Abstract
TKI are standard therapy for pts with CML. Although generally safe, they are associated with some adverse events, most of them manageable and transient. Renal dysfunction has been reported anecdotally on pts with CML treated with TKI. We investigated the incidence of acute renal failure (ARF) and chronic renal failure (CRF) among the CML pts treated with TKI, and analyzed possible relationship between treatment duration with TKI and changes in estimated glomerular filtration rate (GFR).
Four hundred and seventy-five pts treated with imatinib (255 pts; 49 at 400 mg daily; 206 at 800 mg daily), dasatinib (101 pts) and nilotinib (119 pts) in prospective clinical trials at a single institution were evaluated. Pts were followed routinely with blood chemistries including renal function tests, at least weekly during the first 2-3 months, then every 2-4 weeks for 6-12 months, then every 8-12 weeks. GFR was estimated using the Modification of Diet in Renal Disease (MDRD) equation and recorded from the onset of TKI treatment until last follow up. ARF was defined as an increase in serum creatinine of ≥0.3 mg/dl, and CRF defined as an estimated GFR ≤ 60 ml/min/1.73 m2 persisting for at least 90 days.
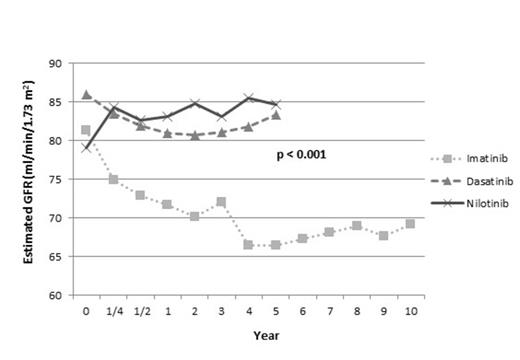
After a median follow-up of 50 months (range, 2 to 138 months), 19 pts (4%) developed ARF. The median time of onset for ARF was 9 days (range 4-84 days). Sixteen of 19 pts (84%) were on imatinib, 2 on nilotinib, and 1 on dasatinib (p=0.006). There was no association between imatinib dose and incidence of ARF (2% with 400mg vs 7% with 800mg) (p=0.174). The median age for pts with ARF was 58 yrs compared to 48 for pts with no ARF (p=0.009). Seventy-nine percent (15 out of 19) of ARF pts and 59% (269 of 456) of pts without ARF were male (p=0.063). During study time, estimated GFR decreased significantly in pts treated with imatinib compared to dasatinib (Figure 1) (p<0.001). Interestingly, in pts treated with nilotinib, we observed significant increase in GFR when we compare baseline GFR to the GFR in all other time points (p<0.05). 442 pts (94%) had no CRF at baseline, and 48 of these pts (11%) developed CRF over the course of TKI treatment. Among them, 39 pts (81%) were on imatinib compared to 19% on other TKIs (5 dasatinib, 4 nilotinib) (p<0.001). There was no association between imatinib dose (400mg and 800mg) and CRF incidence (p=0.591). The median age for pts who developed CRF was 61 yrs compared to 47 for those with no CRF (p=<0.001). Fifty-eight percent (28 out of 48) of CRF pts and 60 % (256 of 427) of pts without CRF were male (p=0.828). Overall CCyR rate was the same (89%) in pts who had ARF and no ARF, and overall MMR rate was 79% in pts with ARF and 83% in pts with no ARF (p=0.401). Overall CCyR rate was 98% in pts who developed CRF over the course of TKI therapy compared to 89% in pts who did not develop CKD (p=0.026). Similarly, overall MMR rate was higher (96%) in pts developed CKD compared to pts who did not have CKD (82%) (p=0.013). Overall survival and transformation free survival was not statistically different when compared pts with ARF vs no ARF and CRF vs no CRF. However, pts with ARF had decreased event free survival (EFS) when compared to no ARF pts (p=0.019). There was no EFS difference in CRF pts (0.966).
Mean Estimated Glomerular Filtration Rates
Long-term treatment with imatinib may cause a significant decline in estimated GFR. Interestingly, treatment with nilotinib may cause a slight improvement in GFR. It is important that pts are monitored for renal function during therapy with TKI, with particular attention to those with risk factors for renal dysfunction.
Ravandi:Pfizer and Novartis: Honoraria; BMS: Research Funding. Jabbour:Novartis, BMS, Ariad, and Pfizer: Consultancy. Cortes:Ariad, BMS, Novartis, Pfizer and Teva: Research Funding; BMS, Pfizer and Teva. : Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal