Abstract
PAM score is a simple tool for predicting mortality risk in patients undergoing allogeneic bone marrow transplantation. Originally developed and validated at Fred Hutchinson Cancer Research Center, Seattle, this has recently been validated in 276 patients from Japan. We retrospectively evaluated this in our cohort of T deplete myeloablative allogeneic transplants as there are no published studies validating PAM score in T deplete myeloablative setting.
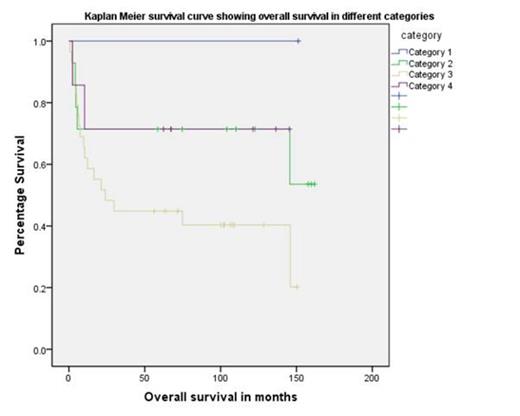
After a median follow up of 63 months 51% of the patients are alive. The predicted mortality for category 2, 3 and 4 patients via PAM score was 28%, 48% and 28% respectively. This was concordant with the predicted mortality at 2yrs for category 2 and 3. Category 4 was not concordant (Chi-square test, P=0.0035).
| Characteristics . | N=51 . | ||
|---|---|---|---|
| Age-yr | |||
| Median | 35 | ||
| Range | 17-57 | ||
| Sex-No (%) | |||
| Male | 29 (57) | ||
| Female | 22 (43) | ||
| Donor type-No (%) | |||
| Related | 29 (57) | ||
| Unrelated | 22 (43) | ||
| Disease type-No (%) | |||
| ALL | 12 (24) | ||
| AML | 24 (48) | ||
| CML | 3 (6) | ||
| MDS | 4 (7) | ||
| Others | 8 (15) | ||
| Disease risk category-No (%) | |||
| Low | 3 (6) | ||
| Intermediate | 32 (63) | ||
| High | 16 (31) | ||
| Conditioning Regimen-No (%) | |||
| TBI >12Gy | 43 (84) | ||
| TBI<12 | 3 (6) | ||
| Non TBI | 5 (10) | ||
| Alemtuzumab dose in stem cell bag (mg) | |||
| 0 | 4 | ||
| 7.5 | 6 | ||
| 10 | 18 | ||
| 15 | 4 | ||
| 20 | 19 | ||
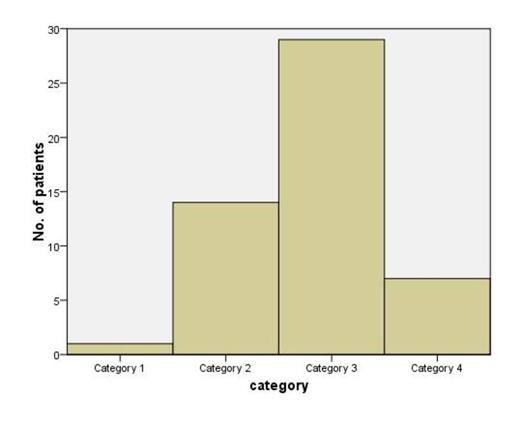
| PAM score categories (2 yr probability of mortality) | Predicted(%) | Observed(%) | |
| 1 | 1 | <25 | 0 |
| 2 | 14 | 25-50 | 28 |
| 3 | 29 | 50-75 | 48 |
| 4 | 7 | >75 | 28 |
| Follow up (months) | |||
| Median | 63 | ||
| Range | 1-162 | ||
| Characteristics . | N=51 . | ||
|---|---|---|---|
| Age-yr | |||
| Median | 35 | ||
| Range | 17-57 | ||
| Sex-No (%) | |||
| Male | 29 (57) | ||
| Female | 22 (43) | ||
| Donor type-No (%) | |||
| Related | 29 (57) | ||
| Unrelated | 22 (43) | ||
| Disease type-No (%) | |||
| ALL | 12 (24) | ||
| AML | 24 (48) | ||
| CML | 3 (6) | ||
| MDS | 4 (7) | ||
| Others | 8 (15) | ||
| Disease risk category-No (%) | |||
| Low | 3 (6) | ||
| Intermediate | 32 (63) | ||
| High | 16 (31) | ||
| Conditioning Regimen-No (%) | |||
| TBI >12Gy | 43 (84) | ||
| TBI<12 | 3 (6) | ||
| Non TBI | 5 (10) | ||
| Alemtuzumab dose in stem cell bag (mg) | |||
| 0 | 4 | ||
| 7.5 | 6 | ||
| 10 | 18 | ||
| 15 | 4 | ||
| 20 | 19 | ||
| PAM score categories (2 yr probability of mortality) | Predicted(%) | Observed(%) | |
| 1 | 1 | <25 | 0 |
| 2 | 14 | 25-50 | 28 |
| 3 | 29 | 50-75 | 48 |
| 4 | 7 | >75 | 28 |
| Follow up (months) | |||
| Median | 63 | ||
| Range | 1-162 | ||
Milligan:Celgene: unrestricted educational grant Other.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal