In this issue of Blood, Jodele and colleagues1 report that defective complement regulation contributes to the development of thrombotic microangiopathy (TMA) after hematopoietic stem cell transplantation (HSCT) with important implications for diagnosis and management of this severe clinical complication.
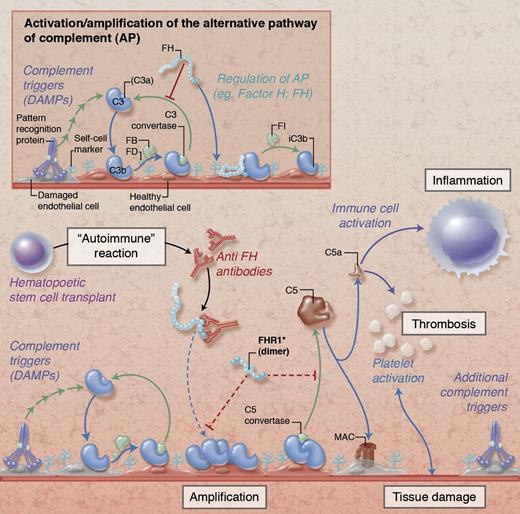
Simplified illustration of complement activation and regulation on damaged endothelial cells. Under physiological conditions (insert), initial tissue injury with exposure of damage-associated molecular patterns (DAMPs) that trigger complement activation leads to opsonization with C3b and formation of C3 convertases that amplify the response by generating additional C3b. Together with membrane regulators of complement (not shown), circulating FH protects host cells by binding to cell surface components (eg, glycosaminoglycans), promoting decay of the convertases and degrading C3b via factor I (FI). In HSCT-associated TMA (main figure), these regulatory mechanisms are potentially impaired by autoantibodies against FH (anti-FH) and/or deletions in genes encoding FH-related protein 1 (FHR1). While the exact pathophysiological involvement of anti-FH and FHR1 remains to be explored, the expected resulting dysregulation facilitates amplification of the complement response with subsequent cleavage of C5 and generation of the anaphylatoxin C5a and the membrane attack complex (MAC). These effectors contribute to thromboinflammatory consequences by activating immune cells, endothelium, and platelets and by affecting membrane integrity, thereby fueling a vicious cycle of tissue damage that fosters additional complement activation. Proposed physiological mechanisms of FHR1 are indicated with dotted red lines in the main figure; the functional implication of FHR deletions in HSCT-associated TMA are not yet known. Professional illustration by Alice Y. Chen.
Simplified illustration of complement activation and regulation on damaged endothelial cells. Under physiological conditions (insert), initial tissue injury with exposure of damage-associated molecular patterns (DAMPs) that trigger complement activation leads to opsonization with C3b and formation of C3 convertases that amplify the response by generating additional C3b. Together with membrane regulators of complement (not shown), circulating FH protects host cells by binding to cell surface components (eg, glycosaminoglycans), promoting decay of the convertases and degrading C3b via factor I (FI). In HSCT-associated TMA (main figure), these regulatory mechanisms are potentially impaired by autoantibodies against FH (anti-FH) and/or deletions in genes encoding FH-related protein 1 (FHR1). While the exact pathophysiological involvement of anti-FH and FHR1 remains to be explored, the expected resulting dysregulation facilitates amplification of the complement response with subsequent cleavage of C5 and generation of the anaphylatoxin C5a and the membrane attack complex (MAC). These effectors contribute to thromboinflammatory consequences by activating immune cells, endothelium, and platelets and by affecting membrane integrity, thereby fueling a vicious cycle of tissue damage that fosters additional complement activation. Proposed physiological mechanisms of FHR1 are indicated with dotted red lines in the main figure; the functional implication of FHR deletions in HSCT-associated TMA are not yet known. Professional illustration by Alice Y. Chen.
Approximately 10% to 25% of the 11 000 autologous, 3000 sibling donor, and 4000 unrelated HSCTs performed annually in the United States are complicated by TMA.2 TMA is associated with a high rate of renal failure and significant mortality. Older age, advanced underlying disease, unrelated donors, high-dose radiation for conditioning, calcineurin inhibitors, cytomegalovirus, and possibly mutations in thrombomodulin and diacylglycerol kinase-ε, human herpesvirus 6, and graft-versus-host disease are putative risk factors, but the lack of consensus diagnostic criteria precludes precise estimates of incidence, outcome, or effectiveness of intervention with rituximab and plasma exchange. TMA is undoubtedly a multifactorial syndrome, but this study delineates a subset of patients for whom early diagnosis and treatment that restores physiological complement activity may be helpful.
Each of 3 patients with TMA following allogeneic HSCT developed antibodies that reacted with the complement regulator factor H (FH), potentially as an “autoimmune” reaction to genotypic differences between recipient and donor. Moreover, in 5 of 6 HTSC recipients who developed TMA, heterozygous deletions in the gene cluster that encodes the FH-related (FHR) proteins FHR1 to FHR5 were identified.1 This strong correlation between development of TMA and FH/FHR status implicates complement, and in particular the alternative pathway, in the pathogenesis of TMA in these patients.
Complement contributes to immune surveillance by clearing foreign or apoptotic cells, but disruptions in this triage system can direct its deleterious actions against host tissue and trigger or exacerbate diverse inflammatory, degenerative, and immune-related disorders.3 Under physiological conditions, circulating FH acts together with membrane regulators to protect healthy human cells from complement attack by binding to host cell surfaces and inhibiting alternative pathway activity (see figure insert). Polymorphisms in or autoantibodies against FH that tamper with cell surface recognition or its regulatory activities reduce the host’s ability to tame events that trigger complement, leading to untoward activation of immune cells, platelets, and endothelium, which eventuates in tissue damage (see figure). In contrast to FH, the function of FHR proteins is only beginning to unfold. Whereas initial reports suggested a role for FHR1 in the regulation of C5 activation,4 newer studies are more indicative that FHR1 and other FHRs primarily act as oligomeric counterregulators that compete with FH for surface recognition sites.5 The ratio between FH and FHR may therefore be critical for fine-tuning complement regulation. Deletions in the CFHR3-CFHR1 gene cluster are surprisingly prevalent (∼33% in the general population and HSCT donors) and have been reported to lower the risk of age-related macular degeneration and immunoglobulin A nephropathy but increase susceptibility for systemic lupus erythematosus.5 While the exceptional prevalence of the heterozygous deletion in HSCT patients with TMA (83%) points toward a damaging effect, the exact involvement of FHR1 in this syndrome needs to be further clarified, which may shed light on the (patho)physiological role of FHRs.
Based on this study, HSCT-related TMA joins other microangiopathic syndromes in which complement dysregulation appears to play an important pathogenic role, including atypical hemolytic uremic syndrome (aHUS), Shiga-toxin–associated HUS (STEC-HUS), thrombotic thrombocytopenic purpura (TTP), antiphospholipid antibody syndrome, and the preeclampsia-related HELLP syndrome.6,7 Each is characterized by an imbalance in the interrelated complement and coagulation cascades that fuels a vicious cycle of tissue damage, which furthers the activation of potentially deleterious defense systems. However, the clinical diversity of these disorders highlights important differences in the triggers and outcomes of complement dysregulation. In aHUS, FH autoantibodies and/or FHR deletions are comparatively uncommon, and dysregulation is linked primarily to mutations in FH, membrane cofactor protein, factor I, and factor B6 that were not found in HSCT recipients in this study. Generation of FH autoantibodies has been associated with FHR1 deficiency in aHUS, whereas the 3 HSCT recipients with such autoantibodies studied here had detectable FHR1. Conversely, TMA is not typical of complement-mediated membranous glomerulonephritis. This heterogeneity suggests important differences in the sites and intensity of complement activation and the involvement of additional pathogenic pathways (eg, ADAMTS-13 deficiency in TTP) in causing vascular injury.
The newly discovered involvement of complement dysregulation in the pathogenesis of HSCT-associated TMA suggests a need to extend current therapeutic approaches (ie, antibody suppression, plasma exchange/replacement) to include complement-targeted treatment strategies. Eculizumab (Soliris, Alexion), an antibody that blocks the activation of C5 and thereby prevents inflammatory signaling via the anaphylatoxin C5a and tissue damage via the membrane attack complex, has already been approved for use in aHUS and has been shown to reduce thrombin generation and prevent thrombosis and hemolysis in patients with paroxysmal nocturnal hemoglobinuria.8 However, eculizumab is among the most expensive drugs in the clinic and does not prevent ongoing opsonization and amplification via the alternative pathway, and treatment success in STEC-HUS during a recent Escherichia coli outbreak in Germany was less conclusive than for aHUS.6,9 It will be important to monitor how C5-targeted intervention will perform in this and other TMA syndromes and whether inhibitors that block at an earlier stage of complement activation will provide greater benefit. Fortunately, diverse complement inhibitors, including some based on engineered forms of FH, are already in preclinical or clinical development.9
The identification of complement-related disease markers and risk factors (eg, FH autoantibodies, polymorphisms in regulatory genes, FHR deletions) may serve as important diagnostic tools to identify patients who may benefit most from complement regulatory therapies. However, this outcome also leaves us with several important clinical management issues. Does the incidence of complement regulatory abnormalities justify pretransplant screening of donor and recipient? If not, what clinical criteria should prompt serological and genetic evaluation? Is the presence of anti-FH antibodies diagnostic or is evidence of function-blocking activity required? What criteria should be used to initiate eculizumab, a very expensive but safe agent, and should it be used alone or in conjunction with rituximab and/or plasma exchange, and how should therapy be monitored? Thus, the paper by Jodele and colleagues will undoubtedly prompt further study of the complement system in additional posttransplant patients and in other important syndromes such as graft-versus-host disease.10
Conflict-of-interest disclosure: D.R. is an inventor on 2 patent applications that describe novel complement inhibitors. The remaining author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal