In this issue of Blood, Rossi et al provide convincing evidence that extends and generalizes the importance of trogocytosis, a process in which effector cells use Fcγ receptors to remove Immunoglobulin G (IgG)-chelated antigens from donor cells. Their work suggests that the process may be beneficial in the context of epratuzumab treatment of autoimmune diseases.1
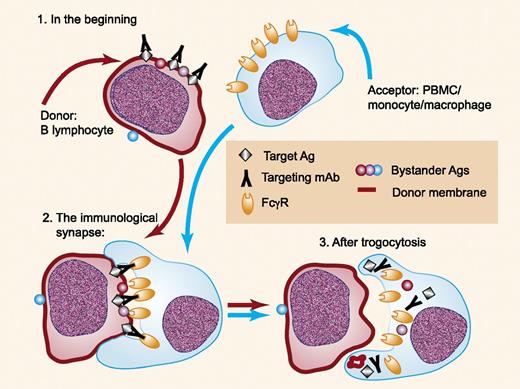
Trogocytosis of IgG bound to targeted antigens is mediated by Fcγ receptors on acceptor cells. Interaction of IgG bound to target antigens on the donor cell (1) with Fcγ receptors on the acceptor cell leads to formation of an immunologic synapse (2). The acceptor cell then ingests the immune complex and portions of the donor cell membrane, along with the participating Fcγ receptors (3). Other surface antigens in close proximity to the target immune complex are also taken up by the acceptor cell. Ag, antigen; PBMC, peripheral blood mononuclear cell. Professional illustration by Paulette Dennis.
Trogocytosis of IgG bound to targeted antigens is mediated by Fcγ receptors on acceptor cells. Interaction of IgG bound to target antigens on the donor cell (1) with Fcγ receptors on the acceptor cell leads to formation of an immunologic synapse (2). The acceptor cell then ingests the immune complex and portions of the donor cell membrane, along with the participating Fcγ receptors (3). Other surface antigens in close proximity to the target immune complex are also taken up by the acceptor cell. Ag, antigen; PBMC, peripheral blood mononuclear cell. Professional illustration by Paulette Dennis.
Textbooks of immunology, as well as immunology courses taught in medical and graduate schools, tell us about the many interesting activities promoted by Fcγ receptors on effector cells.2 Fcγ receptor–mediated binding of IgG-containing immune complex substrates by monocytes/macrophages and neutrophils can lead to phagocytosis of large substrates (IgG-opsonized particles and cells) and endocytosis of smaller, soluble immune complexes. Natural killer (NK) cells are not supposed to swallow their prey, but they can be quite effective at killing IgG-opsonized cells due to recognition by FcγRIIIA (CD16), thus leading to antibody-dependent cell-mediated cytotoxicity.3 However, another reaction is possible, which can occur if the effector (acceptor) cell does not engulf an entire substrate cell but instead chooses to nibble (gnaw; or trogo4 [ancient Greek]) off the most attractive material, which is IgG bound to a targeted antigen on the donor cell (see figure).5,6 That is, the acceptor cell first forms an immunologic synapse with the donor cell, based on interaction between acceptor cell Fcγ receptors with Fc regions of IgG antibodies bound to the targeted antigen (black cubes) on the donor cell. Then, instead of surrounding and engulfing the donor cell, the acceptor cell pinches off a small piece of the donor cell. The acceptor cell then internalizes what it caught; this includes portions of the cell membrane of the donor cell, the antibody/antigen immune complex, and Fcγ receptors of the acceptor cell that participated in the trogocytosis reaction. This system was not designed by a meticulous neurosurgeon because innocent bystander antigens (red and purple spheres) can be caught in the cross fire at the immunologic synapse and then are also taken up by the acceptor cell.5,7 Figure 6 in the article by Rossi et al presents clear evidence for both the immunologic synapse and the transfer of green membrane dye from epratuzumab-opsonized Daudi cells to monocytes. The fate of the various internalized materials remains to be delineated.
The evolutionary and biological significance of Fcγ receptor–mediated trogocytosis is not clear, but it is reasonable to hypothesize that this process could provide a means for acceptor cells to remove antibody-targeted particles and pathogens bound to normal cells without destroying them in the process. On the other hand, our laboratory has demonstrated that trogocytosis of cell-bound immune complexes, composed of B cell–associated CD20 along with rituximab or ofatumumab, may substantially compromise the therapeutic efficacy of these mAb’s (monoclonal antibodies) at high burdens of circulating malignant cells, often found in patients with chronic lymphocytic leukemia.8,9 This adverse event occurs when the usual very high doses of mAb are infused, which can lead to exhaustion of effector systems that are required for the immunotherapeutic action of these mAb’s.
The study by Rossi et al1 is important because it demonstrates that epratuzumab, an mAb that targets CD22 on B lymphocytes, can promote either internalization of CD22 (by the B cell) or trogocytosis of CD22 by acceptor cells; CD19, CD21, and several other B-cell surface molecules are among the innocent bystander antigens that are also removed from the B lymphocytes. The mechanism of action of epratuzumab in autoimmune diseases is not clearly understood because it does not promote rapid and substantial clearance of B cells. The study by Rossi et al suggests that an alternative outcome may result, which spares the B cells but compromises their (auto)immune activity. That is, B cells that are opsonized by epratuzumab are not subject to efficient clearance, but they are instead subject to trogocytosis of CD22, and it is removal of innocent bystanders CD19 and CD21, and possibly other functional proteins as well, that downregulates the autoimmune activity of the live, but damaged B cells. Rossi et al provide support and some validation for this idea based on careful correlative studies and analyses of B cells of systemic lupus erythematosus patients who had been treated with epratuzumab. Indeed, CD19 and CD21, along with CD22, were reduced considerably on patients’ B cells. Presumably, future studies will attempt to examine relevant immunologic functions of such B cells to determine if they have indeed suffered immunologic damage as a result of trogocytosis.
Rossi et al found that monocytes, neutrophils, and NK cells were all capable of promoting trogocytosis of B cells opsonized at CD22 with epratuzumab. Their results follow the same patterns we reported for trogocytosis of rituximab-CD20 complexes from B cells by acceptor cells, which also specifically included macrophages.6 Metchnikoff first studied these interesting “big eaters” more than a century ago,10 and he might have been pleased to know that under certain conditions they prefer to nibble. The factors that influence which path is followed are currently not known. Future investigations should address this difficult question because it is clear that under certain conditions phagocytosis would be a more favorable outcome, but in the case of epratuzumab therapy, trogocytosis might be the best result in autoimmune disease therapy.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal