Key Points
Allogeneic CD19-CAR VSTs are well tolerated by patients with relapsed B-cell malignancies post-HSCT.
At periods of CD19-CAR VST persistence, these cells demonstrate antitumor activity.
Abstract
Autologous T cells expressing a CD19-specific chimeric antigen receptor (CD19.CAR) are active against B-cell malignancies, but it is unknown whether allogeneic CD19.CAR T cells are safe or effective. After allogeneic hematopoietic stem cell transplantation (HSCT), infused donor-derived virus-specific T cells (VSTs) expand in vivo, persist long term, and display antiviral activity without inducing graft-vs-host disease; therefore, we determined whether donor VSTs, engineered to express CD19.CAR, retained the characteristics of nonmanipulated allogeneic VSTs while gaining antitumor activity. We treated 8 patients with allogeneic (donor-derived) CD19.CAR-VSTs 3 months to 13 years after HSCT. There were no infusion-related toxicities. VSTs persisted for a median of 8 weeks in blood and up to 9 weeks at disease sites. Objective antitumor activity was evident in 2 of 6 patients with relapsed disease during the period of CD19.CAR-VST persistence, whereas 2 patients who received cells while in remission remain disease free. In 2 of 3 patients with viral reactivation, donor CD19.CAR-VSTs expanded concomitantly with VSTs. Hence CD19.CAR-VSTs display antitumor activity and, because their number may be increased in the presence of viral stimuli, earlier treatment post-HSCT (when lymphodepletion is greater and the incidence of viral infection is higher) or planned vaccination with viral antigens may enhance disease control. This study is registered at clinicaltrials.gov as #NCT00840853.
Introduction
Although allogeneic hematopoietic stem cell transplant (HSCT) may be a curative option for patients with high-risk B-cell malignancies,1-3 opportunistic infections and disease relapse remain significant causes of morbidity and mortality.4,5 Donor lymphocyte infusion may control infections and, to a limited extent, leukemia/lymphoma relapse, but the associated graft-versus-host disease (GVHD) significantly limits the clinical success of this procedure.6-10 We and others have previously demonstrated that life-threatening viral infections with pathogens such as Epstein-Barr virus (EBV), cytomegalovirus (CMV), and adenoviruses (AdV) occurring after allogeneic HSCT can be treated without toxicity (including GVHD) by infusing ex vivo–expanded, donor-derived, virus-specific cytotoxic T cells (VSTs).7,11-13 In addition, these VSTs are capable of persisting several years after infusion.14
Unfortunately, adoptively transferred ex vivo–expanded leukemia/lymphoma antigen-specific T cells (for example T cells specific for minor histocompatibility antigens) have shown limited persistence and produced transient antitumor responses.15 By contrast, autologous T lymphocytes genetically modified to express CD19.CARs have shown promise as a highly effective way of treating even advanced CD19+ B-cell malignancies.16-19 However, the adaption of this methodology to the allogeneic setting has not been evaluated. Given that donor-derived VSTs are capable of expanding and persisting in HSCT recipients, we determined whether these cells could be safely engrafted with CD19.CAR and infused in patients with residual B-cell malignancies after HSCT, without inducing GVHD. We hypothesized that CAR-VSTs would be activated by endogenous viral antigens, increasing their expansion and persistence irrespective of the presence of CD19-expressing normal or malignant B cells. This approach should therefore provide activity that is both antiviral (through the native T-cell receptor [TCR]) and antitumor (through the CD19.CAR) from a single T-cell product.
We now show that CD19.CAR-engrafted VSTs capable of recognizing both virus-infected and malignant target cells can be safely administered to patients with high-risk CD19+ malignancies after allogeneic HSCT. The effects of these infusions on viral infections and malignant disease were also analyzed.
Materials and methods
Clinical study
This phase 1 study was conducted in accordance with the Declaration of Helsinki and was approved by the institutional review board of Baylor College of Medicine. It was designed to assess the feasibility and safety of infusing escalating doses of donor-derived VSTs (CMV, EBV, and AdV-specific) genetically modified to express a CD19-specific CAR (CD19.CAR-VSTs) in patients with B-cell malignancies who have either disease relapse or are at high risk for disease relapse after allogeneic HSCT. No preconditioning regimens were given to the patients before T-cell infusions. T-cell products were administered using a dose escalation schedule of 1.5 × 107/m2, 4.5 × 107/m2, and 1.2 × 108/m2 on the basis of total cell numbers and not on CD19.CAR+ cells. We used an interpatient dose escalation that followed a continual reassessment method, which required safety to be demonstrated 45 days after infusion in 2 patients at each dose level.20 Patients receiving additional doses of CD19.CAR-VSTs received the same number of cells as they did at their initial dose. Adverse events during and after T-cell infusions were graded according to National Institutes of Health criteria (Common Terminology Criteria for Adverse Events, version 3), and responses were assessed by week 6 after T-cell infusion and were defined as complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD).
Generation of CD19.CAR-VSTs
VSTs were generated as previously described.13,21 Briefly, peripheral blood mononuclear cells (PBMCs) from transplant donors were obtained by Ficoll density and used first to generate EBV-transformed lymphoblastoid B-cell lines (LCLs) for use as antigen-presenting cells by infection with the B95-8 laboratory strain of EBV derived from a B95-8 master cell bank.13,22 For the first VST stimulation, adherent monocytes were infected with the Ad5f35pp65 vector (from a master virus bank produced by our vector production facility) at a multiplicity of infection of 10 particle-forming units per cell. Nine days after infection, cells received a second stimulation with irradiated LCLs infected with the same Ad5f35pp65 vector at an multiplicity of infection of 100 particle-forming units per cell. Three days later and twice weekly thereafter, the medium was supplemented with interleukin (IL)-2 (Proleukin; Chiron Therapeutics, Emeryville, CA). After the third stimulation, with Ad5f35pp65-transduced LCLs, VSTs were transduced with the clinical-grade retroviral supernatant encoding the CD19-specific CAR, which also contains the CD28 costimulatory endodomain, using 24-well plates coated with retronectin (TaKaRa Bio, Inc.).20,21 VSTs were then further expanded using transduced LCLs and IL-2 to reach sufficient numbers for clinical use. Thus, CD19.CAR-VSTs were in culture for at least 5 to 6 weeks before being deemed ready for infusion and subsequent clinical freeze.
IFNγ enzyme-linked immunospot (ELISpot)
This assay was used to evaluate the virus specificity of T cells in the VSTs and the PBMCs collected from patients at different time points after T-cell infusions. Peptide mixtures (Pepmixes) spanning the CMV protein pp65, the Adv proteins hexon and penton, and the EBV proteins BZLF1, LMP1, LMP2, EBNA1, EBNA3a, EBNA3b, and EBNA3c were used to stimulate the VSTs as previously described.13,21 Staphylococcal enterotoxin B or phytohemagglutinin (PHA) were used as positive controls. The ability of VSTs to secrete interferon (IFN) γ was then independently assessed by Zellnet Consulting (Fort Lee, NJ) and compared with input cell numbers to obtain the frequency of VST precursors.
51Chromium release assay
This assay was used to evaluate the cytotoxic activity of CD19.CAR-VSTs against CD19+ targets (CD19+ tumor cell line or LCLs) and PHA blasts derived from the recipient, as previously described.13,21 Briefly, 51Cr-labeled target cells were cocultured with effector cells that had been serially diluted to produce the effector-to-target (E:T) ratios of 5:1, 10:1, 20:1, and 40:1. Target cells incubated in complete medium or 1% Triton X-100 were used to determine spontaneous and maximal 51Cr release, respectively. After 4 to 6 hours, supernatants were harvested and radioactivity was measured on a γ counter. Mean percentages of specific lysis of triplicate wells were calculated as 100 × (experimental release – spontaneous release)/(maximal release – spontaneous release).
Flow cytometry
CD19.CAR-VSTs were phenotypically characterized using a panel of fluorochrome-conjugated monoclonal antibodies including CD3, CD4, CD8, CD16, CD19, CD25, CD27, CD28, CD45RA, CD45RO, CD56, CD62L, and CCR7 (Becton Dickinson, Franklin Lakes, NJ). Cells were acquired on a FACSCalibur flow cytometer. Data were analyzed using Cell Quest software (Becton Dickinson). Flow cytometry of blood samples collected from patients was performed at the Center for Cell and Gene Therapy or by an accredited clinical laboratory.
Real-time quantitative polymerase chain reaction of CD19.CAR transgene and viremia
The integrated genome of the retrovirus encoding the CD19.CAR was quantified by real time quantitative polymerase chain reaction (Q-PCR) as previously described.20 After DNA extraction from peripheral blood (PB) samples with the QIAamp DNA Blood Mini Kit (Qiagen, Hamburg, Germany) following the manufacturer’s instructions, we amplified the DNA with primers and probes (Applied Biosystems, Foster City, CA) complementary to specific sequences within the retroviral vector.20 The standard curve was established using serial dilutions of the plasmid encoding the transgene. Similarly, the presence of the EBV in PBMCs was determined in DNA samples extracted from PBMC using specific primers and probes targeting EBER.21 Amplifications were performed using the ABI7900HF (Applied Biosystems) according to the manufacturer’s instructions.
Multiplex enzyme-linked immunosorbent assay
Plasma samples taken before or after CD19.CAR-VSTs infusions were analyzed using a multiplex enzyme-linked immunosorbent assay (Millipore) according to the manufacturer’s instructions. GM-CSF, sCD137, IFNγ, sFas, sFasL, granzyme A, granzyme B, IL-2, IL-4, IL-5, IL-6, IL-10, IL-13, MIP-1α, MIP-1β, tumor necrosis factor-α (TNF-α), and perforin were quantified.
Statistical analyses
Data points corresponding to the first CD19.CAR-VST infusion for each patient were plotted against the number of days postinfusion, and an area under the curve was calculated using the trapezoidal method using MATLAB software.
Results
Characteristics of infused VSTs
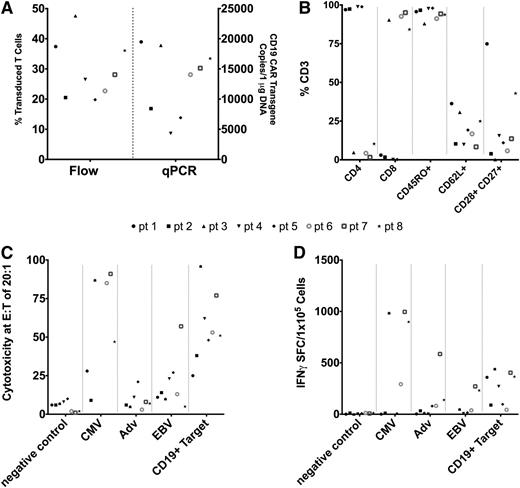
We manufactured CD19.CAR-VSTs from PBMCs collected from 8 hematopoietic stem cell donors. Generation of CD19.CAR-VSTs required 40 ± 12 days of culture, and the characteristics of the T-cell products are summarized in Figure 1. CD19.CAR transgene expression by flow cytometry ranged from 20% to 48% of CD3+ T cells and met the release criterion of ≥20% CAR expression (Figure 1A). Five CD19.CAR-VST lines were composed of predominantly CD8+ cells, whereas three were predominantly CD4+ cells (Figure 1B). The majority of CD19.CAR-VSTs were CD45RO+ and lacked CCR7, but a residual fraction retained the expression of the memory-associated phenotypic markers CD62L, CD27, and CD28 (Figure 1B). Natural killer cells (CD3–CD56+) were not detectable. Each VST line recognized at least 1 viral antigen and the CD19+ target as assessed by 51Cr release assay (Figure 1C) and IFNγ ELISpot (Figure 1D). The cytotoxic activity against recipient-derived PHA blasts was negligible, indicating a lack of alloreactive T-cell precursors.
Characteristics of CD19.CAR-VSTs. (A) Transduction of VSTs with the CD19.CAR is shown as both percentages of T cells on flow cytometry and number of copies on Q-PCR. Each symbol represents a patient, with the marker style corresponding to the same patient across the characteristics measured. (B) Phenotypic characteristics of CD19.CAR-VSTs as measured by flow cytometry. (C) Cytotoxic specificity of CD19.CAR-VSTs as measured by 51Cr release assays against different targets: control PHA blasts, PHA blasts loaded with virus-derived peptides or CD19+ cells. (D) IFNγ ELISpot of CD19.CAR-VSTs against different viral-antigen–loaded and CD19+ target cells.
Characteristics of CD19.CAR-VSTs. (A) Transduction of VSTs with the CD19.CAR is shown as both percentages of T cells on flow cytometry and number of copies on Q-PCR. Each symbol represents a patient, with the marker style corresponding to the same patient across the characteristics measured. (B) Phenotypic characteristics of CD19.CAR-VSTs as measured by flow cytometry. (C) Cytotoxic specificity of CD19.CAR-VSTs as measured by 51Cr release assays against different targets: control PHA blasts, PHA blasts loaded with virus-derived peptides or CD19+ cells. (D) IFNγ ELISpot of CD19.CAR-VSTs against different viral-antigen–loaded and CD19+ target cells.
Patient characteristics and clinical outcome postinfusion
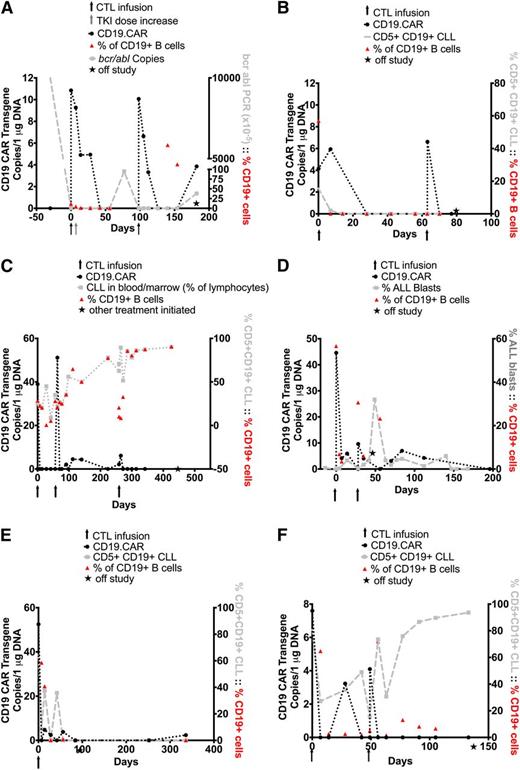
Eight patients were infused with CD19.CAR-VSTs and their characteristics are summarized in Table 1. Initially, only patients who had relapsed after HSCT were permitted in the study and thus they were treated 4 months to 13 years after HSCT. The first 6 patients (2 with B-cell acute lymphoblastic leukemia [B-ALL], 2 with B-cell chronic lymphocytic leukemia [B-CLL]/Richter’s transformation, and 2 with B-CLL/17p deletion) were enrolled after failing multiple salvage regimens to control their disease relapse post–allogeneic HSCT. All had detectable leukemic cells or minimal residual disease at the time of the CD19.CAR-VST infusion. After a protocol amendment, however, we also enrolled patients 7 and 8, with B-ALL who were at high risk for relapse but who were in remission at the time of CD19.CAR-VST infusion, more than 8 months and 3 months, respectively, after allogeneic HSCT. All infusions were well tolerated, and the donor-derived CD19.CAR-VSTs did not induce GVHD. No patient had any elevation of IL-6, TNFα, or IFNγ levels in plasma samples obtained before or during the first 6 weeks after CD19.CAR-VST infusions (data not shown). Patient 2, who presented with mesenteric lymph node involvement, had an episode of diarrhea 4 weeks after infusion that was later determined to be unrelated to T-cell infusion. Five of 6 patients demonstrated transient changes in disease activity, and an objective response was obtained in 2 of 6 patients (1 and 2) at the 6-week evaluation period. Patients 1 and 2 achieved a CR and PR that were durable for 3 months and 8 weeks, respectively (Table 2). Patient 1 with Philadelphia-positive B-ALL had detectable bcr/abl transcripts in the bone marrow (4 weeks pre-CD19.CAR-VSTs). She received the cell product in her nadir after hyperCVAD therapy, at which time she had detectable blasts in the PB. Because of the hyperCVAD, normal B cells were undetectable at the time of infusion. The patient subsequently achieved CR within 2 weeks after the first CD19.CAR-VST infusion. After a week, the patient’s dosage of dasatinib was increased at an outside institution as additional prophylaxis. Unfortunately, prolonged administration at this increased dose of dasatinib was likely toxic to the T cells23 and was associated with a fall in the CD19.CAR-VST signal and subsequent return of detectable bcr/abl transcripts 2 months later (Figure 2A). A second infusion of CD19.CAR-VSTs was again followed by clearance of detectable bcr/abl, but florid relapse supervened 4 months later (Figure 2A). Patient 2 had B-CLL with Richter’s transformation with circulating leukemic cells (16% CD19+CD5+ cells) at the time of CD19.CAR-VST infusion. Leukemic cells became undetectable in the PB after a week and remained undetectable for 8 weeks, with concomitant depletion of normal B cells (Figure 2B). However, there was continued progression of his Richter’s transformation, with continued enlargement of his lymph nodes. He received a second dose of CD19.CAR-VSTs, but he did not respond to the treatment and was placed on palliative care. In addition, a third patient (patient 3) with B-CLL who had 25% circulating CD19+CD5+ cells maintained SD for 15 months after 3 CD19.CAR-VST infusions and with no additional therapy (Figure 2C). Patients 4 and 5 with pre–B-ALL and B-CLL/Richter’s, respectively, showed initial transient responses to cell infusions that were not durable. Patient 4 did not respond to a second infusion of CD19.CAR-VSTs (Figure 2D). In patient 5, infusion of CD19.CAR-VST was followed by a decrease of CD5+CD19+ leukemic and CD19+ normal B lymphocytes in the PB, but lymph node enlargement progressed and the patient was subsequently treated with salvage chemotherapy (Figure 2E). Patient 6 with B-CLL/17q deletion did not respond to the treatment, despite a decreasing normal B-cell count in the PB, and received alternative chemotherapy treatments (Figure 2F). In all patients with recurrent/persistent disease after CD19.CAR-VST infusions, the tumor cells remained CD19+.
Patient characteristics
| Pt . | Age/Sex . | Primary diagnosis . | Time to relapse after transplant/type . | HLA match with donor . | Treatment received after relapse posttransplant . | Absolute T cell count/ALC at time of infusion (per uL) . | Status of disease at T-cell infusion . |
|---|---|---|---|---|---|---|---|
| 1 | 23/F | B-ALL/Ph+ | 9 mo/MRD | 6/6 | Hyper CVAD (MTX and Ara-C) | 992/1269 | Detection of bcr/abl transcripts |
| Dasatinib | |||||||
| 2 | 59/M | B-CLL/Richter’s | 3 mo/MUD | 13/14 | Lenalidomide | 35/1154 | Lymphadenopathy; 16% circulating CD19+CD5+ cells |
| Hyper CVAD with rituximab × 2 | |||||||
| Ofatumumab with methylprednisolone | |||||||
| Radiation | |||||||
| Hyper CVAD without vincristine | |||||||
| 3 | 57/M | B-CLL/17p deletion | 11 y/MRD | 6/6 | DLI | 4897/10155 | 25% Circulating CD19+CD5+ cells |
| Rituximab | |||||||
| Lenalidomide | |||||||
| Bendamustine | |||||||
| 4 | 9/M | Pre–B-ALL | 1 y/MRD | 10/10 | AALL07p1 (standard 4-drug induction with bortezomib) | 313/4962 | 0.5% Circulating blasts |
| Steroids | |||||||
| Vincristine | |||||||
| 5 | 49/M | B-CLL/Richter’s | 14 mo/MRD | 14/14 | Hyper CVAD | 731/4501 | Lymphadenopathy; 64% circulating CD19+CD5+ cells |
| Rituximab | |||||||
| Dexamethasone | |||||||
| Methylprednisolone | |||||||
| Lenalidomide | |||||||
| Ofatumumab | |||||||
| 6 | 59/M | B-CLL/17p deletion | Immediate/MRD | 10/10 | DLI × 4 | 993/12232 | Lymphadenopathy; splenomegaly; 81% circulating CD19+CD5+ cells |
| Lenalidomide | |||||||
| Ofatumumab × 8 | |||||||
| 7 | 40/F | Pre-BALL/Ph+ | No relapse/MUD | 10/10 | — | 2921/4500 | CR |
| 8 | 12/F | B-ALL | No relapse/MUD | 9/10 | — | 1913/4030 | CR |
| Pt . | Age/Sex . | Primary diagnosis . | Time to relapse after transplant/type . | HLA match with donor . | Treatment received after relapse posttransplant . | Absolute T cell count/ALC at time of infusion (per uL) . | Status of disease at T-cell infusion . |
|---|---|---|---|---|---|---|---|
| 1 | 23/F | B-ALL/Ph+ | 9 mo/MRD | 6/6 | Hyper CVAD (MTX and Ara-C) | 992/1269 | Detection of bcr/abl transcripts |
| Dasatinib | |||||||
| 2 | 59/M | B-CLL/Richter’s | 3 mo/MUD | 13/14 | Lenalidomide | 35/1154 | Lymphadenopathy; 16% circulating CD19+CD5+ cells |
| Hyper CVAD with rituximab × 2 | |||||||
| Ofatumumab with methylprednisolone | |||||||
| Radiation | |||||||
| Hyper CVAD without vincristine | |||||||
| 3 | 57/M | B-CLL/17p deletion | 11 y/MRD | 6/6 | DLI | 4897/10155 | 25% Circulating CD19+CD5+ cells |
| Rituximab | |||||||
| Lenalidomide | |||||||
| Bendamustine | |||||||
| 4 | 9/M | Pre–B-ALL | 1 y/MRD | 10/10 | AALL07p1 (standard 4-drug induction with bortezomib) | 313/4962 | 0.5% Circulating blasts |
| Steroids | |||||||
| Vincristine | |||||||
| 5 | 49/M | B-CLL/Richter’s | 14 mo/MRD | 14/14 | Hyper CVAD | 731/4501 | Lymphadenopathy; 64% circulating CD19+CD5+ cells |
| Rituximab | |||||||
| Dexamethasone | |||||||
| Methylprednisolone | |||||||
| Lenalidomide | |||||||
| Ofatumumab | |||||||
| 6 | 59/M | B-CLL/17p deletion | Immediate/MRD | 10/10 | DLI × 4 | 993/12232 | Lymphadenopathy; splenomegaly; 81% circulating CD19+CD5+ cells |
| Lenalidomide | |||||||
| Ofatumumab × 8 | |||||||
| 7 | 40/F | Pre-BALL/Ph+ | No relapse/MUD | 10/10 | — | 2921/4500 | CR |
| 8 | 12/F | B-ALL | No relapse/MUD | 9/10 | — | 1913/4030 | CR |
MUD, matched unrelated donor; MRD, matched related donor.
Patient outcomes
| Pt . | Diagnosis . | Time with detectable T cells in PB . | AUC (CD19.CAR signal vs days of first infusion) . | Tumor response . | Number of cells infused (number of infusions) . |
|---|---|---|---|---|---|
| 1 | B-ALL/Ph+ | 12 wk | 223.11 | CR × 3 mo | 3.15 × 107 (2 infusions) |
| 2 | B-CLL/Richter’s | 1 wk | 97.38 | PR × 8 wk | 3.4 × 107 (2 infusions) |
| 3 | B-CLL/17p deletion | 2 wk | 136.395 | SD × 15+ months | 3.3 × 107 (3 infusions) |
| 4 | Pre–B-ALL | 12 wk | 247.45 | PD | 1.9 × 107 (2 infusions) |
| 5 | B-CLL/Richter’s | 8 wk | 348.215 | PD | 1 × 108 (1 infusion) |
| 6 | B-CLL/17p deletion | 4 wk | 71.82 | PD | 1.13 × 108 (2 infusions) |
| 7 | Pre–B-ALL/Ph+ | 12 wk* | 223.826 | CCR × 8 mo* | 9.7 × 107 (1 infusion) |
| 8 | B-ALL | 8 wk* | 946.19 | CCR × 2 mo* | 5.8 × 107 (1 infusion) |
| Pt . | Diagnosis . | Time with detectable T cells in PB . | AUC (CD19.CAR signal vs days of first infusion) . | Tumor response . | Number of cells infused (number of infusions) . |
|---|---|---|---|---|---|
| 1 | B-ALL/Ph+ | 12 wk | 223.11 | CR × 3 mo | 3.15 × 107 (2 infusions) |
| 2 | B-CLL/Richter’s | 1 wk | 97.38 | PR × 8 wk | 3.4 × 107 (2 infusions) |
| 3 | B-CLL/17p deletion | 2 wk | 136.395 | SD × 15+ months | 3.3 × 107 (3 infusions) |
| 4 | Pre–B-ALL | 12 wk | 247.45 | PD | 1.9 × 107 (2 infusions) |
| 5 | B-CLL/Richter’s | 8 wk | 348.215 | PD | 1 × 108 (1 infusion) |
| 6 | B-CLL/17p deletion | 4 wk | 71.82 | PD | 1.13 × 108 (2 infusions) |
| 7 | Pre–B-ALL/Ph+ | 12 wk* | 223.826 | CCR × 8 mo* | 9.7 × 107 (1 infusion) |
| 8 | B-ALL | 8 wk* | 946.19 | CCR × 2 mo* | 5.8 × 107 (1 infusion) |
Follow-up still ongoing.
Outcome after CD19.CAR-VST infusions in patients infused with disease. Panels A-F represent single patients. Panels A-C illustrate patients with sustained objective clinical response, and panels D-F illustrate patients with transient or no response. In all panels, black arrows denote the time of CD19.CAR-VST infusions, black circles indicate the detection of CD19.CAR-VSTs in the PB by Q-PCR, and red triangles represent the values of normal B-cell counts in the PB. (A) Data from patient 1. Detection of bcr/abl transcripts are indicated as light gray squares. The gray arrow indicates the increase in dose of dasatinib (TKI). (B-C) Data from patients 2 and 3, respectively. In both subjects, circulating B-CLL cells (CD19+CD5+) were measured by flow cytometry and are shown as light gray squares. (D-F) Data from patients 4, 5, and 6, respectively. Leukemic cells, measured in the PB by flow cytometry, are represented as light gray squares. The star indicates the time when patients received additional/alternative treatments or were considered out of study.
Outcome after CD19.CAR-VST infusions in patients infused with disease. Panels A-F represent single patients. Panels A-C illustrate patients with sustained objective clinical response, and panels D-F illustrate patients with transient or no response. In all panels, black arrows denote the time of CD19.CAR-VST infusions, black circles indicate the detection of CD19.CAR-VSTs in the PB by Q-PCR, and red triangles represent the values of normal B-cell counts in the PB. (A) Data from patient 1. Detection of bcr/abl transcripts are indicated as light gray squares. The gray arrow indicates the increase in dose of dasatinib (TKI). (B-C) Data from patients 2 and 3, respectively. In both subjects, circulating B-CLL cells (CD19+CD5+) were measured by flow cytometry and are shown as light gray squares. (D-F) Data from patients 4, 5, and 6, respectively. Leukemic cells, measured in the PB by flow cytometry, are represented as light gray squares. The star indicates the time when patients received additional/alternative treatments or were considered out of study.
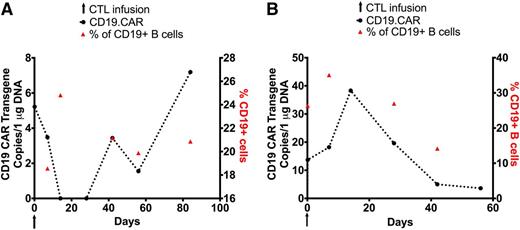
Of the 2 patients (7 and 8) treated in remission, patient 7 remains in CR more than 8 months after T-cell infusion, although the role of CD19.CAR-VSTs is unclear because normal B cells were not eliminated (Figure 3A), whereas patient 8, who was infused 3 months posttransplant, remains in CR 8 weeks after CD19.CAR-VST infusion and has decreasing numbers of normal B cells (Figure 3B).
Outcome after CD19.CAR-VST infusions without evidence of disease. Each panel represents a single patient. In all panels, black arrows denote the time of CD19.CAR-VST infusions, black circles indicate the detection of CD19.CAR-VSTs in the PB by Q-PCR, and red triangles represent the values of normal B-cell counts in the PB. (A-B) Data from patients 7 and 8 who received CD19.CAR-VSTs 8 months and 3 months after allogeneic HSCT, respectively.
Outcome after CD19.CAR-VST infusions without evidence of disease. Each panel represents a single patient. In all panels, black arrows denote the time of CD19.CAR-VST infusions, black circles indicate the detection of CD19.CAR-VSTs in the PB by Q-PCR, and red triangles represent the values of normal B-cell counts in the PB. (A-B) Data from patients 7 and 8 who received CD19.CAR-VSTs 8 months and 3 months after allogeneic HSCT, respectively.
CD19.CAR-VST persistence and trafficking
Molecular signals derived from infused CD19.CAR-VSTs were detectable in the PB, as summarized in Table 2. Transgene levels were generally low (range, 4.2-53 copies per 1000 ng DNA) as detected approximately 3 hours after infusion by specific Q-PCR. Transgene signals were detected from 1 to 12 weeks after each infusion and were always below the level needed to detect a distinct CAR+ population by flow cytometry.20,24 There was no correlation between transgene levels in the PB and clinical response. We also consistently identified transgene signals at disease sites (bone marrow or lymph nodes) in all biopsy samples available for analysis (Figure 4A). Importantly, in 3 patients (1, 3, and 7), Q-PCR signals were detectable in the bone marrow or the lymph nodes even when no signals were measurable in the PB, indicating preferential accumulation of the infused T cells at the disease site (Figure 4B).
In vivo trafficking of CD19.CAR-VSTs. (A) CD19.CAR-VSTs accumulate at the sites of disease. Immunohistochemistry stain of B-CLL cells (top row) and CD3+ T cells (bottom row) from patient 3. (B) Summary table documenting the presence of detectable CD19.CAR-VSTs at the sites of disease as assessed by Q-PCR compared with concomitant PB samples measured as copies per 1000 ng of DNA.
In vivo trafficking of CD19.CAR-VSTs. (A) CD19.CAR-VSTs accumulate at the sites of disease. Immunohistochemistry stain of B-CLL cells (top row) and CD3+ T cells (bottom row) from patient 3. (B) Summary table documenting the presence of detectable CD19.CAR-VSTs at the sites of disease as assessed by Q-PCR compared with concomitant PB samples measured as copies per 1000 ng of DNA.
Response to viruses
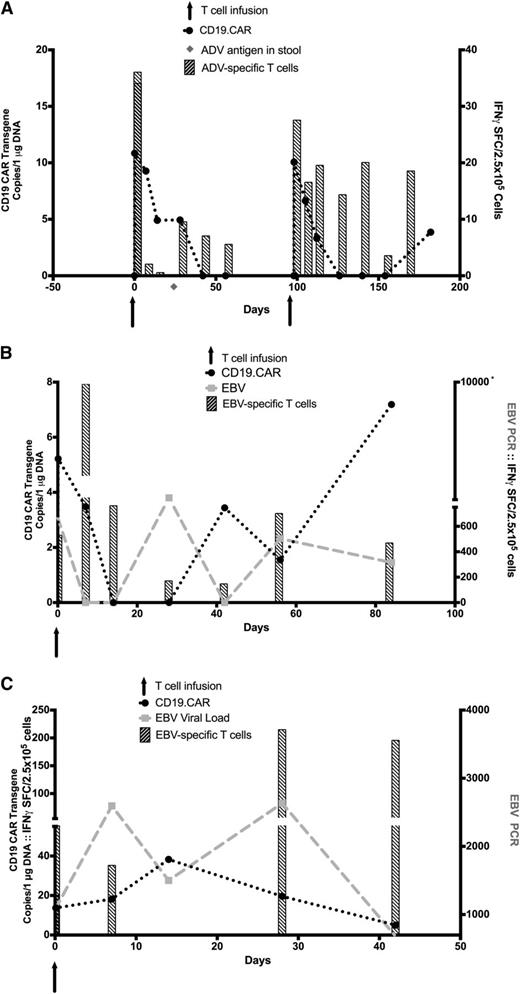
Because this study was initially approved only for patients who developed measurable disease after allogeneic HSCT, CD19.CAR-VST infusions were given late after HSCT, at a time when the frequency of viral infections had dwindled as a result of immune recovery.25,26 Thus, only 3 of 8 patients had viral reactivation during the course of the trial, one of whom (patient 1) had an adenovirus infection and 2 of whom (patients 7 and 8) reactivated EBV. Nine months after an allogeneic HSCT for relapsed B-ALL, patient 1 was infused with CD19.CAR-VSTs that included cells with reactivity to AdV antigens. We detected AdV antigen in stool 4 weeks after infusion, and this increased antigenemia was associated with an increase of AdV-specific precursors by INFγ ELISpot in the PB and subsequent clearance of the virus. Expansion of AdV-specific T cells, however, was not associated with an increase in CD19.CAR signal (Figure 5A). Two patients (patients 7 and 8) with EBV infection received CD19.CAR-VSTs, showing reactivity ex vivo to EBV antigens and EBV-transformed lymphoblastoid cell lines. Patient 7 with B-ALL received CD19.CAR-VSTs in remission at 8 months posttransplant and developed an increase in EBV-DNA in PBMCs 4 weeks after T-cell infusion, indicating EBV reactivation. This rising viral load both promoted the expansion of circulating EBV-specific precursors as assessed by IFNγ ELISpot and also increased the level of circulating CD19.CAR-VSTs, as indicated by the increase of the molecular signal with a corresponding reduction in EBV load (Figure 5B). Patient 8 with B-ALL received CD19.CAR-VSTs in remission at 3 months posttransplant, when she had a rise in EBV-DNA viral load in the absence of detectable EBV–T cell precursors in PB. The CD19.CAR-VST infusion was followed by a concomitant increase of in vivo EBV reactivity in PB and control of the viremia that was again associated with an increase of CD19.CAR transgene in the PB (Figure 5C) and a substantially increased AUC for detectable transgene when compared with other subjects (Table 2).
Viral reactivation. (A) AdV reactivation and monitoring of the specific immune response in patient 1. Detection of AdV-specific T-cell responses by IFNγ ELISpot (striped bars) and CD19.CAR transgene by Q-PCR (black circles) are illustrated. The light gray diamond denotes the time of adenovirus antigen detection in stool. (B-C) EBV reactivation and immune responses in patients 7 and 8, respectively. Detection of EBV-DNA viral load by Q-PCR (light gray squares), CD19.CAR transgene by Q-PCR (black circles), and EBV-specific T-cell responses (striped bars) by IFNγ ELISpot in the PB are illustrated. Arrows denote time of CD19.CAR-VSTs infusions.
Viral reactivation. (A) AdV reactivation and monitoring of the specific immune response in patient 1. Detection of AdV-specific T-cell responses by IFNγ ELISpot (striped bars) and CD19.CAR transgene by Q-PCR (black circles) are illustrated. The light gray diamond denotes the time of adenovirus antigen detection in stool. (B-C) EBV reactivation and immune responses in patients 7 and 8, respectively. Detection of EBV-DNA viral load by Q-PCR (light gray squares), CD19.CAR transgene by Q-PCR (black circles), and EBV-specific T-cell responses (striped bars) by IFNγ ELISpot in the PB are illustrated. Arrows denote time of CD19.CAR-VSTs infusions.
Discussion
We report the first clinical trial infusing donor-derived VSTs engrafted with a CD19.CAR into patients with B-cell malignancies who relapsed after allogeneic HSCT. We show that CD19.CAR-VST infusions were safe in all patients, did not induce GVHD, expanded in the presence of EBV reactivation, and in 2 of 6 patients could produce objective clinical responses.
In all previously reported clinical trials of CD19 or CD20.CAR-modifed T cells, the infused cells were autologous in origin,16-20,27-29 so it was unclear whether donor-derived T cells could be given safely (eg, after allogeneic HSCT). To reduce the risk of GVHD developing from such allogeneic cells and to ensure that the donor-derived CD19.CAR–T cells had selective antileukemia/lymphoma effects, we expressed the CD19.CAR in VSTs because these cells lack demonstrable alloreactivity in vivo when given to HSCT recipients.13,14,30 The presence of the CD19.CAR did not alter the virus specificity of these cells (which is mediated by their native TCRs) and, as anticipated, CD19.CAR-VSTs did not induce GVHD.
In addition to avoiding GVHD, the inherent virus specificity of the native TCRs in the allogeneic CD19.CAR-VSTs was also expected to promote the proliferation and persistence of these CAR-VSTs in vivo in response to endogenous viruses (EBV, CMV, AdV) and associated co-stimulatory molecules presented on antigen-presenting cells infected with virus or cross-presenting viral antigens.31-33 We anticipated that this additional TCR stimulus would complement the stimulation received by engagement of the CD19.CAR by B cells and the associated costimulation received through the incorporated CD28 endodomain. Our studies showed that CD19.CAR-VSTs can be detected at low levels in the PB for up to 12 weeks, but because of the length of time that had elapsed after allogeneic HSCT before these patients received CD19.CAR-VSTs (3 months to 13 years), only 3 patients had viral reactivations that allowed us to assess the influence of viral-directed native TCR specificity on the persistence and numbers of CD19.CAR-VSTs. At such a late time after HSCT, endogenous immune reconstitution with VSTs has usually occurred25,26,34 and may dominate the antiviral response, reducing the likelihood that CD19.CAR-VSTs receive activation and expansion signals through their native TCRs.
Of the 3 patients who had viral reactivation, two with EBV reactivation had concomitant increase of both EBV-specific precursors and of the CD19.CAR signal, suggesting virus-induced expansion of the CD19.CAR-VSTs. The third patient, who had AdV infection, showed an increase in AdV-specific precursors with no concomitant rise in the CD19.CAR signal. We do not know whether the observed differences between the effects of EBV reactivation and AdV infection on CD19.CAR-VST expansion will occur consistently or whether they represent a biological difference between the immunostimulatory capacity of AdV vs EBV, attributable, for example, to the contrast between a systemic infection of B cells (EBV reactivation) vs a localized infection of epithelial cells in the gastrointestinal tract (AdV infection). Nonetheless, the limited data from these 3 patients support the concept that CD19.CAR-VSTs retain antiviral activity in vivo and that this activity may be exploited to increase the number of CD19.CAR effector cells.
Irrespective of the effects of native TCR engagement by viral antigens, CD19.CAR-VSTs can engage the CD19 antigen on normal and malignant B cells and receive costimulation through the CD28 signaling domain incorporated in this receptor. The in vivo persistence of CD19.CAR-VSTs in the current study is similar to that previously reported by our own20 and other groups18,19,27,28 using autologous polyclonal-activated (using CD3 and CD28 antibodies) T cells expressing a similar second-generation CD19.CAR encoding the CD28 endodomain. The results described here indicate that the CD28 signaling mediated by the CAR is equally effective irrespective of whether it is expressed in activated T cells or VSTs, or in autologous or allogeneic T cells.21
Although the infused allogeneic CD19.CAR-VSTs accumulated at disease sites and produced transient antitumor activity in 5 of 6 patients with measurable disease, in only 2 of 6 patients were objective responses noted at the 6-week evaluation. The effects on normal B cells were similarly incomplete, with only 4 of 8 patients showing measurable reduction. Paralleling the modest in vivo expansion of CD19.CAR-VSTs, patients had no symptoms suggestive of systemic inflammatory response syndrome (SIRS),16,18 and we found no increase in systemic cytokine levels, including TNFα, IL-6, and IFNγ after cell infusions.
There are several explanations for the modest expansion and antitumor activity of infused CAR-VSTs. The first is that the delayed treatment of these patients after allo-HSCT meant that viral reactivation was less frequent than anticipated, thus the benefits of native receptor stimulation were limited. However, data from the 3 patients who reactivated AdV or EBV described before suggest that EBV reactivation may indeed favor expansion and persistence of the CAR-VSTs posttransplant. Second, our patients received no additional lymphodepletion before VST infusion, which is in contrast to the preinfusion chemotherapy given during many other studies of CAR–T cells. Hence our patients lacked the homeostatic expansion signals associated with recovery from the lymphopenic state.35 Finally, there is evidence that the expansion/persistence of CD19.CAR–T cells incorporating the CD28 endodomain is less than expansion obtained with T cells whose CD19.CAR incorporates the 4-1BB endodomain,16 although it remains to be demonstrated whether such a distinction would apply when the 4-1BB endodomain is incorporated in a CD19.CAR expressed by VSTs.
We believe it less likely that the limited in vivo expansion reflects CAR-VST “exhaustion.” Although the VSTs generated for this clinical trial required several rounds of expansion ex vivo to reach sufficient numbers for adoptive transfer,20 the VSTs retain a population with memory-associated markers and can evidently expand in vivo in the presence of EBV viral antigens, as can nonmodified VSTs.11 It will be of interest to discover whether newer methodologies enabling faster generation of these cells36 will further augment their functionality in vivo. Of note, we found no evidence that the 4 of 8 lines with predominant CD4 phenotype were functionally inferior, and indeed the presence of a CD4 subset may be beneficial for both VST persistence and clinical outcome.37,38
In conclusion, the results of our study using allogeneic CD19.CAR-VSTs show that this strategy is apparently safe after allogeneic HSCT and that allogeneic VSTs expressing CD19.CARs can produce both antitumor and antiviral activity in the absence of GVHD. Earlier administration of CD19.CAR-VSTs after HSCT, when the host is lymphodepleted and the incidence of viral infection is higher, may allow these cells to better capture the putative advantages of native TCR stimulation (and associated costimulation) for expansion and persistence, and thereby produce a higher frequency of longer sustained tumor responses. Alternatively, intentional stimulation of the native TCRs by viral vaccines may produce equal benefit, with greater predictability.39,40
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
The authors would like to thank Dr Hao Liu for the statistical support in designing the clinical study.
This work was supported in part by the Leukemia and Lymphoma Society Specialized Center of Research 7018-04, RP100484 CPRIT, and a William Lawrence & Blanche Hughes Foundation Grant; and the Cancer Institute of New South Wales International Clinical and Research Fellowship (K.P.M.).
Authorship
Contribution: C.R.Y.C. designed and performed the in vitro experiments, analyzed data, and wrote the manuscript; K.P.M. designed the research, performed preclinical experiments, and contributed to data analysis and manuscript preparation; S.L., S.K., and E.L. performed in vitro experiments; A.P.G., Z.M., and B.J.G. ensured compliance with regulatory requirements for the clinical trial; O.D. supervised the manufacture of cells used in infusion; A.J.B., S.I., E.J.S., R.A.K., R.T.K., C.M.H., H.E.H., G.C., and C.M.B. enrolled patients in the study and monitored clinical responses; C.R. and B.S. contributed to experimental design; B.S., H.E.H., C.M.R., and M.K.B. contributed to preparation of the manuscript; and C.M.B. and G.D. designed the study, analyzed data, and wrote and reviewed the manuscript and Bluebird Bio.
Conflict-of-interest disclosure: The Center for Cell and Gene Therapy has a collaborative research agreement with Celgene.
Correspondence: Gianpietro Dotti, Center for Cell and Gene Therapy, Baylor College of Medicine, 1102 Bates St, Feigin Center Suite 1750, Houston, TX 77030; e-mail: gxdotti@txch.org or gdotti@bcm.edu.
References
Author notes
C.M.B. and G.D. share senior coauthorship of this study.
C.R.Y.C. and K.P.M. contributed equally to this study.