Abstract
In the present analyses, we sought to determine the impact of disease characteristics at diagnosis and at HSCT including pre-transplant MDS therapy and depth of response, stem cell source and intensity of conditioning regimen on disease outcomes after HSCT.
Between 2000 and 2012, 291 MDS patients with a median age of 55 years (range, 18-71) were transplanted with a matched related donor (MRD, n=131), matched unrelated donor (MUD, n=114) or mismatched donors (MMD (n=46). The study cohort had high-risk features including 117 patients (40.2%) with therapy-related MDS (tx-MDS) and 78 (27.3%) with MK+. Histological subtype was RAEB-1 and -2 in 122 (41.9%) and CMML in 26 (8.9%) patients. Therapy prior to HSCT was chemotherapy only (chemo, n=81), hypomethylating agents only (HMA, n=100) and both (chemo+HMA, n=50). There were 74 untreated patients prior to HSCT. Of 74, 45 (60.8%) had tx-MDS, 48 (64.9%) had MRD and proceeded with HSCT with a median of 4.7 months. Among different therapy groups; histological subtype and disease status at HSCT, donor type and conditioning intensity were not different (Table 1). The median age among therapy groups were different with 54 vs. 57 and 59 observed in chemo, HMA and chemo+HMA (p<0.001). Compared with chemo and chemo+HMA, HMA had more monosomal karyotype (MK) (40.4% vs. 16% in chemo and 11.4% in chemo+HMA, p<0.001) and higher risk groups by Revised International Prognostic Score (IPSS-R) (58% vs. 40.7% in chemo and 27.8% in chemo+HMA, p=0.006)
Demographic of therapy groups
| . | Untreated (%) n=74 . | Chemo (%) n=81 . | HMA (%) n=100 . | Chemo+HMA (%) n=36 . | P* . |
|---|---|---|---|---|---|
| Median age | 51 | 54 | 57 | 59 | <0.001 |
| RAEB-1 and -2 | 19 (25.7) | 41 (50.6) | 52 (52) | 15 (41.7) | 0.1 |
| CMML | 2 (2.7) | 10 (12.5) | 9 (9) | 3 (13.9) | 0.1 |
| MDS-tx | 45 (60.8) | 29 (35.8) | 37 (37) | 6 (16.7) | 0.07 |
| MK+ | 21 (29.6) | 13 (16.1) | 40 (40.4) | 4 (11.4) | <0.001 |
| High&very high risk by IPSS-R | 30 (40.5) | 33 (40.7) | 58 (58) | 10 (27.8) | 0.006 |
| CR at HSCT | 0 | 21 (25.9) | 25 (25) | 3 (8.3) | 0.08 |
| MUD/MMD | 18/8 (24.3/10.8) | 34/17 (42/21) | 44/17 (44/17) | 18/4 (50/11.1) | 0.8 |
| Myeloablative conditioning | 55 (74.3) | 56 (69.1) | 69 (69) | 21 (58.3) | 0.5 |
| . | Untreated (%) n=74 . | Chemo (%) n=81 . | HMA (%) n=100 . | Chemo+HMA (%) n=36 . | P* . |
|---|---|---|---|---|---|
| Median age | 51 | 54 | 57 | 59 | <0.001 |
| RAEB-1 and -2 | 19 (25.7) | 41 (50.6) | 52 (52) | 15 (41.7) | 0.1 |
| CMML | 2 (2.7) | 10 (12.5) | 9 (9) | 3 (13.9) | 0.1 |
| MDS-tx | 45 (60.8) | 29 (35.8) | 37 (37) | 6 (16.7) | 0.07 |
| MK+ | 21 (29.6) | 13 (16.1) | 40 (40.4) | 4 (11.4) | <0.001 |
| High&very high risk by IPSS-R | 30 (40.5) | 33 (40.7) | 58 (58) | 10 (27.8) | 0.006 |
| CR at HSCT | 0 | 21 (25.9) | 25 (25) | 3 (8.3) | 0.08 |
| MUD/MMD | 18/8 (24.3/10.8) | 34/17 (42/21) | 44/17 (44/17) | 18/4 (50/11.1) | 0.8 |
| Myeloablative conditioning | 55 (74.3) | 56 (69.1) | 69 (69) | 21 (58.3) | 0.5 |
Comparison was between different therapy groups
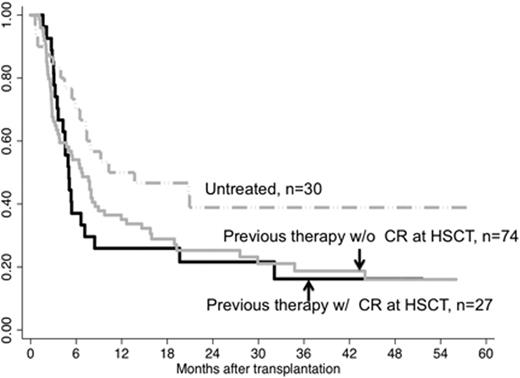
EFS by previous therapy and disease status at HSCT in patients with high and very high risk disease by IPSS-R.
EFS by previous therapy and disease status at HSCT in patients with high and very high risk disease by IPSS-R.
The summary outcomes
| . | Untreated . | Chemo . | HMA . | Chemo+HMA . |
|---|---|---|---|---|
| 3 year EFS | 43.4% | 31.2% | 31.1% | 31.5% |
| 3 year relapse incidence | 21.7% | 32.3% | 30.3% | 29.6% |
| 3 year TRM | 18.8% | 30.9% | 32.1% | 27.% |
| . | Untreated . | Chemo . | HMA . | Chemo+HMA . |
|---|---|---|---|---|
| 3 year EFS | 43.4% | 31.2% | 31.1% | 31.5% |
| 3 year relapse incidence | 21.7% | 32.3% | 30.3% | 29.6% |
| 3 year TRM | 18.8% | 30.9% | 32.1% | 27.% |
Multivariate analyses for EFS**
| . | EFS . | . | . |
|---|---|---|---|
| Variable | HR | I | p |
| MK | |||
| CN | Ref | ||
| MK- | 1.8 | 1.2-2.7 | 0.003 |
| MK+ | 4.5 | 2.9-7.0 | <0.001 |
| Histology per WHO | |||
| RA/RCMDS | Ref | ||
| RAEB | 1.4 | 0.9-2.0 | 0.2 |
| CMML | 2.0 | 1.1-3.7 | 0.03 |
| Undifferentiated | 1.2 | 0.8-1.9 | 0.4 |
| . | EFS . | . | . |
|---|---|---|---|
| Variable | HR | I | p |
| MK | |||
| CN | Ref | ||
| MK- | 1.8 | 1.2-2.7 | 0.003 |
| MK+ | 4.5 | 2.9-7.0 | <0.001 |
| Histology per WHO | |||
| RA/RCMDS | Ref | ||
| RAEB | 1.4 | 0.9-2.0 | 0.2 |
| CMML | 2.0 | 1.1-3.7 | 0.03 |
| Undifferentiated | 1.2 | 0.8-1.9 | 0.4 |
Adjusted for BM blast count, platelet transfusion, ferritin level and disease status at HSCT, previous MDS therapy and donor type.
In summary, MK and histology were the only prognostic factors for EFS in MDS HSCT patients. Therapy prior to HSCT and disease status at HSCT were not found to be prognostic for any disease outcome in our analyses. Our results suggest that high-risk MDS patients should proceed with HSCT without any delay given that more therapy with the goal to achieve better disease control may not lead to more favorable disease outcomes after HSCT. These data need to be confirmed in larger, controlled clinical trials.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal