Abstract
Despite recent therapeutic advances, multiple myeloma (MM) remains primarily an incurable cancer. Patients experiencing rapid recovery of T cells post autologous stem cell transplant (auto-SCT) may have improved outcomes, and spontaneous cellular responses to tumor can occur, suggesting immune mediated control of tumor is possible. We and others have investigated therapeutic cancer vaccines that have shown promise in pilot studies, in particular following post-transplant infusion of activated autologous T cells. However, efficacy of these approaches may be limited by thymic selection which restricts the repertoire of T cell receptors (TCRs) to low affinity TCRs that cannot recognize the low level of antigen present on most tumor cells. We hypothesized that incorporation of affinity-enhanced tumor antigen-specific TCRs into autologous T cells infused post-transplant would overcome this limitation and improve response rates in the post auto-SCT setting.
We report interim results of a Phase II clinical trial (NCT01352286) to evaluate the safety and activity of autologous T cells genetically engineered to express an affinity-enhanced TCR that recognizes the NY-ESO-1/LAGE-1 peptide complex HLA‐A*0201‐SLLMWITQC; these cells are infused in the setting of profound lymphodepletion that accompanies high dose chemotherapy administered during auto-SCT. Patients with high risk or relapsed MM, who are HLA‐A*0201 positive, and whose tumor is positive for NY-ESO-1 and/or LAGE-1 by RT-PCR are eligible. CD25 depleted CD4 and CD8 T cells are activated and expanded using anti-CD3/CD28 antibody conjugated microbeads, and genetically modified with a lentiviral vector containing the TCR construct at a multiplicity of infection of 1. Engineered T cells are administered four days after high dose melphalan and two days following auto-SCT, at a dose range of 1-10 billion total cells with a minimum gene modification requirement of 10%. Patients are evaluated for MM responses in accordance with the IMWG criteria at 6 weeks, and 3 and 6 months post infusion. At 3 months, patients start lenalidomide maintenance. The initial 6 patient phase is complete and a 20 patient extension phase is ongoing.
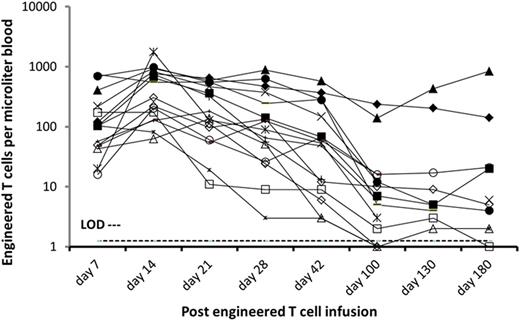
Prior to enrollment on study, patients had received a median of 3 prior therapies including 6 with prior transplant. 50% of tumors contained high risk chromosomal abnormalities, and NY-ESO-1 expression is correlated with adverse prognosis. 20 patients (average age of 57) have been infused with an average of 2.3 X 109 engineered T cells (range 4.5 X 108-3.9 X 109); this reflects an average clinical scale transduction efficiency of 34% (range 18% – 49%). Infusions have been well tolerated, and the majority of adverse events were related to the high dose melphalan. Possibly related SAEs were neutropenia and thrombocytopenia, and GI and metabolism disorders including diarrhea, colitis, hyponatremia and hypomagnesemia. 10, 4, 2, and 2 patients have reached the 1 year, 9 month, 6 month and 3 month assessment timepoints, respectively, and 17/20 patients are alive. Best response by day 100 is sCR/CR in 2/15 (13%), nCR in 10/15 (67%), and PR in 3/15% (20%), which compares favorably to historic responses in patients undergoing first or second transplant. Engineered T cells expanded and persisted in blood and marrow at 180 days by Q-PCR and flow-cytometry in all but one case (Figure). 7 patients progressed after day 100, which was accompanied either by loss of engineered T cells or loss of tumor antigen. Detailed phenotyping and functional analysis of engineered T cells, and correlates with clinical responses, is underway.
This is the first clinical evaluation of engineered T cells in the MM setting. Infusions are safe, well tolerated, and are associated with encouraging responses in a high risk myeloma population. A study evaluating the engineered T cells in a non-transplant study is underway.
Stadtmauer:Celgene: Consultancy. Binder-Scholl:Adaptimmune: Employment. Smethurst:Adaptimmune: Employment. Brewer:Adaptimmune: Employment. Bennett:Adaptimmune: Employment. Gerry:Adaptimmune: Employment. Pumphrey:Adaptimmune: Employment. Tayton-Martin:Adaptimmune: Employment. Ribeiro:Adaptimmune: Employment. Levine:Novartis: cell and gene therapy IP, cell and gene therapy IP Patents & Royalties. Jakobsen:Adaptimmune: Employment. Kalos:Novartis corporation: CART19 technology, CART19 technology Patents & Royalties; Adaptive biotechnologies: Member scientific advisory board , Member scientific advisory board Other. June:Novartis: Patents & Royalties, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal