Key Points
NOD-specific Sirpa polymorphism is the genetic determinant of highly efficient xenograft activity in NOD-based immunodeficient mouse models.
Abstract
Current mouse lines efficient for human cell xenotransplantation are backcrossed into NOD mice to introduce its multiple immunodeficient phenotypes. Our positional genetic study has located the NOD-specific polymorphic Sirpa as a molecule responsible for its high xenograft efficiency: it recognizes human CD47 and the resultant signaling may cause NOD macrophages not to engulf human grafts. In the present study, we established C57BL/6.Rag2nullIl2rgnull mice harboring NOD-Sirpa (BRGS). BRGS mice engrafted human hematopoiesis with an efficiency that was equal to or even better than that of the NOD.Rag1nullIl2rgnull strain, one of the best xenograft models. Consequently, BRGS mice are free from other NOD-related abnormalities; for example, they have normalized C5 function that enables the evaluation of complement-dependent cytotoxicity of antibodies against human grafts in the humanized mouse model. Our data show that efficient human cell engraftment found in NOD-based models is mounted solely by their polymorphic Sirpa. The simplified BRGS line should be very useful in future studies of human stem cell biology.
Introduction
Immunodeficient mice are widely used to reconstitute human hematopoiesis by xenotransplantation of hematopoietic stem cells (HSCs).1,2 This “humanized” mouse model provides a powerful tool with which to evaluate the biologic properties of human HSCs and progenitors in vivo.3,4 Such xenotransplantation systems have also been used to study human cancer stem cells.5-8
Elimination of the lymphoid system is the first step to achieving reconstitution of human hematopoiesis. To deplete T and B cells, the scid mutation in the Prkdc gene9-11 or disruption of the recombination activating gene 1 or 2 (Rag1 and Rag2)12,13 has been introduced into various mouse strains. In addition, to deplete natural killer (NK) cells or their functions, the IL-2 receptor common γ chain subunit (Il2rg)14-16 or beta-2-microglobulin (B2m)17-19 is disrupted.
However, depletion of lymphoid cells is not sufficient and it has been shown empirically that additional strain-specific factors modulate human hematopoietic engraftment in the xenotransplantation setting. For example, within the SCID strain, the SCID with the NOD background was the gold standard for the xenotransplantation assay based on its high efficiency.11 In fact, recent studies have shown that among the lymphoid-depleted mouse strains, the NOD-scid Il2rgnull (NSG/NOG)14,15 and NOD.Rag1nullIl2rgnull (NOD-RG)20 strains are the most efficient; the BALB/c.Rag2nullIl2rgnull (BALB-RG) strain is the next efficient21,22 ; and the C57BL/6 strains with scid,23 Rag2null, Rag2nullB2mnull, Rag2nullPrfnull,24 or Rag2nullJak3null25 mutations are unable to reconstitute human hematopoiesis. The NOD strain has multiple immune deficiencies, including defects of appropriate regulation of the T-lymphocyte repertoire, antigen presenting cell function, NK cell function,26 and hemolytic complement (C5) and cytokine production from macrophages,27 and these abnormalities are presumed to collaborate to cause the development of autoimmune diabetes and hemolytic anemia.26,28 To establish xenotransplantation models, lymphoid-depleted strains have been backcrossed into the NOD/ShiLt–inbred strain multiple times to introduce such numerous NOD-specific abnormalities.14,15 However, it was unknown whether we could select a genetic determinant(s) specially required to achieve the NOD-specific high engraftment capability for human cells.
Previously, we used positional genetics to characterize the molecular basis for this capability in the NOD strain by measuring the ability of mouse BM stromal layers to support hematopoietic long-term culture-initiating cell activity (LTC-IC) in vitro and identified the strain differences as the polymorphism of the Sirpa gene located within the insulin-dependent diabetes (Idd-13) locus.24 Stroma cells from the NOD BM supports LTC-IC of human cells, but those from C57BL/6 could not. Enforced expression of the NOD-type SIRPA enabled C57BL/6 stroma cells to support human LTC-IC.24 This in vitro finding is also applicable to the in vivo setting, as shown by another study in which a human SIRPA BAC transgene introduced into Rag2nullIl2rgnull mice on a mixed 129;BALB/c background significantly improved the efficiency of human hematopoietic engraftment.29
SIRPA is a transmembrane protein that contains 3 Ig-like domains within the extracellular region. It is expressed in macrophages, myeloid cells, and neurons, and interacts with its ligand CD47 through its respective IgV-like domains, where the NOD strain has specific polymorphism. CD47 is a member of the Ig superfamily that is ubiquitously expressed in hematopoietic and nonhematopoietic cells. The cytoplasmic region of SIRPA has immunoreceptor tyrosine–based inhibitory motifs, and binding cell-surface CD47 with SIRPA on macrophages provokes inhibitory signals through phosphorylation of these inhibitory motifs of SIRPA,30 preventing their phagocytic activity.31-33 A recent study also showed that transgenic expression of mouse CD47 into CD34+CD38− human fetal liver cells significantly enhanced the human cell engraftment into BALB-RG mice.34 Based on these data, the binding of NOD-SIRPA with human CD47 might produce signals for mouse macrophages not to engulf human HSCs, which presumably makes the strain permissive for human HSC engraftment.24
The most important question was whether the NOD-specific highly efficient human cell engraftment in vivo could be explained solely by the NOD-Sirpa polymorphism. In the present study, we established a C57BL/6.Rag2nullIl2rgnull (C57BL/6-RG) mouse line harboring the NOD-type Sirpa. Our data show clearly that replacement of the C57BL/6-type Sirpa with the NOD-type Sirpa is sufficient for the C57BL/6-RG strain to be endowed with the xenotransplantation capability that is at least equal to NOD-RG mice. Therefore, we successfully segregated the genetic abnormality responsible for efficient human cell engraftment from multiple genetic abnormalities in the NOD strain. The simplified humanized mouse system established by the new C57BL/6.Rag2nullIl2rgnullNOD-Sirpa (BRGS) strain should be very useful in improving xenotransplantation strategies in future studies of human cell biology.
Methods
Mice
C57BL/6, C57BL/6.NOD-Idd13, NOD, NOD.CB17-Prkdcscid (NOD-scid), and NOD.Cg-Rag1tm1MomIl2rgtm1Wjl/Sz (NOD-RG) mice were purchased from the Jackson Laboratory; C57BL/6.Rag2tm1FwaIl2rgtm1Wjl (C57BL/6-RG) mice were purchased from Taconic. All mice were bred and maintained in individual ventilated cages at the Kyushu University Animal Facility and fed with autoclaved food and water. BRGS mice were generated by breeding C57BL/6-RG and C57BL/6.NOD-Idd13 mice and backcrossed with C57BL/6-RG mice. Rag2 gene and Sirpa gene are located on chromosome 2 with 17.1 cM. First, we repeated the breeding of C57BL/6-RG and C57BL/6.NOD-Idd13 mice, and after 10 breedings, we obtained the recombination between the Rag2− and the SirpaNOD loci by chromosomal crossover. This was examined by genotyping by the microsatellite markers D2Mit447 and D2Mit338, which are 0.63 cM apart on chromosome 2, during interbreeding. In addition, Sirpa, Rag2, and Il2rg were genetically typed by PCR and direct sequencing. In C57BL/6.NOD-(D2Mit447-D2Mit338) Rag2nullIl2rgnull mice, the region between D2Mit447 and D2Mit338 contains 33 genes, including Sirpa, but Sirpa is the only gene within the Idd13 locus that is expressed in BM stromal cells and macrophages and had coding sequence polymorphism between the NOD and other strains.24 Therefore, we refer to our established mouse line as BRGS herein. Sequences of the oligonucleotide primers used are provided in supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). All experiments were conducted following the guidelines of the institutional animal committee of Kyushu University.
Binding affinity of mouse macrophages to human CD47-Fc
Mouse macrophages were obtained by peritoneal lavage. Cells were stained with purified anti–mouse Sirpa (P84; BD Biosciences) conjugated with PE and anti–mouse CD11b (3A33; Beckman Coulter) conjugated with FITC. CD11b+SIRPA+ cells were defined as mature macrophages. The binding between SIRPA and CD47 was assessed by staining with biotinylated human CD47-Fc conjugated with streptavidin-allophycocyanin (APC),35 and analyzed with a FACSAria III cell sorter (BD Biosciences).
In vitro mouse macrophage phagocytosis assays for human hematopoietic stem cells
Phagocytic activity of mouse macrophages against the human CD34+CD38− population that contains the majority of human HSCs was evaluated in vitro, as described previously.36 In brief, mouse peritoneal-derived macrophages were incubated at 1.0 × 104 cells in 200 μL of RPMI 1640 medium in Falcon culture tubes (2058; BD Biosciences). Cells were opsonized with CD34 antibody (sc-19621; Santa Cruz Biotechnology), incubated with mouse IFN-γ (100 ng/mL; R&D Systems) for 24 hours, and then lipopolysaccharide (0.3 μg/μL) for 1 hour. Human cord blood (CB) HSCs were then added to the tubes. Two hours after coincubation with macrophages and target cells, the phagocytic index was calculated using the following formula: phagocytic index = number of ingested cells/(number of macrophages/100). At least 200 macrophages were counted by a blinded observer.
Sensitivity of BRGS mice to irradiation
Cohorts of BRGS mice were exposed to varying doses of the whole-body irradiation from a 137Cs γ-irradiator. The mice were examined daily and euthanized when moribund. Surviving mice were euthanized at 8 weeks after irradiation. NOG/NSG mice are highly radiosensitive because of the scid mutation. To examine the radiosensitivity of BRGS mice, 6- to 10-week-old BRGS mice were irradiated with 550-670 cGy, and monitored for 8 weeks. Early deaths were observed in the mouse group irradiated with more than 620 cGy, whereas those irradiated with 550-580 cGy survived at the end of 8 weeks. Based on these data, we irradiated BRGS mice at 580 cGy in all xenotransplantation experiments. The irradiation doses for experiments with NOD-RG (420 cGy) and C57BL/6-RG (670 cGy) were decided by radiosensitivity experiments.
Transplantation of human HSCs into mice
CB cells were collected during normal full-term deliveries after obtaining informed consent in accordance with the Declaration of Helsinki (provided by the Kyushu Block Red Cross Blood Center, Japan Red Cross Society). Mononuclear cells were separated by Ficoll-Hypaque density-gradient centrifugation. Lineage-depleted CB cells were obtained magnetically using a lineage cell depletion kit (Miltenyi Biotec). A total of 5 × 103 CD34+CD38− cells were injected intrafemorally into mice. Within an individual experiment, mice of each strain received CD34+CD38− cells purified from the same mixture of CB cells from multiple donors. After transplantation, mice were given sterile water containing prophylactic enrofloxacin (Baytril; Bayer HealthCare). Mice were killed 8, 16, or 24 weeks after transplantation.
Antibodies, cell staining, and sorting
For the analyses of mouse T, B, and NK cells, mouse peripheral blood cells were stained with PE-conjugated anti-CD3 (145-2C11), FITC-conjugated anti-CD19 (1D3), APC-conjugated anti-NK1.1 (PK136; BD Biosciences), and Pacific Blue–conjugated anti–Gr-1 (RB6-8C5; BioLegend). Sorting of CD34+CD38− subfractions was accomplished by staining lineage-depleted CB cells with FITC-conjugated anti-CD34 (581/CD34) and PE-conjugated anti-CD38 (HIT2; BD Biosciences). For analysis and sorting of human cells in the immunodeficient mice, FITC-conjugated anti-CD4 (RPA-T4), CD33 (HIM3-4), CD41a (HIP8), TCR αβ (WT31), TCR γδ (11F2), Igλ L chain (JDC-12), Igκ L chain (G20-193; BD Biosciences), anti-CD10 (SS2/36; Dako), PE-conjugated anti-CD8 (RPA-T8), CD20 (2H7), NKp46 (9E2; BD Biosciences), CD235a (JC159; Dako), PE-Cy7–conjugated anti-CD3 (SK7; BD Biosciences), CD19 (HIB19; BioLegend), APC-conjugated anti-CD45 (J33; Beckman Coulter), and PaB-conjugated anti–mouse CD45 (30-F11; BioLegend) monoclonal antibodies were used in addition to the antibodies described in the preceding paragraph. Nonviable cells were excluded by propidium iodide staining. The cells were analyzed and sorted with a FACSAria cell sorter (BD Biosciences).
Complement-dependent hemolytic activity
To estimate the serum complement activity of mice, the peripheral blood of mice were collected in 1.5-mL tubes and allowed to stand at room temperature for 1 hour. The serum was collected after centrifugation of the blood at 200g for 15 minutes at 4°C and stored −80°C until use. The mixtures of each diluted sera of mice, 3.75 × 106 erythrocytes of sheep and 2.5μg of zymosan (Imgenex) were incubated 10 hours at 37°C. After incubation, the absorbance of each sample at 415 nm was measured.
In vivo antibody treatment in a disseminated lymphoma xenograft model
A total of 8 × 105 Raji cells (Burkitt lymphoma cell line; American Type Culture Collection) were injected into BRGS or NOD-RG mice (6-10 weeks of age) via the tail vein. Raji cells proliferated predominantly in the BM. Ten days after injection, these mice were IP injected daily with 200 μg of rituximab or mouse IgG2a control for 1 week and then BM cells were collected and analyzed with the FACSAria III.
Statistical analysis
Data are presented as means ± SD. The significance of the differences between groups was determined via the Student t test. For comparison of complement-dependent hemolytic activity among the mouse strains, repeated-measures ANOVA was performed.
Results
Establishment of the BRGS mouse
The BRGS mouse line was established by breeding the C57BL/6-RG with the C57BL/6.NOD-Idd13 mouse that is congenic for NOD-derived Idd13 locus within which the Sirpa is the only gene that is polymorphic and is expressed in the BM stromal cells.24 BRGS mice were all born healthy and displayed good fertility. They showed a median life span of 65 weeks without the development of lymphoma that usually occurs in the NOD-scid strain after the age of > 5 months.11
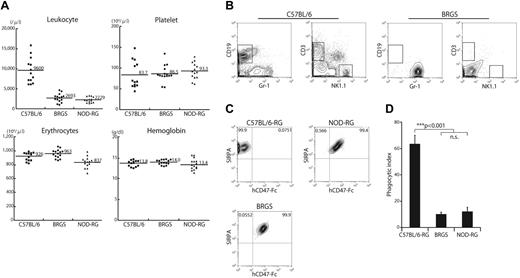
As shown in Figure 1A, BRGS mice had normal levels of hemoglobin and platelets, but a low number of leukocytes. This is because of the lack of CD3+ T cells, CD19+ B cells, and NK1.1+ NK cells (Figure 1B). IP macrophages from either C57BL/6-RG, BRGS or NOD-RG mice were evaluated for the binding to human CD47 on FACS. CD11b+ peritoneal macrophages strongly expressed SIRPA in all of these strains. As shown in Figure 1C, both macrophages from the BRGS and those from the NOD-RG strain bound to the human CD47-Fc protein, whereas those from the C57BL/6 strain did not, confirming that BRGS mice have the NOD-type SIRPA that can bind to human CD47. Consistent with these binding data, when macrophages of each strain were cultured with human CD34+CD38− cells, macrophages from C57BL/6-RG mice, but not those from BRGS or NOD-RG mice, actively engulfed human CD34+CD38− cells, as shown by the significant elevation of the phagocytic index in the C57BL/6-RG mice (Figure 1D).
BRGS mice lack lymphocytes and SIRPA recognizes human CD47-Fc. (A) Frequencies of blood leukocytes, erythrocytes, hemoglobin, and platelets in BRGS mice. Leukocyte counts in BRGS (2.69 ± 1.01 × 103/μL) and NOD-RG mice (2.23 ± 0.7 × 103/μL) are significantly decreased compared with that in C57BL/6 mice (9.6 ± 0.32 × 103/μL). BRGS mice have normal erythrocyte (9.63 ± 0.63 × 106/μL), hemoglobin (14.0 ± 0.6 g/dL), and platelet (8.7 ± 2.0 × 105/μL) counts. (B) Representative FACS plots of blood in C57BL/6 and BRGS mice. BRGS mice lacked T, B, and NK cells. (C) Binding activity of human CD47-Fc to SIRPA expressed in peritoneal macrophages derived from C57BL/6-RG, BRGS, or NOD-RG mice. Macrophages from BRGS and NOD-RG mice, but not those from C57BL/6-RG mice, were stained with human CD47-Fc on FACS. (D) Phagocytosis assay of C57BL/6-RG, BRGS, or NOD-RG macrophages against human CD34+CD38− CB HSCs (n = 3). The phagocytic index was determined as the number of engulfed cells per 100 macrophages. Bars indicate mean ± SD.
BRGS mice lack lymphocytes and SIRPA recognizes human CD47-Fc. (A) Frequencies of blood leukocytes, erythrocytes, hemoglobin, and platelets in BRGS mice. Leukocyte counts in BRGS (2.69 ± 1.01 × 103/μL) and NOD-RG mice (2.23 ± 0.7 × 103/μL) are significantly decreased compared with that in C57BL/6 mice (9.6 ± 0.32 × 103/μL). BRGS mice have normal erythrocyte (9.63 ± 0.63 × 106/μL), hemoglobin (14.0 ± 0.6 g/dL), and platelet (8.7 ± 2.0 × 105/μL) counts. (B) Representative FACS plots of blood in C57BL/6 and BRGS mice. BRGS mice lacked T, B, and NK cells. (C) Binding activity of human CD47-Fc to SIRPA expressed in peritoneal macrophages derived from C57BL/6-RG, BRGS, or NOD-RG mice. Macrophages from BRGS and NOD-RG mice, but not those from C57BL/6-RG mice, were stained with human CD47-Fc on FACS. (D) Phagocytosis assay of C57BL/6-RG, BRGS, or NOD-RG macrophages against human CD34+CD38− CB HSCs (n = 3). The phagocytic index was determined as the number of engulfed cells per 100 macrophages. Bars indicate mean ± SD.
BRGS mice are capable of multilineage reconstitution of human hematopoiesis with efficiency at least equal to that of NOD-RG mice
A recent study has shown that intrafemoral injection is more efficient than IV injection in the xenotransplantation setting.37 We used intrafemoral injection into adult mice in the present study because our preliminary data also showed that human cell chimerisms of adult BRGS by intrafemoral injection was significantly better than those with IV injection (data not shown). We transplanted 5 × 103 CD34+CD38− human CB cells intrafemorally into C57BL/6-RG, BRGS or NOD-RG mice at the age of 6-8 weeks. Before transplantation, C57BL/6-RG, BRGS, and NOD-RG mice were irradiated with 670, 580, and 420 cGy, respectively. Each dose was set by irradiation tolerance experiments (see the Methods).
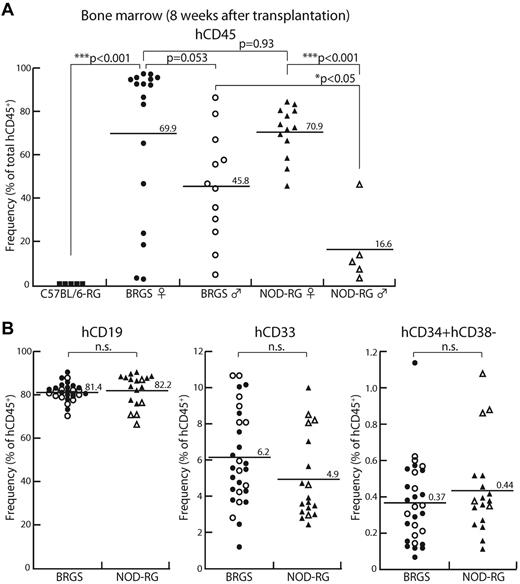
At 8 weeks after transplantation, human CD45+ cells were not detectable in C57BL/6-RG mice (Figure 2A). Both BRGS and NOD-RG showed successful reconstitution and their average frequencies of human CD45+ cells were 59.9% and 55.8%, respectively. Recent studies have shown that in the NSG strain,15 female recipients better support the reconstitution of human hematopoiesis, although the underlying mechanism for this remains unclear.38,39 As shown in Figure 2A, NOD-RG and BRGS female mice showed equally excellent human CD45+ reconstitution at approximately 70% chimerism. NOD-RG male mice, however, showed significantly poor reconstitution (16.6% of human cell chimerism on average) compared with NOD-RG female mice. In contrast, the percentages of human cell chimerisms in BRGS male mice (approximately 45%) were only slightly lower than those in BRGS female mice and, as a result, BRGS male mice showed significantly better engraftment compared with NOD-RG male mice.
BRGS mice show efficient engraftment of human HSCs comparable to NOD-RG mice. In the BM, human HSC engraftment was examined by flow cytometric analysis 8 weeks after transplantation. C57BL/6-RG mice (■; n = 5), female BRGS mice (●; n = 17), male BRGS mice (○; n = 12), female NOD-RG mice (▴; n = 13), and male NOD-RG mice (▵; n = 5) mice were analyzed. (A) Both BRGS and NOD-RG female mice showed excellent human CD45+ reconstitution. BRGS male mice showed significantly better engraftment compared with NOD-RG male mice. (B) Frequencies of CD19+ B cells, CD33+ myeloid cells, and CD34+CD38− HSCs in BRGS and NOD-RG mice.
BRGS mice show efficient engraftment of human HSCs comparable to NOD-RG mice. In the BM, human HSC engraftment was examined by flow cytometric analysis 8 weeks after transplantation. C57BL/6-RG mice (■; n = 5), female BRGS mice (●; n = 17), male BRGS mice (○; n = 12), female NOD-RG mice (▴; n = 13), and male NOD-RG mice (▵; n = 5) mice were analyzed. (A) Both BRGS and NOD-RG female mice showed excellent human CD45+ reconstitution. BRGS male mice showed significantly better engraftment compared with NOD-RG male mice. (B) Frequencies of CD19+ B cells, CD33+ myeloid cells, and CD34+CD38− HSCs in BRGS and NOD-RG mice.
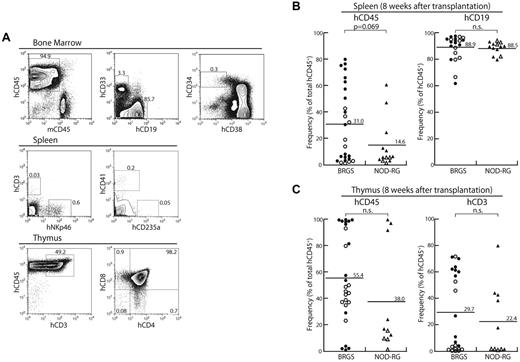
In the BM, the percentages of CD19+ B cells, CD33+ myeloid cells, and CD34+CD38− cells that contain the majority of human HSCs were almost equal between the BRGS and the NOD-RG strains irrespective of sex (Figure 2B). Representative FACS plots at 8 weeks after injection are shown in Figure 3A. In the spleen, small numbers of CD3+ T cells and CD3−NKp46+ NK cells, as well as CD41+ megakaryocytes and CD235a+ erythrocytes, were found in both BRGS and NOD-RG mice (Figure 3A) and there was no significant difference in the percentages of these cells between the 2 strains regardless of sex. The majority of human cells in the spleen were CD19+ B cells (Figure 3B). Although BM human CD19+ cells were mainly CD10+CD20− immature B cells, the majority (approximately 90%) of human spleen CD19+ cells were CD10−CD20+ mature B cells (data not shown). Thymic T cells were found in both the BRGS and NOD-RG strains, and the majority of human CD3+ T cells in the thymus were CD4+CD8+ immature T cells (Figure 3A,C).
Multilineage human HSC reconstitution in BRGS mice. (A) Representative FACS plots at 8 weeks after transplantation in the BM, spleen, and thymus. (B) Human hematopoietic reconstitution in the spleens of BRGS and NOD-RG recipients (●: BRGS female; ○: BRGS male; ▴: NOD-RG female; ▵: NOD-RG male). There were no significant differences in the percentages of human CD45+ cells and human CD19+ B cells between these mice. (C) Human hematopoietic reconstitution in the thymi of BRGS and NOD-RG recipients. There were no significant differences in the percentages of human CD45+ cells and human CD3+ T cells between these mice. Symbols are as in panel B.
Multilineage human HSC reconstitution in BRGS mice. (A) Representative FACS plots at 8 weeks after transplantation in the BM, spleen, and thymus. (B) Human hematopoietic reconstitution in the spleens of BRGS and NOD-RG recipients (●: BRGS female; ○: BRGS male; ▴: NOD-RG female; ▵: NOD-RG male). There were no significant differences in the percentages of human CD45+ cells and human CD19+ B cells between these mice. (C) Human hematopoietic reconstitution in the thymi of BRGS and NOD-RG recipients. There were no significant differences in the percentages of human CD45+ cells and human CD3+ T cells between these mice. Symbols are as in panel B.
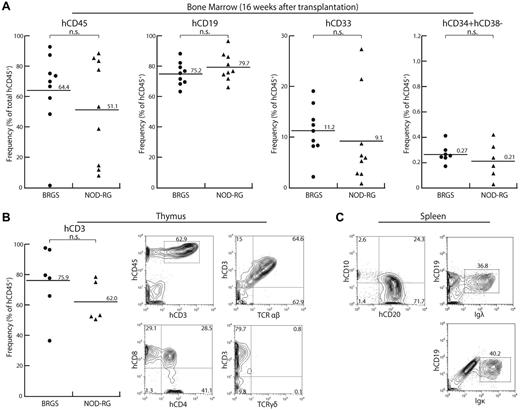
Figure 4 shows the analysis of reconstitution of human HSCs at 16 weeks after transplantation. In this analysis, we used only female BRGS and NOD-RG mice. In the BM, both BRGS and NOD-RG mice showed sustained human cell engraftment and the frequencies of human CD45+ cells were 64.4% and 51.1% in average, respectively, which were comparable to their levels at 8 weeks after transplantation. The percentages of CD33+ myeloid cells, CD19+ B cells, and CD34+CD38− HSCs were comparable to those at 8 weeks after transplantation (Figure 4A).
Human hematolymphoid reconstitution at 16 weeks after transplantation. (A) In the BM, BRGS mice showed sustained multilineage engraftment of human hematopoiesis at a level comparable to that in NOD-RG mice (●: BRGS female; ▴: NOD-RG female). (B) In the thymus, CD3+ T cells were developed and their frequencies were comparable in BRGS and NOD-RG mice. On FACS analysis, cells were differentiated into CD4+ and CD8+ single-positive T cells expressing the surface TCR-αβ chain. (C) In the spleen, CD10−CD19+CD20+ mature B cells expressing surface Ig light chain λ or κ chain were present.
Human hematolymphoid reconstitution at 16 weeks after transplantation. (A) In the BM, BRGS mice showed sustained multilineage engraftment of human hematopoiesis at a level comparable to that in NOD-RG mice (●: BRGS female; ▴: NOD-RG female). (B) In the thymus, CD3+ T cells were developed and their frequencies were comparable in BRGS and NOD-RG mice. On FACS analysis, cells were differentiated into CD4+ and CD8+ single-positive T cells expressing the surface TCR-αβ chain. (C) In the spleen, CD10−CD19+CD20+ mature B cells expressing surface Ig light chain λ or κ chain were present.
In the thymus, the percentage of CD3+ T cells was increased up to approximately 80% and approximately 60% in the BRGS and NOD-RG strains, respectively. In addition to CD4+CD8+ thymic precursors, both CD4+ and CD8+ single-positive T cells were present and expressed surface TCR-αβ or TCR-γδ, suggesting that human T-cell maturation occurs in the BRGS thymus, as has been shown previously in the NOG, NSG, and NOD-RG mouse lines14-16,20 (Figure 4B). The number of CD20+ mature B cells in the spleen was increased and they expressed surface Ig light chain λ/κ, reflecting their normal maturation (Figure 4C).
The BRGS mouse maintains self-renewal of human HSCs in the long term
Figure 5A shows the changes in human cell chimerism in female BRGS mice in the long term. The frequency of human CD45+ cells was maintained at a high level at least until 24 weeks after transplantation. B-cell frequencies gradually declined, but human myeloid, T, and NK cells progressively increased after engraftment (Figure 5B). The delayed reconstitution of these lineages of human cells has also been reported in studies using NSG mice.40,41
Evaluation of self-renewal of human HSCs in the BRGS mouse model. (A) Change in frequency of human CD45+ cells after transplantation. The level of human CD45+ cells was maintained at a high level until 24 weeks after transplantation (8 weeks, n = 29; 16 weeks, n = 17; and 24 weeks, n = 4). (B) Change in the frequency of human CD33+ myeloid cells, B cells, T cells, and NK cells in the BM and spleen during the 24 weeks after transplantation (□: 8 weeks; ■: 16 weeks; and ■: 24 weeks). Note that the B-cell numbers gradually decreased and were compensated for by myeloid, T, and NK cells. (C) To test the self-renewal ability of human HSCs maintained in the first recipient mice, 1 × 106 human CD45+ cells were sorted from first-recipient mice and injected into second-recipient mice. Only female mice were used as recipients. After another 8 weeks, 4 of 6 BRGS secondary recipients showed multilineage engraftment of human CD33+, CD19+, and CD3+ cells. Representative FACS plots are shown.
Evaluation of self-renewal of human HSCs in the BRGS mouse model. (A) Change in frequency of human CD45+ cells after transplantation. The level of human CD45+ cells was maintained at a high level until 24 weeks after transplantation (8 weeks, n = 29; 16 weeks, n = 17; and 24 weeks, n = 4). (B) Change in the frequency of human CD33+ myeloid cells, B cells, T cells, and NK cells in the BM and spleen during the 24 weeks after transplantation (□: 8 weeks; ■: 16 weeks; and ■: 24 weeks). Note that the B-cell numbers gradually decreased and were compensated for by myeloid, T, and NK cells. (C) To test the self-renewal ability of human HSCs maintained in the first recipient mice, 1 × 106 human CD45+ cells were sorted from first-recipient mice and injected into second-recipient mice. Only female mice were used as recipients. After another 8 weeks, 4 of 6 BRGS secondary recipients showed multilineage engraftment of human CD33+, CD19+, and CD3+ cells. Representative FACS plots are shown.
Figure 5C shows the results of the serial transplantation analysis. After confirmation of human cell engraftment at 8 weeks after the first transplantation, 1 × 106 human CD45+ cells were purified from primary BRGS recipients. These cells were transplanted into irradiated secondary BRGS recipients by intrafemoral injection and tested for engraftment after another 8 weeks. Four of 6 secondary BRGS recipients showed multilineage engraftment of human CD33+, CD19+, and CD3+ cells (Figure 5C). These data strongly suggest that BRGS mice can support long-term reconstitution and self-renewal of human HSCs.
The BRGS mouse is useful for experiments using CDC of antibodies in the xenotransplantation setting
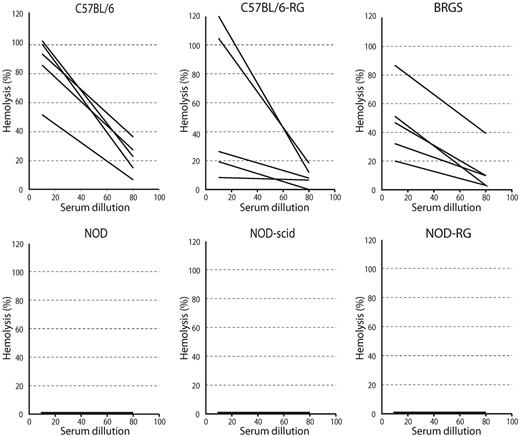
One of the problems in NOD-based xenograft models is that the cytotoxic activities of antibodies are unable to be evaluated in vivo in humanized mice. First, antibody-dependent cell-mediated cytotoxicity (ADCC) does not operate efficiently in xenotransplantation experiments because these strains of mice are deficient in NK cells, the major player for ADCC. In addition, immunodeficient phenotypes of the NOD strain include complement-dependent hemolytic activity due to a deficiency of C5,42 which is essential for antibodies to exert complement-dependent cytotoxicity (CDC). All NOD-based immunodeficient strains have this abnormality, whereas the BRGS strain does not because it has a C57BL/6 background except for the NOD-type SIRPA. We tested CDC activity in C57BL/6-based strains, including the C57BL/6, C57BL/6-RG, and BRGS mice, and in NOD-based strains such as NOD, NOD-scid, and NOD-RG. As shown in Figure 6, sera from all of the C57BL/6-based strains, including the BRGS strain, showed CDC activities on sheep RBCs, whereas this was not found in any of NOD-based strains. There were no significant differences in CDC activities among the C57BL/6, C57BL/6-RG, and BRGS strains.
BRGS mice had CDC activity. Sera from BRGS and C57BL/6-based mice showed CDC activity, whereas none of the NOD-based strains did. Five mice were analyzed in each strain. There were no statistical differences in CDC activities among the C57BL/6-based strains.
BRGS mice had CDC activity. Sera from BRGS and C57BL/6-based mice showed CDC activity, whereas none of the NOD-based strains did. Five mice were analyzed in each strain. There were no statistical differences in CDC activities among the C57BL/6-based strains.
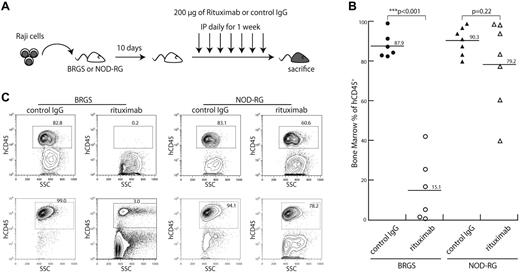
To determine whether BRGS mice had restored CDC in vivo, 8 × 105 cells of Raji, a Burkitt lymphoma cell line expressing human CD45, was injected into BRGS or NOD-RG mice. Ten days after transplantation, either rituximab, an anti-CD20 antibody that has both CDC and ADCC activities, or a control IgG2a antibody was administered IP for 7 days (Figure 7A) and the effect of antibody injection on elimination of Raji cells was evaluated. Representative results are shown in Figure 7B. In mice injected with control IgG2a, Raji cells rapidly proliferated up to approximately 90% in the BM of both BRGS and NOD-RG mice. In contrast, by injection of rituximab, percentages of human CD45+ Raji cells were significantly decreased in BRGS mice (15.1%), whereas the percentages of human CD45+ cells in NOD-RG mice were only slightly reduced by rituximab treatment (79.2%). Representative FACS data are shown in Figure 7C. These data clearly show that the CDC activity of antibodies was able to operate in the BRGS strain.
CDC activity of antibodies is evaluable in vivo in the BRGS xenogeneic model. (A) Experimental scheme of this experiment. Raji cells were injected into mice via the tail vein. Ten days after the injection, either rituximab or control IgG2a antibody (200 μg each) was injected IP daily for 1 week. (B) Frequencies of human CD45+ Raji cells in the BM of BRGS and NOD-RG mice with or without rituximab injection. A significant reduction of Raji cells was found only in BRGS mice injected with rituximab. (C) Representative FACS plots of the BM cells of BRGS and NOD-RG mice after injection of rituximab or control IgG.
CDC activity of antibodies is evaluable in vivo in the BRGS xenogeneic model. (A) Experimental scheme of this experiment. Raji cells were injected into mice via the tail vein. Ten days after the injection, either rituximab or control IgG2a antibody (200 μg each) was injected IP daily for 1 week. (B) Frequencies of human CD45+ Raji cells in the BM of BRGS and NOD-RG mice with or without rituximab injection. A significant reduction of Raji cells was found only in BRGS mice injected with rituximab. (C) Representative FACS plots of the BM cells of BRGS and NOD-RG mice after injection of rituximab or control IgG.
Discussion
The NOD/ShiLt inbred mouse strain, which was originally developed by selecting cataract-prone strains,27 exhibits susceptibility to the spontaneous development of autoimmune insulin-dependent diabetes mellitus (IDDM) and many other autoimmune disorders. The susceptibility to IDDM is polygenic and genetic loci associated with susceptibility to IDDM have been identified through the development of congenic mouse strains. More than 20 Idd loci have been identified. NOD mice display multiple aberrant immunophenotypes, and introduction of these abnormalities into immunodeficient mouse lines by multiple backcrossing accelerated human cell engraftment in xenotransplantation assays.11,14,15
In the current study, we present formal proof that under disruption of T, B, and NK cells, NOD-specific Sirpa polymorphism could explain the efficient human cell engraftment in the NOD strain. We replaced the Idd13 locus of C57BL/6-RG mice with that of B6. The NOD-Idd13 mouse has the C57BL/6 background but is congenic for the NOD-derived 23cM segment of chromosome 2 extending from microsatellite marker D2Mit274 through D2Mit343.43 In a previous study, we resolved the sequence corresponding to the phenotype of support of human LTC-IC to a region of 960 kilobases, within which coding regions of 14 genes reside. Sirpa was the only gene within the Idd13 locus expressed in BM stromal cells and macrophages and had coding sequence polymorphism between the NOD and other strains.24 To determine whether the efficient human cell engraftment in the NOD strains was completely dependent on the NOD-type SIRPA polymorphism, we compared the engraftment efficiency of the BRGS mouse with the NOD-RG mouse as a control, because in both strains RAG and γc genes are disrupted to disturb lymphoid cell development. The NOD-RG strain displays the excellent human cell engraftment comparable to the NOG/NSG strain21 in which the SCID mutation instead of RAG-1 disruption is introduced. Our data show that the reconstitution activity of human hematopoiesis in BRGS mice is at least equal to that in NOD-RG mice in terms of engraftment levels and multilineage reconstitution. Therefore, replacement of the C57BL/6-Sirpa with the NOD-Sirpa is sufficient for the C57BL/6-RG strain to gain the human cell engraftment capability equal to the NOD-RG strain. NOD-SIRPA is able to bind human CD47, signaling of which inhibits activation of host macrophages to engulf human HSCs (Figure 1D), and therefore this signaling might be able to inhibit xenograft rejection.24
The polymorphism of Sirpa could explain the strain-specific trend toward human cell acceptability in xenotransplantation experiments. There are 20 amino acid differences in the sequences of Sirpa IgV domain between the NOD and B6 strains. Among these, 5 amino acid residues are unique for NOD compared with C57BL/6, BALB/c, ICR, and C3H. By testing their binding affinity to human CD47 and their ability to support human LTC-IC, we found that the xenograft capability–related NOD-specific polymorphism can be aggregated to a single location of polymorphism (C.I., K.T., S.U., T.Y., K.I., J.K., T.M., K.A., The efficient engraftment of human hematopoiesis in the BALB/c strain is mounted by BALB/c-specific Sirpa polymorphism that enhances binding affinity to human CD47, manuscript in preparation). In addition, we found recently that Balb/c mice also have another polymorphism at the Sirpa IgV domain. Protein-binding assays show that C57BL/6-SIRPA never binds to human CD47, but Balb/c-SIRPA and NOD-SIRPA showed modest and very high binding affinity, respectively, correctly reflecting their strain-specific graft efficiencies.44 Furthermore, a recent study has shown that the enforced expression of human SIRPA by a human BAC transgene enables the 129;Balb/c.Rag1nullIl2rgnull mouse to engraft human cells as efficiently as the NSG mouse.29 Therefore, in xenograft models, the degree of SIRPA-CD47 interaction decided by Sirpa polymorphism is one of the most critical factors to achieve efficient human cell engraftment. Further study is required to understand how the different binding affinity between these mouse polymorphic SIRPAs and human CD47 is translated into cytoplasmic signaling that leads to respective efficiency for xenotransplantation capabilities.
Several recent studies have shown that, in xenograft models, female mice somehow present significantly better reconstitution than do male mice.38,39 It remains unclear whether sex-related factors such as steroid hormones can affect the engraftment of human HSCs. In the present study, the human cell chimerism obtained in the BRGS strain was quite high, reaching > 90% in 9 of 17 BRGS female mice, but none of the 13 NOD-RG female mice achieved that level at 8 weeks after transplantation (Figure 2A). Furthermore, although BRGS male mice displayed lower levels of human chimerism (approximately 45%), NOD-RG male mice showed significantly lower levels than did NOD-RG female mice, reaching only < 20% of human cell chimerism in average on our conditions (Figure 2A). As a result, the human cell chimerism in BRGS male mice was significantly better than that in NOD-RG male mice. Therefore, the BRGS mice showed a trend toward higher levels of human chimerism in both the males and the females. These results may suggest that unknown genetic abnormalities antagonizing human cell reconstitution can exist outside of the Idd13 locus in the NOD strain.
There remain many unknown factors that affect the efficiency of human cell reconstitution in mouse xenotransplantation models. For example, the BRGS model is capable of long-term, multilineage human hematopoietic reconstitution, but human myeloid, T, and NK cell reconstitution were significantly delayed compared with the B-cell lineage (Figure 5B). This pattern of reconstitution is commonly observed in other xenotransplantation models.40,41 Since the introduction of human cytokines such as thrombopoietin and membrane-bound SCF into humanized mouse models,45,46 myeloid reconstitution has been accelerated, so the delay could have been due to insufficient cross-reactivity of mouse cytokines with human cytokine receptors. It is also possible that the mouse hematopoietic microenvironment, including putative myeloid or lymphoid niches, is not appropriate for human HSC development. The elucidation of such unknown factors is necessary to develop further efficient xenotransplantation models for future studies.
We have also shown herein the usefulness of the BRGS line in testing the function of killing antibodies via CDC activity. Because rituximab has both ADCC and CDC activity47 and because NK cells, the major player for ADCC, are absent in efficient xenograft models such as NOG,14 NSG,15 and NOD-RG20 mice, the disappearance of Raji cells after rituximab injection in the BRGS system must have been dependent largely on its CDC activity. Selective cell depletion by killing antibodies should be very useful in xenograft experiments, for example, in targeting cancer stem cells,48 and in removing specific human cell component(s) from reconstituted human hematolymphopoiesis in vivo. Therefore, the BRGS humanized mouse model is applicable to future, more sophisticated xenograft experiments.
In summary, in the present study, we selected NOD-type polymorphic Sirpa from multiple abnormalities within the NOD background and introduced it into the common C57BL6 mouse line together with Rag2nullIl2rgnull mutations. The xenograft efficiency of the BRGS line was equal to, or even better than, the NOD-RG line, which is currently one of the best xenograft models. This result formally proves that NOD-specific Sirpa polymorphism is the genetic determinant of highly efficient xenograft activity in NOD-based immunodeficient mouse models. Sparing other NOD-specific abnormalities in this model also resulted in normalized C5 function, which should help in future studies using CDC activity of antibodies in vivo. The use of the BRGS line should also save time in introducing other genes for further modification of the line, keeping the high efficiency corresponding to the NOD-based models without performing multiple backcrosses. Therefore, this simplified mouse model should be very useful in future xenotransplantation experiments using human cells.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Atsushi Odawara and Yasuyuki Okawa for purification of the CD47-Fc protein and the Kyushu Block Red Cross Blood Center for providing the CB samples.
This work was supported in part by a grant-in-aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan (to K.A. and K.T.), a grant-in-aid from the Ministry of Health, Labor and Welfare of Japan (to K.A.), the Takeda Science Foundation (to K.T.), the Cell Science Research Foundation (to K.T.), the Sumitomo Foundation (to K.T.), the Japan Leukeamia Research Fund (to K.T.), and the Uehara Memorial Foundation (to K.A.).
Authorship
Contribution: T.Y., K.T., and S.U. coordinated the project, designed and performed the experiments, analyzed the data, and wrote the manuscript; T.S., Y.K., T.T., and C.I. performed the experiments; M. Nishihara managed the mice; H.I., T. Miyamoto, and K.A. designed the experiments, reviewed the data, and edited the manuscript; N.H. provided the antibodies and technical advice; M. Nakao performed the experiments and provided technical advice; and T. Matozaki provided the antibodies and technical advice.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Koichi Akashi, MD, PhD, Department of Medicine and Biosystemic Science, Kyushu University Graduate School of Medical Sciences, 3-1-1 Maidashi, Higashi-ku, Fukuoka 812-8582, Japan; e-mail: akashi@med.kyushu-u.ac.jp.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal