Abstract
JAK inhibitors (JAKi) currently represent a frontline treatment modality in patients with symptomatic intermediate- or high-risk myelofibrosis (MF). While the efficacy of JAKi in decreasing spleen size and improving disease-related symptoms is well documented, the effect of this drug class on overall survival and risk of leukemic transformation in MF patients is less well understood, primarily due to short follow up in individual patient cohorts. Furthermore, there is scant information on baseline predictors of overall and leukemia-free survival, and also spleen and/or anemia responses, at the onset of JAKi therapy. We conducted a sponsor-independent single-center retrospective review of patients treated with JAKi with long follow up to address the aforementioned issues. Here, we report our findings regarding baseline predictors of clinical outcome and treatment response in MF patients treated with JAKi.
Clinical, cytogenetic and molecular data were retrospectively collected on patients treated with one or more JAKi on a clinical trial at the Mayo Clinic, Rochester, MN. Baseline clinical data pertained to the onset of treatment with the first JAKi. Karyotype information was available for all patients. Screening for JAK2V617F, MPLW515, ASXL1, and SRSF2 mutations was performed as previously described (Leukemia. 2013 Apr 26. doi: 10.1038/leu.2013.119). Spleen size was measured by palpation by clinical investigators. Anemia and spleen responses were adjudicated as per IWG criteria (Tefferi, Blood, 2006). Information on survival and leukemic transformation was updated in July 2013.
A total of 157 MF patients were studied (60% male; median age 65 years, range 34-89 years). Ninety seven patients (62%) had primary MF, 42 (27%) post-polycythemia vera MF, and 18 (11%) post-essential thrombocythemia MF. The DIPSS-plus distribution was: Low/Int-1 20 (13%), Int-2 66 (42%), and High 71 (45%). Other characteristics were: JAK2V617F positive 78%, unfavorable karyotype 17%, hemoglobin <10 g/dL 58%, leukocyte count >25 x 109/L 31%, platelet count <100 x 109/L 15%, peripheral blasts ≥1% 69%, red blood cell transfusion-dependent 37%, and constitutional symptoms 56%. Forty one patients (38%) were ASXL1 mutated (n=108) and 20 (18%) SRSF2 mutated (n=110). One hundred and thirty nine patients (89%) were evaluable for spleen response (median spleen size by palpation 19 cm, range 6-32 cm) and 91 (58%) for anemia response by IWG criteria.
The first JAKi was momelotinib (CYT387) in 79 patients (50%), ruxolitinib in 51 (33%) and fedratinib (SAR302503) in 27 (17%). Median follow up from first JAKi treatment was 30 months (range 1-67 months); during this period, 72 deaths (46%) and 14 (9%) leukemic transformations have been documented.
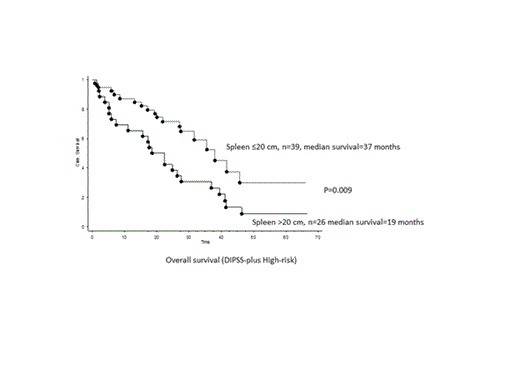
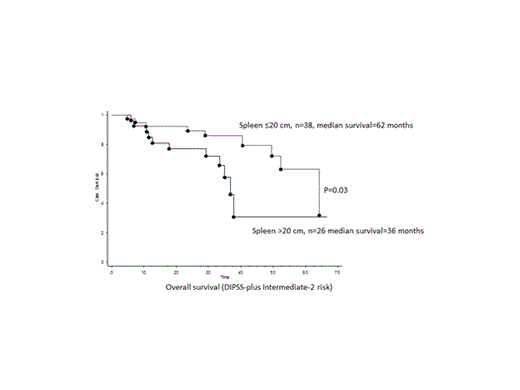
The median overall survival of the entire cohort was 41 months. Multivariable analysis identified the following DIPSS-plus independent predictors for survival: palpable spleen size >20 cm (HR 2.5), ASXL1 mutated status (HR 3.1) and SRSF2 mutated status (HR 3.8) (all p<0.01) (Figure). In contrast, there was no difference in survival with regards to the first JAKi used for MF treatment (p=0.3).
Multivariable analysis identified the following predictors for leukemia-free survival: SRSF2 mutated (HR 9.7), unfavorable karyotype (HR 8.7), and palpable spleen size >20 cm (HR 9.1) (all p<0.01).
Predictors of spleen response included baseline spleen size (≤20 cm 56%, >20 cm 34%; p=0.01), with borderline significance for ASXL1 mutation status (unmutated 51%, mutated 31%; p=0.06).
Predictors of anemia response included RBC transfusion status at baseline (transfusion dependent 38%, transfusion independent 12%; p<0.01) and JAK2V617F status (unmutated 46%, mutated 22%; p=0.02).
Larger baseline spleen size and ASXL1 and SRSF2 mutated status predicted for inferior overall survival, independent of DIPSS-plus, at the onset of first JAKi treatment in MF patients. Similarly, multivariable analysis identified larger baseline spleen size and presence of unfavorable karyotype and SRSF2 mutations as being predictive for inferior leukemia-free survival. Spleen response rates were significantly superior in patients with smaller baseline spleen size and possibly ASXL1 unmutated status. Anemia response rates were significantly higher in patients who were transfusion-dependent at baseline and had JAK2V617F unmutated status.
Pardanani:Bristol Myers Squibb: Clinical trial support Other; Sanofi: Clinical trial support, Publication support services, Clinical trial support, Publication support services Other; PharmaMar: Clinical trial support, Clinical trial support Other; JW Pharmaceutical Corp.: Clinical trial support, Clinical trial support Other. Off Label Use: Use of Ruxolitinib, Momelotinib, Fedratinib and Pomalidomide for treatment of Myelofibrosis in Clinical Trial setting.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal