Abstract
Ibrutinib is an irreversible inhibitor of Bruton’s tyrosine kinase (BTK) with promising clinical activity in phase I/II clinical trials in CD20+ B-cell malignancies for which rituximab-combination chemotherapy is today’s standard of care. Given homology between BTK and interleukin-2 inducible tyrosine kinase (ITK), we recently confirmed based on molecular and phenotypic analysis that ibrutinib irreversibly binds ITK (Dubovsky J, et al: Blood 2013). ITK expression in FcR-stimulated NK cells leads to increased calcium mobilization, granule release, and cytotoxicity (Khurana D et al: J Immunol 2007). As FcR stimulation is requisite for antibody-dependent NK-cell mediated cytotoxicity (ADCC), we investigated if ibrutinib influenced rituximab’s anti-lymphoma activity as well as trastuzumab’s anti-HER2+ breast cancer activity.
Rituximab and trastuzumab-dependent NK-cell mediated cytotoxicity was assessed using lymphoma and HER2+ breast cancer cell lines. In vitro NK cell cytokine secretion, degranulation and cytotoxicity were assessed by IFN-g release, CD107a mobilization and chromium release. Xenotransplant lymphoma and breast cancer models in athymic, nu/nu mice were used to assess efficacy of ibrutinib and rituximab or trastuzumab combination regimens. Using an autologous system from patients with chronic lymphocytic leukemia (CLL), inhibition of NK cell function was compared between ibrutinib and CGI-1746 (Di Paolo JA, et al: Nature Chem Biol 2011), a previously reported selective BTK inhibitor lacking ITK inhibitory function.
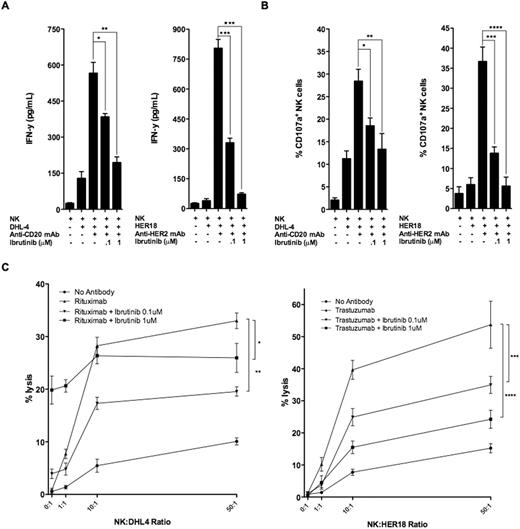
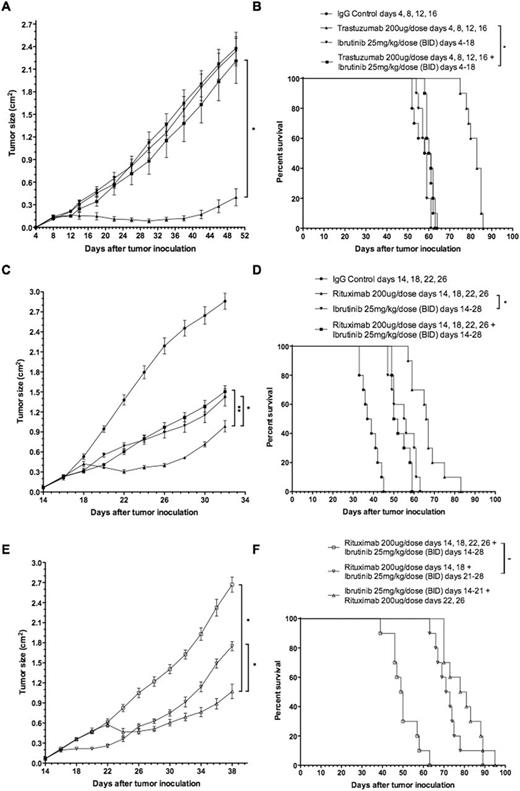
FcR-stimulated NK cells following exposure to rituximab-coated lymphoma cells express high and moderate levels of ITK and BTK, respectively. Ibrutinib inhibited both rituximab- and trastuzumab-induced NK cell cytokine secretion in a dose-dependent manner at 0.1 and 1mM of ibrutinib in vitro (Fig 1A *p=.009, **p=.001, ***p<.001). Similarly, ibrutinib prevented FcR-stimulated NK cell degranulation by ∼60% and ∼90% at 0.1 and 1mM (Fig 1B *p=.013, **p=.017, ***p=.002, ****p=.001). In vitro cytotoxicity of rituximab-coated, chromium-labeled lymphoma cells was observed in an NK cell-independent and ibrutinib-concentration dependent manner (Fig 1C *p<.001, **p=.045). In contrast, in vitro cytotoxicity of trastuzumab-coated, chromium-labeled breast cancer cells was inhibited in an ibrutinib dose-dependent manner (Fig 1C ***p=.036, ****p=.010). Abrogation of trastuzumab-dependent NK cell-mediated cytotoxicity was confirmed in vivo with concurrent ibrutinib daily dosing for 2 weeks during trastuzumab treatment, 4 doses, as measured by tumor growth and survival (Fig 2A, *p<.001; Fig 2B *p=.18). Concurrent ibrutinib daily dosing for 2 weeks during 4 doses of rituximab therapy, similarly, antagonized rituximab’s efficacy in tumor growth and survival, with anti-lymphoma activity of the combination equivalent to ibrutinib monotherapy (Fig 2C *p=.049, **p=.032; Fig 2D *p=.29). Sequential ibrutinib for 1 week followed by 2 doses of rituximab, or sequential rituximab (2 doses) followed by ibrutinib for 1 week resulted in restored anti-lymphoma activity superior to concurrent combination therapy of ibrutinib for 2 weeks and 4 doses of rituximab (Fig 2E *p<.001; Fig 2F *p<.001). Autologous CLL samples, n=6, confirmed similar effect as observed with lymphoma cell lines of 0.1 and 1mM of ibrutinib resulting in in vitro inhibition of NK cell degranulation and cytotoxicity by ∼65% and ∼85%, respectively. Interestingly, in vitro, CGI-1746 had no antagonistic effect on ADCC against rituximab treated targets (CLL cells and Raji lymphoma cell line).
Preclinical investigation of a combination therapy currently in Phase II and III clinical trials, adding a promising novel agent, ibrutinib, to a current standard of care, rituximab, for the treatment of B-cell lymphomas, surprisingly results in antagonistic effects. We demonstrate that the abrogation of both rituximab’s and trastuzumab’s anti-tumor efficacy is a result of ibrutinib’s inhibition of FcR-stimulated NK cell function, specifically ADCC. Selective BTK inhibitors or alternative ibrutinib dosing schedules, sequential versus concurrent, may preserve the anti-lymphoma efficacy of both agents.
Buggy:Pharmacyclics: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal