpG(A)-STAT3 siRNA-treated MV4-11 tumors (Figure 5D). To verify whether the observed effect of STAT3 knockdown in MV4-11 tumors resulted from RNAi, we used the 5′-RACE PCR assay. The presence of the 5′RACE product, which indicates STAT3 mRNA cleavage, was detectable only in tumors treated with CpG(A)-STAT3 siRNA but not CpG(A)-Luc siRNA (Figure 5E). We further confirmed by DNA sequencing that STAT3 mRNA cleavage occurred within the region targeted by STAT3 siRNA.
In vivo delivery of CpG-siRNAs induces RNAi and abrogates target gene expression in TLR9+ leukemia cells. (A) Dose-dependent uptake of IT injected CpG-STAT3 siRNA by TLR9+ MV4-11 cells. Tumor cells were injected SC into NSG mice. After tumors were established, mice were injected IT using 20 or 100 μg of Cy3-labeled CpG-STAT3 siRNA. The percentage of Cy3+ cells was analyzed by flow cytometry in cell suspensions prepared from tumors harvested 3 hours after injection. (B-C) IT injections of CpG(A)-siRNA can effectively silence expression of BCL-XL or STAT3 proteins in xenotransplanted MV4-11 leukemia. NSG mice were injected with MV4-11 AML cells. After tumors were established (day 7), mice were treated 4 times with daily IT injections of CpG(A)-siRNAs targeting BCL-XL (B) or STAT3 (C). Protein levels of both BCL-XL and STAT3 were evaluated using Western blotting with β-actin as a loading control in samples derived from single tumors for both experimental groups. Band intensities were quantified by densitometry using ImageJ Version 1.46 software based on identically exposed images. Shown are results from 1 of 2 independent experiments analyzed for statistical significance. **P = .003; ***P < .0001. (D-E) RNAi-mediated STAT3 gene knockdown in MV4-11 tumors treated using IT injections of CpG(A)-siRNAs as in panel B. (D) STAT3 gene silencing in samples from 4 individual tumors was verified by qPCR and normalized to TBP expression; shown are means ± SE. (E) CpG(A)-STAT3 siRNA-induced cleavage of STAT3 mRNA in MV4-11 tumors in vivo. Tumors injected IT using CpG(A)-siRNAs as indicated were harvested 18 hours later. Total RNA samples were analyzed by the 5′RACE-PCR assay followed by DNA sequencing detecting mRNA cleavage within the targeted region (partial sequences are shown); arrow indicates the major RACE product of the predicted length (300 nt).
In vivo delivery of CpG-siRNAs induces RNAi and abrogates target gene expression in TLR9+ leukemia cells. (A) Dose-dependent uptake of IT injected CpG-STAT3 siRNA by TLR9+ MV4-11 cells. Tumor cells were injected SC into NSG mice. After tumors were established, mice were injected IT using 20 or 100 μg of Cy3-labeled CpG-STAT3 siRNA. The percentage of Cy3+ cells was analyzed by flow cytometry in cell suspensions prepared from tumors harvested 3 hours after injection. (B-C) IT injections of CpG(A)-siRNA can effectively silence expression of BCL-XL or STAT3 proteins in xenotransplanted MV4-11 leukemia. NSG mice were injected with MV4-11 AML cells. After tumors were established (day 7), mice were treated 4 times with daily IT injections of CpG(A)-siRNAs targeting BCL-XL (B) or STAT3 (C). Protein levels of both BCL-XL and STAT3 were evaluated using Western blotting with β-actin as a loading control in samples derived from single tumors for both experimental groups. Band intensities were quantified by densitometry using ImageJ Version 1.46 software based on identically exposed images. Shown are results from 1 of 2 independent experiments analyzed for statistical significance. **P = .003; ***P < .0001. (D-E) RNAi-mediated STAT3 gene knockdown in MV4-11 tumors treated using IT injections of CpG(A)-siRNAs as in panel B. (D) STAT3 gene silencing in samples from 4 individual tumors was verified by qPCR and normalized to TBP expression; shown are means ± SE. (E) CpG(A)-STAT3 siRNA-induced cleavage of STAT3 mRNA in MV4-11 tumors in vivo. Tumors injected IT using CpG(A)-siRNAs as indicated were harvested 18 hours later. Total RNA samples were analyzed by the 5′RACE-PCR assay followed by DNA sequencing detecting mRNA cleavage within the targeted region (partial sequences are shown); arrow indicates the major RACE product of the predicted length (300 nt).
Antitumor effects of CpG(A)-siRNA conjugates
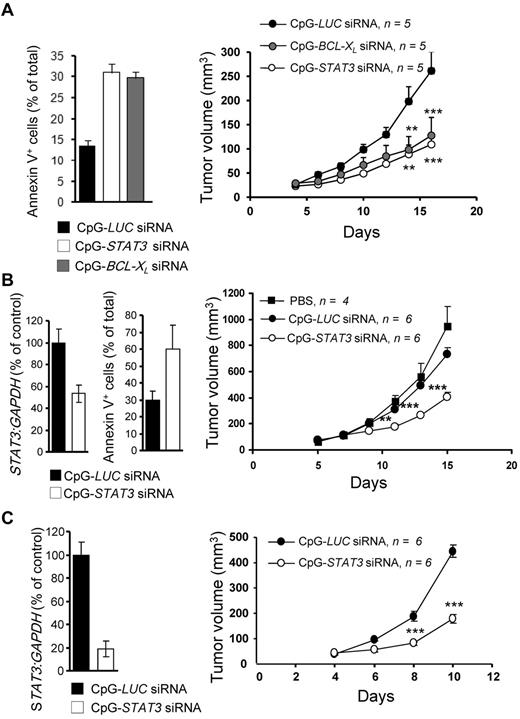
Both STAT3 and BCL-XL are known survival-promoting factors in MM and AML.4,7 Therefore, we assessed the potential of CpG(A)-STAT3 or -BCL-XL siRNAs to induce direct cytotoxic effects in tumor cells. NSG mice with established (> 5 mm in diameter) MV4-11 SC tumors were injected daily with 100 μg of CpG(A)-siRNA directed against STAT3, BCL-XL, or luciferase (as a negative control). Local treatment using CpG(A)-siRNA conjugates targeting STAT3 or BCL-XL inhibited growth of SC growing AML tumors (Figure 6A right). The antitumor effects were correlated with the induction of tumor cell death, as measured by annexin V staining and flow cytometry (Figure 6A left). We further investigated whether CpG-STAT3 siRNA could inhibit the growth of other TLR9+ blood cancers. As shown in Figure 6B and C, IT injections of CpG(A)-STAT3 siRNA effectively silenced STAT3 expression (left panels) and reduced the growth of both KMS-11 myeloma and MonoMac6 leukemia (right panels) cells. In contrast, treatment with control CpG(A)-Luc siRNA had no significant growth-inhibitory effect (Figure 6B-C). We further confirmed that the antitumor activity of CpG(A)-STAT3 siRNA depends on concomitant TLR9 triggering and STAT3 silencing. Control conjugates that did not silence STAT3 (CpG(A)-conRNA) or did not target TLR9 (GpC(A)-STAT3 siRNA) as well as CpG(A) alone were unable to induce antitumor effects against MV4-11 leukemia (supplemental Figure 5).
Therapeutic antitumor effects of CpG(A)-siRNAs targeting STAT3 or BCL-XL in xenotransplanted MM and AML models. (A) IT delivery of CpG(A)-siRNAs targeting STAT3 or BCL-XL inhibits SC growth of MV4-11 leukemia and results in augmented tumor cell death. NSG mice were challenged using 5 × 106 MV4-11 cells injected SC after tumors were established (at the average diameter of 8 mm). Mice were injected IT daily using 100 μg of CpG-siRNAs as indicated. Tumor growth was measured using calipers (right). At day 16, tumors were harvested and the percentage of apoptotic annexin V+ tumor cells was assessed by flow cytometry (left). Statistically significant differences between CpG(A)-STAT3 or BCL-XL siRNAs and CpG(A)-Luc RNA-treated groups (from 2-way ANOVA) are indicated by asterisks. Data are shown as means ± SEM (n = 5). (B-C) Local treatment using CpG(A)-STAT3 siRNA as in panel A, leads to STAT3 gene silencing (B and C left graphs as shown by qPCR), tumor cell death (B middle as shown by flow cytometric analysis of annexin V+ tumor cells), and reduced growth rate of human KMS-11 myeloma (B) and MonoMac6 leukemia (C) in NSG mice. Shown are representative results from 2 independent experiments (A-B) or from a single experiment (C) using 6 mice per treatment group.
Therapeutic antitumor effects of CpG(A)-siRNAs targeting STAT3 or BCL-XL in xenotransplanted MM and AML models. (A) IT delivery of CpG(A)-siRNAs targeting STAT3 or BCL-XL inhibits SC growth of MV4-11 leukemia and results in augmented tumor cell death. NSG mice were challenged using 5 × 106 MV4-11 cells injected SC after tumors were established (at the average diameter of 8 mm). Mice were injected IT daily using 100 μg of CpG-siRNAs as indicated. Tumor growth was measured using calipers (right). At day 16, tumors were harvested and the percentage of apoptotic annexin V+ tumor cells was assessed by flow cytometry (left). Statistically significant differences between CpG(A)-STAT3 or BCL-XL siRNAs and CpG(A)-Luc RNA-treated groups (from 2-way ANOVA) are indicated by asterisks. Data are shown as means ± SEM (n = 5). (B-C) Local treatment using CpG(A)-STAT3 siRNA as in panel A, leads to STAT3 gene silencing (B and C left graphs as shown by qPCR), tumor cell death (B middle as shown by flow cytometric analysis of annexin V+ tumor cells), and reduced growth rate of human KMS-11 myeloma (B) and MonoMac6 leukemia (C) in NSG mice. Shown are representative results from 2 independent experiments (A-B) or from a single experiment (C) using 6 mice per treatment group.
The immunodeficient NSG mice lack most of the immune cell populations, such as T, B, and NK cells, but retain granulocyte activity.40 Innate immunity contributes to antitumor responses, so we assessed whether treatment with CpG-STAT3 siRNA would lead to neutrophil recruitment into the tumor tissue. As shown in the Figure 7, CpG-siRNA targeting STAT3 but not Luc resulted in increased tumor infiltration by neutrophils. In addition, neutrophils partly colocalized with areas of MV4-11 tumors actively undergoing apoptosis as indicated by caspase-3 activity (Figure 7). These results suggest that even in mostly immunodeficient NSG mice, CpG(A)-STAT3 siRNA therapy mobilized residual innate immune cells that potentially contributed to the overall antitumor effect. Our present results validate the feasibility of using this strategy against TLR9+ hematologic malignancies in vivo and emphasize the need for the development of CpG-siRNA conjugates for systemic delivery.
Local delivery of CpG(A)-STAT3 siRNA into xenotransplanted leukemia induces tumor cell death and stimulated neutrophil infiltration. (A-B) Effects of IT delivery of CpG(A)-STAT3 siRNA on tumor cell apoptosis and neutrophil infiltration. Mice xenotransplanted with MV4-11 leukemia were treated by 4 IT injections of CpG(A)-siRNA targeting STAT3 or Luc as a negative control. Tumors were harvested 1 day after the last treatment, frozen, and sectioned. (A) Tissue cryosections were immunofluorescently stained using antibodies to neutrophils (7/4, green) and activated caspase-3 (red). Slides were counterstained using Hoechst 33342 to visualize nuclei (blue) and analyzed by fluorescent microscopy. Shown are representative results from 2 independent experiments using tumor sections from 4 mice per group; arrows indicate contact sites between neutrophils and cells undergoing apoptosis. (B) Quantification of neutrophil infiltration using at least 20 randomly chosen fields. Statistically significant differences between treatment groups (from 2-way ANOVA) are indicated by asterisks. Data are shown as means ± SEM (n = 4). ***P < .001.
Local delivery of CpG(A)-STAT3 siRNA into xenotransplanted leukemia induces tumor cell death and stimulated neutrophil infiltration. (A-B) Effects of IT delivery of CpG(A)-STAT3 siRNA on tumor cell apoptosis and neutrophil infiltration. Mice xenotransplanted with MV4-11 leukemia were treated by 4 IT injections of CpG(A)-siRNA targeting STAT3 or Luc as a negative control. Tumors were harvested 1 day after the last treatment, frozen, and sectioned. (A) Tissue cryosections were immunofluorescently stained using antibodies to neutrophils (7/4, green) and activated caspase-3 (red). Slides were counterstained using Hoechst 33342 to visualize nuclei (blue) and analyzed by fluorescent microscopy. Shown are representative results from 2 independent experiments using tumor sections from 4 mice per group; arrows indicate contact sites between neutrophils and cells undergoing apoptosis. (B) Quantification of neutrophil infiltration using at least 20 randomly chosen fields. Statistically significant differences between treatment groups (from 2-way ANOVA) are indicated by asterisks. Data are shown as means ± SEM (n = 4). ***P < .001.
Discussion
In the present study, we developed a strategy for siRNA delivery to normal and malignant hematopoietic cells in vivo that is suitable for targeting both tumor cells and the tumor microenvironment. The CpG(A)-siRNA approach allows for efficient cell-specific target gene silencing without complex formulations or delivery vehicles. Our studies confirmed the feasibility of using CpG(A)-siRNAs for targeting human TLR9-expressing cells such as pDCs, mDCs, B cells, and several myeloid and B-cell malignancies.25-27,37-39 We have demonstrated that although TLR9− cells such as monocytes are still able to quickly internalize CpG(A)-siRNA, in the absence of TLR9 expression, conjugates do not induce RNAi. Our independent studies delineating the molecular mechanism of intracellular CpG-siRNA processing suggest that TLR9 may in fact participate in the uncoupling and release of siRNA from endosomes (S.N. and M.K., unpublished results). We showed previously that type-B CpG-ODNs, which have been tested in numerous clinical studies, control TLR9 activity using negative feedback regulation through STAT3.33 The mechanism of STAT3 activation by class-B CpG-ODNs in human hematopoietic cells is likely to depend on up-regulation of cytokines such as IL-6 and IL-10, as shown in this and other studies.23,26,32 The signaling cross-talk within the tumor microenvironment further enhances STAT3 signaling, dampening the immunostimulatory activity of TLR agonists.33 In contrast, in the present study, we did not detect increased levels of STAT3-inducing cytokines in human PBMCs cultured with unconjugated CpG(A) ODNs or CpG(A)-siRNAs. The typical feature of class-A CpG-ODNs is induction of type I IFNs, which restricted their clinical application to viral and allergic diseases.29 Although unconjugated CpG(A) potently activated IFNα, the CpG(A)-STAT3 siRNA conjugates did not exert such activity on cultured human PBMCs. Type I IFN responses were shown to depend on TLR9 triggering by CpG(A)-ODN tetramers formed through the interaction of guanine-rich 3′ ends.38,41 Conjugation of the siRNA molecule to CpG(A)-ODN is likely to reduce spontaneous multimerization of the conjugate, thereby preventing TLR9-mediated induction of IFNα expression. However, the CpG(A)-siRNA conjugates retained the potent immunostimulatory activity on human DCs. The combined effect of TLR9 triggering and targeting of the STAT3 immune checkpoint gene strongly augmented maturation of human APCs similar to previous results in Stat3-deficient mice.33 We did not detect mitochondrial toxicity or general cytotoxicity of CpG(A)-STAT3 siRNA on primary human PBMCs in vitro.
Our findings validated that TLR9 expressed by human tumor cells provides a delivery route for therapeutic siRNAs similar to that shown in the normal mouse20 and human immune cells. Previously developed siRNA-delivery strategies used antibodies, oligonucleotides, or RNA aptamers specific to surface receptors on tumor cells.16,36,42 In contrast, our approach used a ligand to intracellular TLR9 receptor that is ubiquitously expressed in B-cell malignancies and AML.25-27 Dicer RNase, which is critical for uncoupling of CpG-siRNA conjugates, is also commonly expressed in normal and transformed B cells and in the majority of clinical AML samples.43,44 Our results demonstrate that repeated administration of CpG(A)-siRNAs specific for oncogenic and/or prosurvival genes such as STAT3 or BCL-XL can induce tumor cell apoptosis and inhibit the growth of several xenotransplanted human MM and AML tumors.
The STAT3 transcription factor plays a dual tumorigenic role in both cancer cells and nonmalignant tumor-associated cells, which do not depend on STAT3 for their survival. It is a highly desirable target for cancer therapy that could induce cancer cell death while activating antitumor immunity with minimal adverse effects.7 The NSG mice used in our xenotransplantation studies are known to be mostly deprived of functional immune cells except for granulocytes.40 Therefore, our experiments did not reflect the immunostimulatory potential of CpG(A)-STAT3 siRNAs, which can greatly contribute to the overall antitumor effect.20 Further studies using tumor models in humanized mice are required to assess the 2-pronged, tumoricidal/immunostimulatory effect of CpG(A)-STAT3 siRNA.
Despite these limitations, our results suggest that treatment of human hematologic malignancies using CpG(A)-STAT3 siRNA also resulted in immune cell activation. Targeting STAT3 in AML tumors resulted in the pronounced infiltration of neutrophils, which are known to contribute to the antitumor immunity after elimination of STAT3-dependent cross-talk in the tumor microenvironment.7,33 The progress in understanding complex interactions within the tumor microenvironment underscores the need to focus on tumor-associated but nonmalignant immune cells for successful anticancer strategies.
Immunomodulatory drugs are making headway in clinical testing for AML, MM, and BCL therapy.30,45,46 It is also becoming apparent that clinical outcome of cancer immunotherapy can be improved by targeted strategies to alleviate tumor-induced immunosuppression without induction of adverse immune effects.47 Recent studies revealed that APCs such as pDCs play a tumor-promoting role in MM patients48 and potentially also in AML patients.49 CpG(A)-STAT3 siRNA provides a new method for targeting abnormal human DC populations in the tumor microenvironment to restore antitumor immunity while reducing survival of TLR9+ tumor cells. Our proof-of-principle studies combining CpG-Stat3 siRNA with local radiotherapy have demonstrated that targeting both tumor and the tumor microenvironment is effective against radiation-resistant mouse BCL.
The results of the present study support the clinical translation of CpG(A)-STAT3 siRNA strategy for in situ immunotherapeutic strategies against BCL and also against certain advanced solid tumors that are TLR9+.26,50 However, for therapy of systemic diseases such as the majority of blood cancers, it is highly desirable to further optimize the serum stability and circulatory half-life of CpG(A)-STAT3 siRNA conjugate. Such optimization includes various chemical modifications of the CpG(A)-siRNA sugar backbone, conjugation to high molecular weight polymers, or encapsulation of the whole molecule. Our results provide a rational basis for further development of the CpG(A)-siRNA approach to target oncogenic and immunosuppressive targets such as STAT3 for clinical application.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Drs D. Tenen and H. Radomska (Beth Israel Deaconess Medical Center, Boston, MA) for providing the AML cell lines and Dr Simon Lacey and the CICSL core staff members at the CoH for support.
This work was supported in part by the National Cancer Institute, National Institutes of Health (grant R01CA155367); the National Institutes of Health (grants R01CA146092 and P50 CA107399); the ThinkCure Foundation (to M.K.); the Kosciuszko Foundation (to A.K.); and a V Foundation Translational Research Grant.
National Institutes of Health
Authorship
Contribution: Q.Z., D.M.S.H., S.N., A.K., W.Z., Y.L., and M.K. performed the research; C.M.K. and P.S. were involved in conjugate design and testing; J.J.R., S.F., S.P., A.R., H.Y., and M.K. designed the experiments and/or analyzed the data; R.B. provided clinical samples; and M.K. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Marcin Kortylewski, PhD, or Hua Yu, PhD, Department of Cancer Immunotherapeutics and Tumor Immunology, Beckman Research Institute at City of Hope, 1500 E Duarte Rd, Duarte, CA 91010; e-mail: mkortylewski@coh.org or hyu@coh.org.
References
Author notes
Q.Z. and D.M.S.H. contributed equally to this work.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal