Key Points
Axl inhibition by BGB324 is active in FLT3-mutated and FLT3 wild-type AML, and presence of Axl and Gas6 are required for therapeutic efficacy.
AML cells educate BMDSCs to secrete Gas6, which mediates leukemia cell proliferation and therapy resistance.
Abstract
Acute myeloid leukemia (AML) represents a clonal disease of hematopoietic progenitors characterized by acquired heterogenous genetic changes that alter normal mechanisms of proliferation, self-renewal, and differentiation.1 Although 40% to 45% of patients younger than 65 years of age can be cured with current therapies, only 10% of older patients reach long-term survival.1 Because only very few novel AML drugs were approved in the past 2 decades, there is an urgent need to identify novel targets and therapeutic strategies to treat underserved AML patients. We report here that Axl, a member of the Tyro3, Axl, Mer receptor tyrosine kinase family,2-4 represents an independent prognostic marker and therapeutic target in AML. AML cells induce expression and secretion of the Axl ligand growth arrest–specific gene 6 (Gas6) by bone marrow–derived stromal cells (BMDSCs). Gas6 in turn mediates proliferation, survival, and chemoresistance of Axl-expressing AML cells. This Gas6-Axl paracrine axis between AML cells and BMDSCs establishes a chemoprotective tumor cell niche that can be abrogated by Axl-targeting approaches. Axl inhibition is active in FLT3-mutated and FLT3 wild-type AML, improves clinically relevant end points, and its efficacy depends on presence of Gas6 and Axl. Axl inhibition alone or in combination with chemotherapy might represent a novel therapeutic avenue for AML.
Introduction
Axl represents a member of the TAM family of receptor tyrosine kinases (TAMRs), which consists of Tyro3 (Sky/Rse), Axl (Ufo/Ark), and Mer (Eyk). Axl has transforming properties because overexpression of a truncated version in premalignant cells induces tumors in mice.5 In line with these data, Axl is overexpressed in different (solid) cancers.6-10 Its ligand, growth arrest–specific gene 6 (Gas6) exerts pleiotropic effects in pathobiology: it amplifies platelet aggregation during thrombus formation,11,12 enhances erythropoiesis,13 and increases leukocyte extravasation in inflammatory conditions,14 among other functions.2,15 Furthermore, Gas6 promotes proliferation and survival of different cancer cell lines in vitro.16,17 In vivo, tumor cells can educate tumor-infiltrating myeloid cells to express Gas6, which in turn fosters their proliferation.18
Members of the TAMR family are abundantly expressed in physiological and malignant hematopoiesis.19,20 Evidence in literature links Axl to acute myeloid leukemia (AML) pathobiology21 and therapy resistance.22 Recent work demonstrated that Axl phosphorylation is important for FLT3 activation and that Axl inhibition suppresses the growth of human FLT3-positive AML in vivo.21 Furthermore, Axl expression conferred worse prognosis to AML patients.23 However, the latter analysis was carried out in a limited number of (n = 19) AML patients. In contrast to Axl, much less is known about the role of the common TAMR ligand Gas6 in AML. Furthermore, a systematic study about prognostic relevance of Gas6 and TAMR has not been carried out until now.
In the present study, we provide evidence for an independent prognostic role of Axl in AML. In addition, the clinically applicable small molecule Axl-inhibitor BGB324 selectively inhibits Axl+ AML independent of FLT3 mutational status. Functional studies point toward a role of stroma-derived Gas6 in chemoresistance of AML cells. Altogether, our data indicate an important role of stroma-derived Gas6 in AML.
Material and methods
All animal experiments were carried out according to institutional guidelines for the welfare of animals and were approved by the local licensing authority (Behörde für Soziales, Gesundheit, Familie, Verbraucherschutz; Amt für Gesundheit und Verbraucherschutz, Hamburg, Germany, project numbers 111/10 and 53/12).
All studies with human samples were carried out in accordance with the Declaration of Helsinki with approval of the local ethical committee (approval numbers PV3997 and PV4107).
Detailed methods are provided in the supplemental data on the Blood Web site.
Animal models
Animal models were performed as published.24-26
Patient samples
We used material from patients with primary diagnosis of cytogenetically normal (Cn) AML included in the multicenter treatment trial AML SHG0199 (www.clinicaltrials.gov #NCT00209833).
In vitro assays
Western blot analysis
Immunoblots were carried out as described elsewhere.28
qRT-PCR
Quantitative reverse transcription-polymerase chain reaction (qRT-PCR) was performed as described elsewhere.27 For primer sequences, refer to supplemental Table 5.
Enzyme-linked immunosorbent assay (ELISA)/flow cytometry/immunohistochemistry
Experiments were performed according to standard protocols.27
Cloning of lentiviral gene ontology vectors and production of lentiviral particles
Cloning of lentiviral gene ontology vectors and lentiviral transductions were performed as described elsewhere.29-32 For primers, refer to supplemental Table 5.
Statistics
Data represent mean ± standard error of the mean (SEM) of representative experiments, unless otherwise stated.
Results
We first studied the prognostic impact of Gas6 and TAMR messenger RNA (mRNA) expression in a cohort of uniformly treated cn AML patients (n = 112) (AML SHG0199; www.clinicaltrials.gov #NCT00209833; for patient characteristics, see supplemental Table 1). We found expression of Axl, Tyro3, Mer, and Gas6 in 57%, 79%, 85%, and 90% of patients, respectively. Upon correlation of expression data with survival data, patients expressing Axl above the median showed a significantly shorter overall survival (OS) than patients expressing Axl below the median (Figure 1A). Tyro3, Mer, and Gas6 lacked prognostic impact in univariate analysis. Axl expression levels represented an independent prognostic factor for OS after correcting for established prognostic factors in multivariate analysis (Table 1). Of note, age was not prognostic in our cohort, which might be explained by the limited patient number resulting from sample availability. Axl expression levels were independent of patient age, French-American-British type or different mutations with prognostic implications (supplemental Table 2). Adjusting for the time-dependent covariate of bone marrow (BM) transplantation (BMT) indicated no influence of BMT status on prognostic impact of Axl (P = .27).
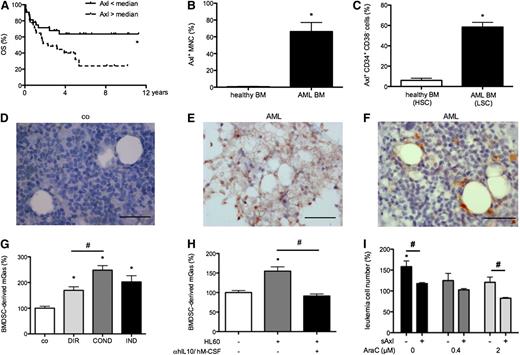
Axl represents a prognostic factor in Cn AML patients and is upregulated by AML cells, whereas its ligand Gas6 is produced by BM stroma cells. (A) Cn AML patients expressing Axl mRNA above the median survived significantly shorter than patients expressing Axl below the median (Kaplan-Meier survival analysis; n = 64; P < .05). (B-C) Quantification of Axl protein expression by flow cytometry of AML and healthy BM MNCs (n = 19/6) and CD34+ CD38− cells (n = 9/6) indicated a higher percentage of Axl+ cells in both populations in AML compared with healthy cells, respectively (*P < .05). (D-F) Representative immunohistochemical Gas6 stainings showing higher percentage of Gas6+ stromal cells in AML compared with control BM (n = 7/5). Bar represents 50 µm. (G) Gas6 ELISA indicating upregulation of mGas6 by OP9 stromal cells induced by direct (DIR) and indirect (IND) coculture with hGas6− HL60 leukemia cells as well as by HL60-conditioned medium (COND) (n = 3; *P < .05; #P < .05). (H) Abrogation of HL60-induced Gas6 upregulation in S17 stroma cells by hIL-10 and hM-CSF neutralizing antibodies (n = 3; *P < .05; #P < .05). (I) Neutralization of Gas6 by sAxl decreased stroma cell–induced resistance of cocultured HL60 cells to cytarabine (AraC) (data normalized to HL60 single culture without treatment; n = 3; *P < .05).
Axl represents a prognostic factor in Cn AML patients and is upregulated by AML cells, whereas its ligand Gas6 is produced by BM stroma cells. (A) Cn AML patients expressing Axl mRNA above the median survived significantly shorter than patients expressing Axl below the median (Kaplan-Meier survival analysis; n = 64; P < .05). (B-C) Quantification of Axl protein expression by flow cytometry of AML and healthy BM MNCs (n = 19/6) and CD34+ CD38− cells (n = 9/6) indicated a higher percentage of Axl+ cells in both populations in AML compared with healthy cells, respectively (*P < .05). (D-F) Representative immunohistochemical Gas6 stainings showing higher percentage of Gas6+ stromal cells in AML compared with control BM (n = 7/5). Bar represents 50 µm. (G) Gas6 ELISA indicating upregulation of mGas6 by OP9 stromal cells induced by direct (DIR) and indirect (IND) coculture with hGas6− HL60 leukemia cells as well as by HL60-conditioned medium (COND) (n = 3; *P < .05; #P < .05). (H) Abrogation of HL60-induced Gas6 upregulation in S17 stroma cells by hIL-10 and hM-CSF neutralizing antibodies (n = 3; *P < .05; #P < .05). (I) Neutralization of Gas6 by sAxl decreased stroma cell–induced resistance of cocultured HL60 cells to cytarabine (AraC) (data normalized to HL60 single culture without treatment; n = 3; *P < .05).
Axl expression represents an independent prognostic factor in cytogenetically normal de novo AML
| Variable . | OR (95% CI) . | P . |
|---|---|---|
| Axl expression below/above median | 5.46 (1.76-16.96) | .003 |
| Age | 0.97 (0.93-1.02) | .245 |
| Sex | 0.99 (0.33-2.95) | .986 |
| ECOG performance status | 0.66 (0.25-1.74) | .405 |
| Karyotype | – | All Cn AML |
| Cytogenetic risk group | – | All Cn AML |
| Leukocyte count | 1.00 (1.00-1.00) | .095 |
| Thrombocyte count | 1.00 (1.00-1.01) | .290 |
| Extramedullary disease | 3.42 (0.80-14.67) | .098 |
| Response to first induction therapy | 1.15 (0.37-3.62) | .808 |
| Blast count BM | 0.98 (0.96-1.00) | .055 |
| FLT3-ITD | 1.70 (0.58-5.00) | .336 |
| NRAS mutation | 0.36 (0.05-2.85) | .333 |
| IDH1/IDH2 | 1.42 (0.36-5.66) | .662 |
| WT1 mutation | 2.57 (0.71-9.26) | .1501 |
| WT1 SNP | 0.47 (0.15-1.48) | .198 |
| Variable . | OR (95% CI) . | P . |
|---|---|---|
| Axl expression below/above median | 5.46 (1.76-16.96) | .003 |
| Age | 0.97 (0.93-1.02) | .245 |
| Sex | 0.99 (0.33-2.95) | .986 |
| ECOG performance status | 0.66 (0.25-1.74) | .405 |
| Karyotype | – | All Cn AML |
| Cytogenetic risk group | – | All Cn AML |
| Leukocyte count | 1.00 (1.00-1.00) | .095 |
| Thrombocyte count | 1.00 (1.00-1.01) | .290 |
| Extramedullary disease | 3.42 (0.80-14.67) | .098 |
| Response to first induction therapy | 1.15 (0.37-3.62) | .808 |
| Blast count BM | 0.98 (0.96-1.00) | .055 |
| FLT3-ITD | 1.70 (0.58-5.00) | .336 |
| NRAS mutation | 0.36 (0.05-2.85) | .333 |
| IDH1/IDH2 | 1.42 (0.36-5.66) | .662 |
| WT1 mutation | 2.57 (0.71-9.26) | .1501 |
| WT1 SNP | 0.47 (0.15-1.48) | .198 |
Multivariate Cox regression survival analysis indicated that Axl represents an independent prognostic factor in Cn AML after correcting for established prognostic factors.
Next, we investigated Axl protein expression in AML BM compared with healthy BM (n = 19/6) by flow cytometry. These analyses indicated (1) presence of Axl protein in 63% of patients (n = 19); (2) higher Axl expression by AML mononucleated cells (MNCs) compared with healthy MNCs (Figure 1B); (3) Axl expression by 73 ± 7% of AML blasts (n = 8); and (4) higher expression of Axl by CD34+CD38− AML stem cells compared with healthy CD34+CD38− BM stem cells (Figure 1C). Finally, we analyzed distribution of Axl ligand Gas6 in BM sections of AML patients and controls (n = 7/5). We found that Gas6 expression was low in AML cells, similar to healthy hematopoietic cells. In contrast, Gas6 was abundant in AML BM stromal cells with fibroblastic/mesenchymal morphology (referred to as BMDSCs), whereas its expression was lower in control BMDSCs (Figure 1D-F). Osteoblasts and endothelial cells exhibited similar Gas6 levels in AML patients compared with controls, whereas fewer osteoclasts expressed Gas6 in AML (supplemental Table 3). Thus, Axl represents a therapeutic target upregulated by AML cells, whereas expression of its ligand becomes increased in AML BM stroma.
Determination of Gas6 and Axl mRNA and protein expression levels in different AML cell lines revealed that, similar to our observations in AML patients, most cell lines expressed Axl, whereas expression of Gas6 was restricted (supplemental Table 4). Congruent with the patient data, Gas6 was secreted by different BMDSC cell lines (S17, OP9) as well as by primary murine BMDSCs (supplemental Table 4). We focused on 2 Gas6+ cell lines (MV4-11, OCI-AML5) and 1 Gas6− cell line (HL60) to dissect impact of autocrine vs paracrine Gas6 supplied by BMDSCs on AML biology.
Interestingly, upon culture of human Gas6− HL60 cells or Gas6+ MV4-11 cells on top of S17 or OP9 cells (direct coculture) or separated by inserts (indirect coculture), Gas6 was specifically upregulated in stromal cells, whereas its levels remained essentially unchanged in AML cells as demonstrated by species-specific ELISAs. Gas6 upregulation in BMDSCs also occurred following incubation with AML cell–conditioned medium (Figure 1G; supplemental Figure 1A-B; and data not shown), suggesting a role for soluble mediators. Therefore, we investigated expression of interleukin-10 (IL-10), IL-4, and macrophage colony-stimulating factor (M-CSF), soluble candidate cytokines implicated in Gas6 upregulation in other cell types,18,33 in BMDSCs, and in AML cells by species-specific qRT-PCR after coculture. We found that HL60 cells upregulated IL-10 and M-CSF, but not IL-4 upon coculture with stromal cells (supplemental Figure 1C). qRT-PCR revealed that BMDSCs expressed IL-10 receptor as well as M-CSF receptor (data not shown). Subsequently, we added human IL-10 and hM-CSF function-blocking antibodies to the cocultures and found that the presence of both anti–hIL-10 and anti-hM-CSF attenuated mGas6 upregulation, whereas each antibody alone had no effect (Figure 1H). Interestingly, this stroma-derived Gas6 was partially responsible for the well-known protective effect of BMDSCs on AML cells34-36 because the pro-survival effect of S17 and OP9 cells on HL60 cells was reduced by sAxl, a Gas6-neutralizing “trap” molecule (supplemental Figure 1D).37 In addition, we found that Gas6 contributed to the chemoprotective effect of BMDSCs on AML cells because sAxl decreased their pro-survival effect upon treatment with cytarabine (Figure 1I). Thus, AML-mediated M-CSF and IL-10 instructs BMDSCs to secrete Gas6, which supports tumor growth and chemoresistance.
Based on these data and on the improved survival of patients expressing low Axl levels, we hypothesized that Axl receptor signaling plays a role in AML pathobiology and chemoresistance. Indeed, presence of Axl confers a growth advantage to AML cells because overexpression of Axl increased numbers of viable cells in comparison with controls, whereas its downmodulation had the opposite effect, in concordance with published literature (Figure 2A-B; supplemental Figure 1E).21 In conditions mimicking adverse conditions in the leukemia microenvironment by serum starvation, Axl phosphorylation was increased in Gas6+ MV4-11 and OCI-AML5 cells (Figure 2C). Furthermore, treatment of Gas6+ AML cells (MV4-11, OCI-AML5) with different concentrations of doxorubicin or cytarabine induced upregulation and increased phosphorylation of Axl in agreement with published data (supplemental Figure 1F and data not shown).22 In contrast, Gas6− HL60 cells did not show enhanced Axl phosphorylation upon chemotherapy and decreased Axl phosphorylation when, after serum starvation, no exogenous Gas6 was present (Figure 2D). Thus, autocrine or paracrine presence of Gas6 might be required for Axl (hyper)activation in AML cell lines. Altogether, we concluded that blockade of Axl signaling holds potential to inhibit AML cells, increase their chemosensitivity, and disrupt their protective niche within the BM.
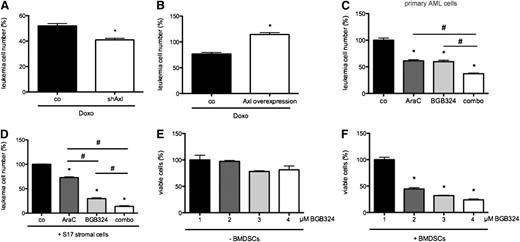
Axl blockade inhibits growth and induces apoptosis of AML cells via Bcl-2 and Puma. (A) Lentiviral overexpression of Axl compared with control-infected cells indicated a growth advantage of MV4-11 leukemia cells (n = 3; *P < .05, #P < .05). (B) In contrast, silencing of Axl by shRNA reduced growth of AML cells (n = 3; *P < .05; #P < .05). (C) Immunoblot showing increased Axl phosphorylation Gas6+ OCI-AML5 and MV4-11 cells induced by serum starvation (SF). Data are also provided as densitometric quantification of (pAxl/β-actin)/(tAxl/β-actin) normalized to cells cultured in normal medium (NM; n = 3; *P < .05). (D) In Gas6− HL60 cells phosphorylation of Axl was not upregulated in SF conditions. Densitometric quantification of (pAxl/β-actin)/(tAxl/β-actin) was normalized to NM cells (n = 3; *P < .05). (E) Treatment with BGB324 induced upregulation of pro-apoptotic Puma mRNA levels in AML cells compared with control (n = 3; *P < .05). (F) Immunoblot of anti-apoptotic Bcl-2 that was decreased by BGB324 treatment. Densitometric quantification of (cleaved caspase 3)/(β-actin) is normalized to untreated cells (n = 3; *P < .05). (G) Downmodulation of Puma reduced numbers of Annexin V+ cells after treatment with BGB324. Data are presented as percentage of mean Annexin V+ cells ± SEM (n = 3; *P < .05). (H) Overexpression of Bcl-2 reduced the fraction of Annexin V+ apoptotic cells after treatment with BGB324. Data are presented as percentage of mean Annexin V+ cells ± SEM (n = 3; *P < .05). (I) BGB324 was more efficient in primary AML cells expressing high levels of Axl (n = 10; r2 = 0.84; P < .05).
Axl blockade inhibits growth and induces apoptosis of AML cells via Bcl-2 and Puma. (A) Lentiviral overexpression of Axl compared with control-infected cells indicated a growth advantage of MV4-11 leukemia cells (n = 3; *P < .05, #P < .05). (B) In contrast, silencing of Axl by shRNA reduced growth of AML cells (n = 3; *P < .05; #P < .05). (C) Immunoblot showing increased Axl phosphorylation Gas6+ OCI-AML5 and MV4-11 cells induced by serum starvation (SF). Data are also provided as densitometric quantification of (pAxl/β-actin)/(tAxl/β-actin) normalized to cells cultured in normal medium (NM; n = 3; *P < .05). (D) In Gas6− HL60 cells phosphorylation of Axl was not upregulated in SF conditions. Densitometric quantification of (pAxl/β-actin)/(tAxl/β-actin) was normalized to NM cells (n = 3; *P < .05). (E) Treatment with BGB324 induced upregulation of pro-apoptotic Puma mRNA levels in AML cells compared with control (n = 3; *P < .05). (F) Immunoblot of anti-apoptotic Bcl-2 that was decreased by BGB324 treatment. Densitometric quantification of (cleaved caspase 3)/(β-actin) is normalized to untreated cells (n = 3; *P < .05). (G) Downmodulation of Puma reduced numbers of Annexin V+ cells after treatment with BGB324. Data are presented as percentage of mean Annexin V+ cells ± SEM (n = 3; *P < .05). (H) Overexpression of Bcl-2 reduced the fraction of Annexin V+ apoptotic cells after treatment with BGB324. Data are presented as percentage of mean Annexin V+ cells ± SEM (n = 3; *P < .05). (I) BGB324 was more efficient in primary AML cells expressing high levels of Axl (n = 10; r2 = 0.84; P < .05).
To investigate the biological effects of Axl inhibition in AML cells, we used a well-characterized, clinically applicable small-molecule Axl kinase inhibitor BGB324 (formerly named R428)38 that inhibits phosphorylation of Axl in AML cells (supplemental Figure 2A). Application of BGB324 monotherapy inhibited proliferation of FLT3-mutated MV4-11 cells and of FLT3 wild-type (WT) OCI-AML-5, THP-1, and Hoxa9/Meis1 cells in a dose-dependent manner with a 50% inhibition/inhibitory concentration (IC50) of 1.75 ± 0.002 µM (MV4-11), 1.35 ± 0.001 µM (OCI-AML5), 2.94 ± 0.003 µM (THP-1), and 0.81 ± 0.001 µM (Hoxa9/Meis1), respectively (supplemental Figure 2B-E). Inhibition of proliferation was validated by 5-bromo-2′-deoxyuridine assays in MV4-11 cells (supplemental Figure 2F). In addition, BGB324 exerted a pro-apoptotic effect on AML cell lines shown by increased Annexin V+ cells (supplemental Figure 2G). Treatment with BGB324 increased expression of the anti-apoptotic Bcl family member Puma (Figure 2E), whereas anti-apoptotic Bcl-2 levels were reduced in BGB324-sensitive cell lines (Figure 2F and data not shown). In contrast to our findings in MV4-11 and OCI-AML5 cells, Bcl-2 levels remained unchanged in BGB324-resistant HL60 cells (supplemental Figure 3A-B). Also, Puma mRNA levels were not significantly increased in HL60 cells treated with BGB324 (supplemental Figure 3C).
To assess functional significance of these perturbations, we silenced Puma by means of lentiviral constructs and subsequently incubated Puma-silenced or control MV4-11 cells and OCI-AML5 cells with different concentrations of BGB324. These experiments revealed that Puma-silenced AML cells underwent less apoptosis in comparison with control cells upon Axl blockade (Figure 2G; supplemental Figure 3D; and data not shown). In line with the mechanism that BGB324 increases Puma levels, which leads to repression of anti-apoptotic Bcl-2 and thereby induces apoptosis, overexpression of Bcl-2 reduced BGB324-mediated apoptosis (Figure 2H; supplemental Figure 3E; and data not shown). These data suggest that Puma and Bcl-2 have functional relevance in mediating induction of apoptosis by BGB324.
BM cells from 7 of 11 patients with primary human AML were sensitive toward Axl inhibition with BGB324 (IC50 1.9 ± 0.5 µM). FLT3-mutational status did not influence response to BGB324 because FLT3 WT AML cells were similarly sensitive to this Axl inhibitor (n = 3/8; P = .4212). Importantly, IC50 values correlated with Axl protein expression levels, indicating specificity of the therapeutic effect because Axl− cells were almost completely resistant (Figure 2I). Thus, after validation in larger cohorts, Axl expression levels could serve as a biomarker for patient stratification for Axl-targeting agents in the clinic. Healthy BM MNCs that express less Axl (described previously) were also almost completely resistant toward BGB324 (IC50 8.9 ± 3.1 μM, n = 5, P = .02) indicating an AML-specific effect of the compound. The role of autocrine Gas6 in primary cells in response to BGB324 is the subject of future studies. Our immunohistochemistry data indicate abundance of stroma-derived Gas6 in AML BM and lower expression in AML cells. qRT-PCR data show that 90% of AML patients express Gas6. Thus, in AML patients, paracrine Gas6 might be more important than autocrine Gas6, but in any case Gas6 protein is present in AML microenvironment.
To determine whether the observed biological effects specifically depend on blockade of Axl and not on (potential) off-target effects of BGB324, we used lentiviral short hairpin RNA (shRNA)-mediated knockdown. Silencing of Axl in MV4-11 cells decreased the sensitivity toward BGB324 (Figure 3A). Conversely, efficacy of BGB324 was enhanced upon overexpression of Axl (Figure 3B).
The small molecule Axl inhibitor BGB324 inhibits Akt and Erk signal transduction pathways. (A) Silencing of Axl with shRNA reduced sensitivity toward 3 µM BGB324 as shown by WST-1 assay of control-infected and shAxl-infected MV4-11 cells. Percentage of viable cells was normalized to untreated control cells (n = 3; *P < .05). (B) The effects of BGB324 on Axl overexpressing MV4-11 cells were more pronounced compared with control-infected cells (n = 3; *P < .05). (C) Immunoblot indicated inhibition of starvation-induced Akt phosphorylation in Gas6+ MV4-11 cells upon treatment with BGB324. Densitometric quantification of (pAkt/β-actin)/(tAkt/β-actin) was normalized to NM cells (n = 3; *P < .05). (D) BGB324 inhibited phosphorylation of Erk upon serum starvation as shown by immunoblot. Densitometric quantification of (pErk/β-actin)/(tErk/β-actin) was normalized to untreated cells (n = 3; *P < .05). (E) Immunoblot showing no inhibition of starvation-induced Akt phosphorylation by BGB324 in Gas6− HL60 cells. Densitometric quantification of (pAxl/β-actin)/(tAxl/β-actin) was normalized to cells in NM (n = 3; *P < .05). (F) Inhibitory effect of 2 µM BGB324 on growth of Gas6+ OCI-AML5 cells was abrogated by Gas6 neutralization via sAxl (n = 3; *P < .05). (G) Expression of Gas6 rendered Gas6− HL60 cells sensitive toward treatment with BGB324 (data normalized to untreated cells; n = 3; *P < .05). (H) Cytarabine (AraC; 4 µM) and BGB324 (2 µM) exerted additive inhibitory effects on MV4-11 cells (n = 3; *P < .05; #P < .05).
The small molecule Axl inhibitor BGB324 inhibits Akt and Erk signal transduction pathways. (A) Silencing of Axl with shRNA reduced sensitivity toward 3 µM BGB324 as shown by WST-1 assay of control-infected and shAxl-infected MV4-11 cells. Percentage of viable cells was normalized to untreated control cells (n = 3; *P < .05). (B) The effects of BGB324 on Axl overexpressing MV4-11 cells were more pronounced compared with control-infected cells (n = 3; *P < .05). (C) Immunoblot indicated inhibition of starvation-induced Akt phosphorylation in Gas6+ MV4-11 cells upon treatment with BGB324. Densitometric quantification of (pAkt/β-actin)/(tAkt/β-actin) was normalized to NM cells (n = 3; *P < .05). (D) BGB324 inhibited phosphorylation of Erk upon serum starvation as shown by immunoblot. Densitometric quantification of (pErk/β-actin)/(tErk/β-actin) was normalized to untreated cells (n = 3; *P < .05). (E) Immunoblot showing no inhibition of starvation-induced Akt phosphorylation by BGB324 in Gas6− HL60 cells. Densitometric quantification of (pAxl/β-actin)/(tAxl/β-actin) was normalized to cells in NM (n = 3; *P < .05). (F) Inhibitory effect of 2 µM BGB324 on growth of Gas6+ OCI-AML5 cells was abrogated by Gas6 neutralization via sAxl (n = 3; *P < .05). (G) Expression of Gas6 rendered Gas6− HL60 cells sensitive toward treatment with BGB324 (data normalized to untreated cells; n = 3; *P < .05). (H) Cytarabine (AraC; 4 µM) and BGB324 (2 µM) exerted additive inhibitory effects on MV4-11 cells (n = 3; *P < .05; #P < .05).
To delineate signaling pathways mediating the biological effects of Axl in AML, we investigated key mediators of leukemia cell proliferation and survival. Here, we found reduced starvation-induced phosphorylation of Akt in Gas6+ MV4-11 and OCI-AML5 cells upon Axl kinase inhibition with BGB324 (Figure 3C and data not shown). Furthermore, BGB324 inhibited baseline and granulocyte macrophage-CSF stimulated mitogen-activated protein kinase (MAPK) pathway signaling in MV4-11 cells (Figure 3D; supplemental Figure 4A).
We conducted additional experiments using the well-described Akt and MAPK inhibitors MK220639-41 and PD98059,42-44 respectively. These experiments revealed that (1) the combination of MAPK inhibition with BGB324 does not exert additive effects at different dose levels (4-8 µM); (2) upon addition of chemotherapy, the therapeutic effect in combination with BGB324 is significantly higher compared with the combination with the MAPK inhibitor; and (3) addition of MAPK inhibitor to the combination of chemotherapy and BGB324 does not result in additive therapeutic efficacy (supplemental Figure 4B and data not shown). These results indicate that BGB324 inhibits MAPK signaling. Furthermore, they underscore the specific implication of Axl in chemoresistance. Similar results were obtained when using the Akt inhibitor (0.5 and 4 µM; supplemental Figure 4C and data not shown).
Next, we sought to elucidate whether the observed AML inhibitory effects of Axl blockade were Gas6-dependent and therefore investigated effects of BGB324 on HL-60 cells (Axl+ Gas6−). Interestingly, Gas6− HL-60 cells were resistant to BGB324; neither proliferation was inhibited nor apoptosis was induced upon treatment (supplemental Figure 5A-B and data not shown). In line with these data, BGB324 did not inhibit Akt phosphorylation or elicit changes in Puma or Bcl-2 expression levels in HL60 cells (Figure 3E; supplemental Figure 3A-C). Specificity of this observation was confirmed in Gas6+ cell lines by knocking down Gas6 via shRNAs and by addition of sAxl. Both treatments induced resistance of OCI-AML-5 cells toward BGB324 (Figure 3F; supplemental Figure 5C). Conversely, expression of Gas6 induced sensitivity to BGB324 in Gas6− HL-60 cells (Figure 3G).
Subsequently, we investigated whether Axl signaling functionally mediates chemoresistance of AML cells. Concomitant inhibition of Axl by BGB324 or Axl shRNA in combination with doses in the range of the IC50 doses of doxorubicin (0.22 ± 0.001 µM) or cytarabine (3.30 ± 0.001 µM) determined by prior dose-response experiments revealed enhanced chemosensitivity of MV4-11 and OCI-AML cells (Figure 3H and data not shown). Again, silencing of Axl phenocopied this effect in MV4-11 cells (Figure 4A). Conversely, overexpression of Axl in these cell lines decreased chemosensitivity (Figure 4B). Isobologram analyses confirmed additive effects of BGB324 in combination with doxorubicin or cytarabine in MV4-11 and OCI-AML cells (supplemental Figure 5D-E and data not shown). The chemosensitivity of Gas6− HL60 cells was not affected by concomitant Axl-inhibition by BGB324 (supplemental Figure 5F). Interestingly, the observed reduction of AML cell chemoresistance by Axl receptor inhibition was mainly the result of increased apoptosis, as evidenced by Annexin V assays and activation of caspase 3 (supplemental Figure 6A-B). Similarly, combination therapy with BGB324 and cytarabine exerted an additive therapeutic effect on 7/11 primary AML patient cells (Figure 4C).
The Axl inhibitor BGB324 inhibits survival of AML cells and induces sensitivity toward chemotherapy. (A) Axl-silencing in MV4-11 leukemia cells increased chemosensitivity of AML cells treated with 200 nM doxorubicin (Doxo). Percentage of viable cells was normalized to untreated control cells, respectively (n = 3; *P < .05). (B) Overexpression of Axl in MV-411 cells increased chemosensitivity of AML cells treated with 200 nM Doxo. Percentage of viable cells was normalized to untreated control cells, respectively (n = 3; *P < .05). (C) AraC (2 µM) and BGB324 (1 µM) elicited additive antileukemic effects in primary AML cells (n = 3; *P < .05; #P < .05). (D) BGB324 treatment reduced stroma-mediated resistance of HL60 cells toward treatment with AraC when HL60 cells were cocultured with S17 cells (n = 3; *P < .05; #P < .05). (E) Gas6-deficient HL60 cells were resistant toward treatment with BGB324 when stroma cells were absent. (F) In the presence of Gas6-producing murine stromal cells (OP9), HL60 cells became sensitive toward treatment with BGB324. Viable cell number was determined by WST-1 assay after 48 hours and normalized to untreated control cells without (E) or with (F) coculture (n = 3; *P < .05).
The Axl inhibitor BGB324 inhibits survival of AML cells and induces sensitivity toward chemotherapy. (A) Axl-silencing in MV4-11 leukemia cells increased chemosensitivity of AML cells treated with 200 nM doxorubicin (Doxo). Percentage of viable cells was normalized to untreated control cells, respectively (n = 3; *P < .05). (B) Overexpression of Axl in MV-411 cells increased chemosensitivity of AML cells treated with 200 nM Doxo. Percentage of viable cells was normalized to untreated control cells, respectively (n = 3; *P < .05). (C) AraC (2 µM) and BGB324 (1 µM) elicited additive antileukemic effects in primary AML cells (n = 3; *P < .05; #P < .05). (D) BGB324 treatment reduced stroma-mediated resistance of HL60 cells toward treatment with AraC when HL60 cells were cocultured with S17 cells (n = 3; *P < .05; #P < .05). (E) Gas6-deficient HL60 cells were resistant toward treatment with BGB324 when stroma cells were absent. (F) In the presence of Gas6-producing murine stromal cells (OP9), HL60 cells became sensitive toward treatment with BGB324. Viable cell number was determined by WST-1 assay after 48 hours and normalized to untreated control cells without (E) or with (F) coculture (n = 3; *P < .05).
Further, we determined whether treatment with BGB324 could reduce Gas6-mediated chemoresistance of AML cells elicited by BMDSCs. To measure this, we added BGB324 at different concentrations ± chemotherapy ± sAxl to cocultures of S17 and HL60 cells and found partial abrogation of the chemoprotective effect of BMDSCs upon Axl inhibition (Figure 4D). Thus paracrine Gas6 can induce chemoresistance in Gas6− cells via Axl. Further experiments revealed that BGB324 inhibited proliferation of HL60 cells in the presence of BMDSCs, whereas HL60 cells alone were resistant to Axl inhibition (Figure 4E-F). Interestingly, additional sAxl could not enhance the inhibitory effect of BGB324 (data not shown) indicating that ligand-mediated effects on other TAM receptors besides Axl most likely play a negligible role in this process. Hence, inhibition of Gas6-Axl signaling in AML cells by pharmacologic or RNA interference agents impedes growth in vitro, increases chemosensitivity, and disrupts BMDSC-derived chemoprotective effects. These effects are specific for the Gas6-Axl axis, because they are abrogated in absence of Gas6 or Axl.
Encouraged by the in vitro data indicating therapeutic potential of Axl inhibition in AML, we investigated the effects of Axl kinase inhibition in vivo. Subcutaneous human AML FLT3 ITD+ MV4-11 xenografts45 displayed inhibition of AML tumor growth and survival of systemic FLT3 WT Hoxa9/Meis1–bearing AML mice26 was significantly prolonged upon treatment with BGB324 (Figure 5A-B). Remarkably, the highest dose caused regression of established MV4-11 tumors by 50.4 ± 22.9% (Figure 5A). To validate that BGB324 affected Axl kinase activity, we determined the phosphorylation status of Axl relative to total Axl in control-treated AML tissue compared with BGB324-treated AML tumors. This analysis demonstrated pronounced inhibition of Axl-phosphorylation in AML tumors by BGB324 treatment, indicating target inhibition by the compound (Figure 5C). Importantly, Axl inhibition reduced anemia, thrombocytopenia, and leukemia cell burden in Hoxa9/Meis1-bearing AML mice (Figure 5D-F). Thus, BGB324 exerts therapeutic effects on clinically relevant end points in localized and systemic preclinical AML models regardless of FLT3 mutational status.
Axl blockade exerts therapeutic potential in vivo. (A) Dose-dependent inhibition of MV4-11 tumor growth by BGB324 (n = 4/6/6; *P < .05). (B) OS benefit of Axl inhibition with BGB324 in vivo in the systemic Hoxa9/Meis1 BMT mouse model (median OS 31 days [control], 38 days [12.5 mg/kg BGB324], and 42 days [25 mg/kg BGB324], respectively; n = 7; *P < .05 vs control, respectively). (C) Immunoblots from MV4-11 tumor protein extracts indicated inhibition of Akt, Erk, and Axl phosphorylation and of Bcl2 expression as well as induction of cleavage of caspase 3 after treatment with 25 mg/kg BGB324. Densitometric quantification of (phosphorylated protein/β-actin)/(total protein expression/β-actin), (Bcl-2/β-actin) and (cleaved caspase 3/β-actin) was normalized to control-treated tumors (n = 4; *P < .05). (D) Anemia and (E) thrombocytopenia, 2 cardinal symptoms of AML, were improved by the treatment with 12.5 mg/kg BGB324 at endstage in the Hoxa9/Meis1 model compared with control-treated animals (n = 7; *P < .05). (D) This treatment also improved the leukemia burden determined by flow cytometric quantification of GFP+ leukemia cells at endstage (n = 7; *P < .05).
Axl blockade exerts therapeutic potential in vivo. (A) Dose-dependent inhibition of MV4-11 tumor growth by BGB324 (n = 4/6/6; *P < .05). (B) OS benefit of Axl inhibition with BGB324 in vivo in the systemic Hoxa9/Meis1 BMT mouse model (median OS 31 days [control], 38 days [12.5 mg/kg BGB324], and 42 days [25 mg/kg BGB324], respectively; n = 7; *P < .05 vs control, respectively). (C) Immunoblots from MV4-11 tumor protein extracts indicated inhibition of Akt, Erk, and Axl phosphorylation and of Bcl2 expression as well as induction of cleavage of caspase 3 after treatment with 25 mg/kg BGB324. Densitometric quantification of (phosphorylated protein/β-actin)/(total protein expression/β-actin), (Bcl-2/β-actin) and (cleaved caspase 3/β-actin) was normalized to control-treated tumors (n = 4; *P < .05). (D) Anemia and (E) thrombocytopenia, 2 cardinal symptoms of AML, were improved by the treatment with 12.5 mg/kg BGB324 at endstage in the Hoxa9/Meis1 model compared with control-treated animals (n = 7; *P < .05). (D) This treatment also improved the leukemia burden determined by flow cytometric quantification of GFP+ leukemia cells at endstage (n = 7; *P < .05).
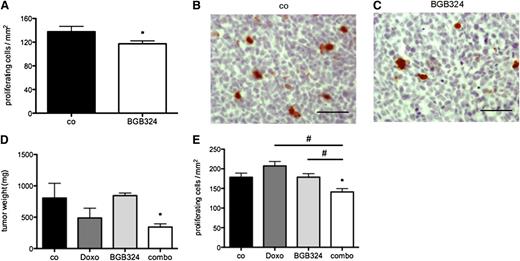
To further elucidate the effects of Axl kinase inhibition on AML tumor cells in vivo, we analyzed proliferation and apoptosis markers in MV4-11 tumors treated with BGB324 compared with control-treated tissue. Histomorphometric analysis of phospho-histone H3 staining46 showed reduced proliferation of AML cells from BGB324-treated tumors compared with controls (Figure 6A-C). Quantification of activated Caspase 3 by immunoblotting revealed enhanced apoptosis in BGB324-treated AML compared with control (Figure 5C). Hence, the observed therapeutic efficacy of BGB324 in vivo is at least partly due to inhibition of AML cell proliferation and increased apoptosis.
Axl blockade chemosensitizes AML cells in vivo. (A) Morphometric analysis of phospho-Histone H3+ cells indicated inhibition of proliferation of MV4-11 AML cells in vivo by BGB324 (25 mg/kg) (n = 4/5; *P < .05). (B-C) Representative immunohistochemistry pictures of phospho-Histone H3 stainings on sections from MV4-11 control- and BGB324-treated tumors (bar represents 50 µm). (D) BGB324 (12.5 mg/kg) exerted a chemosensitizing effect on efficacy of Doxo (3 mg/kg) in MV4-11 xenografts in vivo (n = 4/4/3/6; *P < .05). (E) Analysis of immunohistochemistry for phospho-Histone H3 confirmed an additive effect of Doxo and BGB324 on proliferation of MV4-11 cells in vivo (n = 4/4/3/4; *P < .05; #P < .05).
Axl blockade chemosensitizes AML cells in vivo. (A) Morphometric analysis of phospho-Histone H3+ cells indicated inhibition of proliferation of MV4-11 AML cells in vivo by BGB324 (25 mg/kg) (n = 4/5; *P < .05). (B-C) Representative immunohistochemistry pictures of phospho-Histone H3 stainings on sections from MV4-11 control- and BGB324-treated tumors (bar represents 50 µm). (D) BGB324 (12.5 mg/kg) exerted a chemosensitizing effect on efficacy of Doxo (3 mg/kg) in MV4-11 xenografts in vivo (n = 4/4/3/6; *P < .05). (E) Analysis of immunohistochemistry for phospho-Histone H3 confirmed an additive effect of Doxo and BGB324 on proliferation of MV4-11 cells in vivo (n = 4/4/3/4; *P < .05; #P < .05).
In concordance with our in vitro data, we found decreased Bcl-2, pAkt, and pErk levels in tumor tissue after treatment with BGB324 compared with control tumors (Figure 5C). These results indicate that similar perturbations in signal transduction intermediates might be responsible for increased apoptosis and reduced proliferation induced by BGB324 in vivo. We also analyzed efficacy of BGB324 in Gas6− HL60 cells implanted subcutaneously into NSG mice. Here, we found no difference in tumor growth, corroborating the in vitro finding that HL60 cells are resistant against BGB324 when Gas6 is absent as demonstrated by qRT-PCR of HL60 xenografts (supplemental Figure 7A-B and data not shown).
Our in vitro experiments implicated a role for Axl in mediating chemoresistance of AML cells. We wished to confirm this finding in vivo and combined BGB324 treatment with chemotherapy in our AML models. In the MV4-11 model, we combined BGB324 at a low dose level of 12.5 mg/kg with 3 mg/kg doxorubicin. We deliberately chose subtherapeutic doses of BGB324 and doxorubicin to detect additive effects of the treatments. These experiments showed that combined treatment with BGB324 and doxorubicin inhibits MV4-11 growth, whereas each single treatment did not (Figure 6D). Upon analysis of proliferation and apoptosis, again only the combined approach could inhibit proliferation (Figure 6E).
Discussion
Our study shows that Axl expression represents an independent prognostic factor and provides evidence for a specific role of Gas6-Axl axis as therapeutic target in AML.
By analyzing AML patient samples as well as in vivo and in vitro models, this study yielded the following major findings: (1) high Axl mRNA expression represents an independent poor prognostic factor in Cn AML patients; (2) Axl is a therapeutic target upregulated by primary AML (stem) cells when compared with healthy hematopoietic cells independent of FLT3 mutational status; (3) Axl upregulation in concert with autocrine or paracrine Gas6 specifically induces chemoresistance of AML cells; (4) AML cells instruct BMDSCs to upregulate Gas6, which fosters their growth and chemoresistance; (5) blockade of Axl signaling by a small-molecule Axl kinase inhibitor (BGB324) reduces proliferation and induces apoptosis of AML cells by upregulating Puma expression, which inhibits anti-apoptotic Bcl-2; (6) BGB324 exerts a therapeutic anti-AML effect on relevant clinical end points in FLT3-mutated and -WT AML in systemic and subcutaneous AML models; and (7) presence of Gas6 and Axl might predict for the success of this strategy.
Circumstantial evidence in the literature links Axl expression to prognosis of AML patients.23 However, our study represents the first in which expression of Axl, Sky, Mer, and Gas6 was quantified with qRT-PCR. In a previously published study,23 Axl but not Gas6 or other TAMR expression was determined by semiquantitative conventional PCR. Subsequently, Axl expression was correlated with outcome in a limited sample size yielding no significance in univariate analysis. We now demonstrate that Axl expression levels influence prognosis in univariate and multivariate analysis, whereas Gas6 or other TAMRs have no prognostic relevance. Subsequently we investigated the target potential of Axl.
Although a previous study was primarily focused on the effect of Axl inhibition in FLT3-ITD+ AML,21 we show similar efficacy of Axl targeting approaches in FLT3-ITD+ and FLT3-WT AML. Our data also indicate that >50% of AML patients express Axl protein and that efficacy of BGB324 correlates with Axl expression levels. Thus, a substantial fraction of AML patients is potentially eligible for treatment, and Axl expression could serve as a biomarker for patient stratification. In addition, we show single-agent activity and additive effects of BGB324 when combined with chemotherapy in vitro and in vivo, indicating that BGB324 might be useful for treatment of AML patients alone and in combination with cytotoxic agents.
We discovered that blockade of Axl by BGB324 inhibits Akt and MAPK pathways in AML cells, explaining the antiproliferative and pro-apoptotic effects of BGB324 on AML cells. Functional significance was demonstrated by pharmacologic inhibition of these pathways; however genetic approaches might be required to render the findings more definitive. Furthermore, we demonstrate the novel finding that apoptosis induction by BGB324 is mediated via upregulation of Puma, which in turn suppresses Bcl-2. However, we cannot rule out that Axl signaling exerts its pro-AML effects via additional signaling pathways. For instance, it was demonstrated that Axl is constitutively activated in B-cell chronic lymphocytic leukemia cells and acts as a docking site of intracellular kinases such as Syk,47 which is similar to our findings in AML BGB324-induced apoptosis in chronic lymphocytic leukemia cells.47 Future studies are warranted to dissect the Axl signaling pathway in AML cells in detail.
Our work further indicates that interaction of AML cells with BMDSCs educates the stroma cells to upregulate Gas6, which fosters AML growth and chemoresistance. Until now, Gas6 was known to be an important stroma-derived factor supporting hematopoietic progenitor cells,36 whereas its role in AML biology and therapy resistance represents a novel finding. The Gas6 upregulation occurs via AML cell-derived granulocyte macrophage-CSF and IL-10. These cytokines were already shown to be involved in Gas6 upregulation in other cell types, including different populations of myeloid cells.18,33 Our findings in AML patient BM sections suggest that Gas6 is mainly secreted by BMDSCs, whereas AML cells show relatively little expression. Thus, Gas6 represents a mostly stroma-derived factor supporting AML growth and chemoresistance via Axl.
Interestingly, efficacy of Axl-targeted approaches can depend on presence of Gas6, because HL60 cells grown in vitro or subcutaneously without presence of Gas6-secreting stroma cells are resistant to BGB324. Conversely, overexpression of Gas6 or coculture with BMDSCs renders them sensitive toward BGB324. Future studies with crosstransplantations of Gas6− and Gas6+ AML cells in Gas6-deficient and WT hosts using systemic disease models are warranted. Such studies will be useful to validate the concept with alternative cell lines and to determine impact of autocrine vs paracrine Gas6 on sensitivity of AML to BGB324 in vivo.
The finding that Gas6 is upregulated in the BM stroma upon interaction with neoplastic cells is congruent with previous research showing upregulation of Gas6 by osteoblasts upon contact with prostate carcinoma cells.48 However, in prostate cancer cells residing in the BM, Gas6 exerted an inhibitory effect on proliferation,48 whereas in our study Gas6 fosters proliferation of AML cells. This divergent effect might be due to different biological effects of Gas6 on different (neoplastic) cell types. Further evidence for a proproliferative effect of Gas6 on different murine solid tumor cell lines was provided by an earlier study.18 Thus, Gas6 seems to act in a cell type–specific manner and further research is necessary in order to dissect specific effects of this cytokine on different cell types.
In summary, we postulate that AML cells educate BMDSCs to secrete Gas6, which fosters AML cell growth and chemoresistance. Axl inhibition by BGB324 is active in FLT3-ITD and FLT3-WT AML as single treatment and has additive effects in combination with chemotherapy. Success of Axl inhibition requires presence of Gas6 and Axl. Thus, Axl-targeting agents hold promise to improve current AML therapies.
Our findings might have possible implications beyond AML; for instance, Axl inhibitors might be useful in treating Axl+ solid cancer or target metastatic cancer cells in their BM niches.49
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Christine Gräfin zu Eulenburg (Department of Medical Biometry and Epidemiology, University Hospital Hamburg-Eppendorf, Hamburg, Germany) for help with the statistical analysis; Prof Björn Dahlbäck (Department of Laboratory Medicine, Section for Clinical Chemistry, Lund University, University Hospital Malmö, Sweden) for supplying the anti-Axl antibody; Dr Carol Stocking (Heinrich Pette Institute, Hamburg, Germany) for supplying OP9 cells; Prof Nicolaus Kröger (Department of Stem Cell Transplantation, University Hospital Hamburg, Germany) for providing samples from healthy bone marrow donors; Prof William C. Hahn (Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, MA) and Prof Clark W. Distelhorst (Department of Medicine and Pharmacology, Case Western Reserve University School of Medicine, Case Comprehensive Cancer Center, Cleveland, OH) for providing the Axl and Bcl-2 vectors, respectively; and Nils Jäger on behalf of the Animal Facility at University Hospital Hamburg-Eppendorf for taking care of the mice housing. Fluorescence-activated cell sorter analyses with BD Fortessa were performed at the FACS Sorting Core Unit at University Hospital Hamburg-Eppendorf. BGB324 was obtained from BerGenBio AS (Bergen, Norway).
This work was supported by the Max-Eder group leader program from Deutsche Krebshilfe, the Deutsche Forschungsgemeinschaft (Grant #LO1863/3-1), the Roggenbuck Stiftung, the Hamburger Krebsgesellschaft, the Medical Faculty of the University of Hamburg (FFM program), and the Hamburger Exzellenzinitiative (LEXI program) (S.L.); the Roggenbuck Stiftung and the Hamburger Krebsgesellschaft (A.S.); a Hubertus-Wald fellowship (M.J.); and the ERC Advanced Investigator Grant (269081 “DISSECT”) (K.P.).
Authorship
Contribution: S.L. initiated, conceived, designed, and supervised research, wrote the manuscript, and analyzed data; I.B.B. and A.S. designed experiments, wrote the manuscript, performed experiments, and analyzed data; M.W., R.E., J.S.W., K.R., D.S., S.S., V.W., M.C.-C., M.J., and C.H. performed experiments and data analysis; M.H., M.B., J.W., B.F., J.K., A.G., J.B.L., W.F., P.C., K.P., and C.B. contributed vital new reagents and commented on the research direction and edited the manuscript; and all authors discussed and commented on the manuscript.
Conflict-of-interest disclosure: S.L. receives research funding from BerGenBio AS. J.B.L. is a cofounder of BerGenBio AS. The remaining authors declare no competing financial interests.
Correspondence: S. Loges, Department of Hematology, Oncology and Bone Marrow Transplantation with Section Pneumology, Hubertus Wald Tumorzentrum, Institute of Tumor Biology, University Comprehensive Cancer Center Hamburg, University Medical Center Hamburg-Eppendorf, Martinistrasse 52, D-20246, Hamburg, Germany; e-mail: s.loges@uke.uni-hamburg.de.
References
Author notes
I.B.-B. and A.S. contributed equally to this work.



![Figure 5. Axl blockade exerts therapeutic potential in vivo. (A) Dose-dependent inhibition of MV4-11 tumor growth by BGB324 (n = 4/6/6; *P < .05). (B) OS benefit of Axl inhibition with BGB324 in vivo in the systemic Hoxa9/Meis1 BMT mouse model (median OS 31 days [control], 38 days [12.5 mg/kg BGB324], and 42 days [25 mg/kg BGB324], respectively; n = 7; *P < .05 vs control, respectively). (C) Immunoblots from MV4-11 tumor protein extracts indicated inhibition of Akt, Erk, and Axl phosphorylation and of Bcl2 expression as well as induction of cleavage of caspase 3 after treatment with 25 mg/kg BGB324. Densitometric quantification of (phosphorylated protein/β-actin)/(total protein expression/β-actin), (Bcl-2/β-actin) and (cleaved caspase 3/β-actin) was normalized to control-treated tumors (n = 4; *P < .05). (D) Anemia and (E) thrombocytopenia, 2 cardinal symptoms of AML, were improved by the treatment with 12.5 mg/kg BGB324 at endstage in the Hoxa9/Meis1 model compared with control-treated animals (n = 7; *P < .05). (D) This treatment also improved the leukemia burden determined by flow cytometric quantification of GFP+ leukemia cells at endstage (n = 7; *P < .05).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/122/14/10.1182_blood-2013-03-491431/4/m_2443f5.jpeg?Expires=1764976935&Signature=F5xp780ojsfOlq0Zz17COsSGI~vOffFxO1b8qX~ejjXBwikLl0oW5tIAQozhpq4p-HJTDsUsOBJGG4CAMdbvpKWfbk5LtXeVu9uK9J821RqgnZlfx5MvP3c8WGHjY~~4TLsfGmHCZv9TJnfcqMLCXaM4aS889oqvsx6anhJ-xLaoEoitI8tLGlK-7jFuBeYQ4xTrgazpx4S86P8KP~Qhs3SVKGKzira~Njz46WiUe1lJ-0pcC84mihFFOa-iG0sSSJ~ZwEVZ-ZW2RfFOeBzEhee4vWLYbQ-xeO6768xJCoG4BjrC20NU2-GbpauwYDGWF8QTEHkC7Wn3MGsbFfQKVw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal