Abstract
The prognostic significance of minimal residual disease (MRD) quantification in the post-transplant setting has been demonstrated in multiple lymphoid malignancies, including acute lymphoblastic leukemia (ALL), mantle cell lymphoma (MCL) and chronic lymphocytic leukemia (CLL). Previous studies support the clinical utility of molecular MRD quantification of tumor burden after allogeneic hematopoietic cell transplantation (allo-HCT) (Logan et al, Leukemia 2013). We have developed the ClonoSIGHT™ test, which is based on the deep sequencing of immunoglobulin and T-cell receptor rearrangements and has a detection limit of one cancer cell per million leukocytes in peripheral blood or bone marrow (Faham et al, Blood 2012; Armand et al, Brit J Haematol 2013). In this report, we will discuss the technical performance of the ClonoSIGHT test for routine MRD quantification after allo-HCT and present a case study on a patient with T-cell prolymphocytic leukemia (T-PLL).
A 55 year old female presented with T-PLL including symptomatic CNS disease, received 12 weeks of Alemtuzumab therapy and then 12 weeks following her last Alemtuzumab treatment received an unrelated donor myeloablative allo-HCT using Fludarabine, BCNU and Melphalan conditioning with antithymoglobulin, Mycophenolate mofetil and cyclosporine primary immune prophylaxis. Peripheral blood samples were collected for MRD assessment before and serially after allo-HCT. Using universal primer sets, we amplified T-cell receptor beta (TRB), delta (TRD) and gamma (TRG) variable, diversity, and joining gene segments from genomic DNA isolated from peripheral blood mononuclear cells (PBMC). Amplified products were sequenced and analyzed using standardized algorithms for clonotype determination, and leukemia-specific clonotypes were identified based on their frequency within the T-cell repertoire (>5%). The leukemia-specific clonotype was then quantified in serial peripheral blood samples and reported as the absolute number of leukemic-specific clones among total leukocytes.
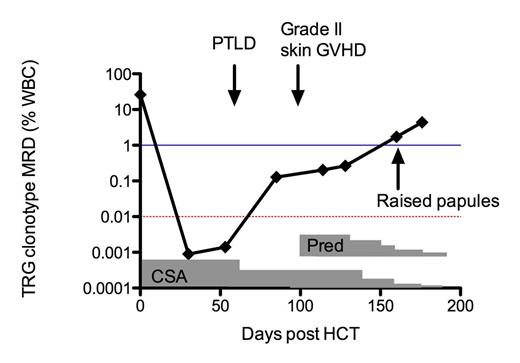
A single clonal TRG gene rearrangement accounting for 26.1% WBC in the pre-transplant sample was identified and quantified in serial peripheral blood samples. A 4-log decline in MRD levels occurred post allo-HCT (Figure 1) thru 56 days following graft infusion; however, serial MRD monitoring demonstrated increasing levels of leukemia-specific clonotypes in the peripheral blood over time (Figure 1). Immunosuppression tapering strategies were employed in response to clinical events and MRD levels. Specifically, the patient developed an EBV+ post-transplant lymphoproliferative disease (PTLD) 60 days post allo-HCT, and cyclosporine was tapered in addition to instituting anti-CD20 rituximab treatment. As per institutional practice, a bone marrow biopsy 84 days post-HCT showed full donor engraftment with normal cellularity and no evidence of PLL was detected by flow cytometry when ClonoSIGHT detected 0.013% PLL in the patient's blood. Unfortunately, in the setting of immune suppression taper at 100 days post allo-HCT, the patient developed Grade II skin GVHD and was treated with 0.5mg/kg prednisone daily and tapered as indicated. At 160 days post allo-HCT, the patient presented with new skin papules suspected to be leukemia cutis. The PLL clonotype was detected in the skin biopsy; however, it was present at lower frequency in the TRG repertoire than in the blood, thus not supporting a diagnosis of leukemia cutis. In agreement, skin pathology revealed Verruca Vulgaris (warts). However, the patient's MRD continued to increase in the blood while immunosuppression was tapered and stopped completely 6 months post-HCT.
MRD levels over time in a T-PLL patient. Red dotted line indicates detection level by 4-color flow cytometry (0.01%), blue line indicates detection level by pathology (1%).
MRD levels over time in a T-PLL patient. Red dotted line indicates detection level by 4-color flow cytometry (0.01%), blue line indicates detection level by pathology (1%).
MRD assessment can be used to monitor a patient's disease progression after immune cellular therapy and aids immune suppression management following allo-HCT. Further, as presented in this case study, ClonoSIGHT detection of the leukemia clone in the blood compared with other tissues can sensitively and specifically assess extramedullary relapse.
Kong:Sequenta, Inc.: Employment, Equity Ownership. Faham:Sequenta, Inc.: Employment, Equity Ownership, Membership on an entity’s Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal