Disease recurrence is the major cause of treatment failure after allogeneic hematopoietic stem cell transplantation (alloHSCT) for acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS). Graft-versus-host disease (GVHD) is the major cause of non-relapse morbidity and mortality after alloHSCT. Decitabine (DAC) is a hypomethylating agent that irreversibly binds to and inhibits DNA methyltransferase-1, leading to loss of DNA methylation. DAC maintenance may help eradicate minimal residual disease and facilitate a graft-versus-leukemia effect. Lower DAC doses are expected to be better tolerated after alloHSCT and equally effective in promoting hypomethylation. Additionally, DAC maintenance may have a favorable effect on the incidence of GVHD by enhancing the effect of T-regulatory lymphocytes (Choi et al, Blood, 2012).
Patients (pts) with AML/MDS in complete remission (CR) after alloHSCT, with ANC> 1,500/mm3, platelets> 50,000/mm3, and without grade III-IV acute GVHD were eligible to receive DAC, starting between day +50 and +100 after alloHSCT. We investigated 4 DAC doses: 5, 7.5, 10 and 15 mg/m2/day IV x 5 days of a 6-week cycle, for a total of 8 cycles. Each cohort contained 4-8 evaluable patients. The Maximum Tolerated Dose (MTD) was defined as the maximum dose at which< 20% of patients experience hematologic or non-hematologic dose limiting toxicities (DLT) during the 1st cycle of treatment. GVHD prophylaxis was at the physician discretion.
19 pts were enrolled to date; the median age was 60 y (22-66); 14 pts had AML and 5 MDS. All conditioning regimens were myeloablative; 14 donors were unrelated and 5 related. 3 cohorts have been completed and a final 4th cohort is currently enrolling. Median follow-up from alloHSCT is 24 mo (7-36). 8 pts (44%) completed all 8 cycles: 7 pts remain in CR with stable counts and full donor chimerism and 1 pt developed CNS-only relapse 26 mo after alloHSCT. 9 pts went off study before cycle 6: 1 pt for poor compliance after 6 cycles, 3 pts for relapsed disease (after 1, 2 and 5 cycles, respectively), 2 pts for sepsis, and 2 pts after physician decision. 6 pts have died: 3 from relapse, 2 from sepsis after 3 cycles of DAC (they were not neutropenic at a time), and 1 form sepsis >1 y after getting off study. 2 pts are still on study passed 3rd cycle. DAC maintenance was well tolerated. Associated hematological toxicities were mostly grade I/II leukopenia and thrombocytopenia. There was one occurrence of hematological DLT. No MTD has been reached. Non-hematological toxicities were grade I/II nausea, fatigue, neuropathy, and transaminase elevation. 2 pts had grade I-II acute GVHD prior to starting DAC and both resolved while on DAC; 1 pt developed grade IV gut GVHD coinciding with first cycle of DAC that completely resolved on DAC; 1 pt developed late acute GVHD of skin and liver around 6th cycle of DAC that resolved after few wks. 2/8 pts who completed 8 cycles of DAC developed very mild skin and oral chronic GVHD not requiring any systemic therapy, 1/8 pt developed late acute GVHD responding to therapy, and 1 pt developed overlap GVHD syndrome. 4/7 pts who went off study prior cycle 6, and did not have an early relapse, developed severe chronic GVHD requiring intensive immunosuppressive therapy.
To our knowledge this is the first report of DAC as maintenance therapy after alloHSCT. DAC at the dose of 15 mg/m2 for 5 days every 6 weeks is safe and can be administered in heavily pretreated pts in the post-alloHSCT setting. Approximately 43% of pts were able to receive all 8 cycles. The lack of toxicities and low incidence of GVHD indicate that a longer period of administration should be investigated. Although there is a trend of increased FOXP3 expression, results were not statistically significant. Further correlative studies, including genome-wide methylation studies, are ongoing.
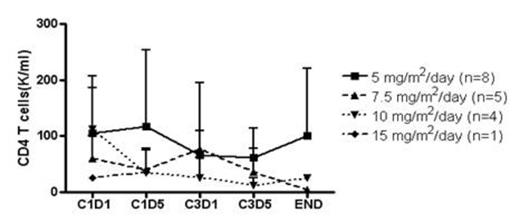
Effect of DAC on FOXP3 and CD4 T cells
Off Label Use: Decitabine maintenance after alloHSCT.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal