Abstract
Hematopoietic stem cell (HSC) transplantation has curative potential for patients with hematological malignancies. Clinically, HSCs derived from mobilized peripheral blood (PB) are used more frequently than bone marrow (BM). However, current standard mobilizing agents yield grafts that may not contain sufficient HSCs or may contain constituents that promote graft-versus-host disease (GVHD). Here we tested the effect of the combination of two clinical grade drugs, FLT3L and AMD3100 (Plerixafor), on stem cell mobilization, and evaluated the transplantation outcomes of the mobilized cells.
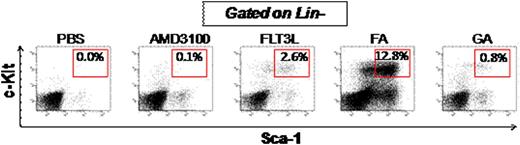
HSCs (Lin-Sca1+c-Kit+, LSK cells) in the PB were analyzed in mice subjected to various mobilization regimens. Compared to PBS-treated mice, AMD3100-treated mice had modest upregulation of LSK cells, while FLT3L-treated mice mobilized significantly more LSK cells into PB. However, mice from the combination of FLT3L and AMD3100 (FA) group mobilized highest frequency and most absolute number of LSK cells compared to the mice from any other groups including G-CSF alone and its combination with AMD3100 (GA) (Fig. 1 and not shown, P < 0.05). FLT3L and AMD3100 showed a synergistic mobilizing effect. Colony formation assays revealed that cells mobilized by FA contained significantly more CFU (colony formation unit) than any other regimens, including GA, FLT3L alone, and AMD3100 alone, and were consistent with the synergistic effect between FLT3L and AMD3100 (P< 0.01).
In addition to LSK cells, other immune cell subsets were also assessed. Mobilization with FA or FLT3L alone significantly increased NK cell percentages to the same extent, while FA resulted in dramatically more NK cells in absolute number than FLT3L alone. FA also led to significantly higher absolute number of Treg cells in mobilized blood compared with the other regimens. Of note, both NK and Treg cells have been demonstrated to suppress GVHD development in mice, and NK cells also possess an antitumor activity. The two major subsets of DCs, plasmacytoid DCs (pDCs) and conventional DCs (cDCs), were also significantly increased in proportion for the FA group when compared with other regimens including GA. Of note, DCs have been reported to modulate GVHD reactions, with cDCs enhancing and pDCs inhibiting GVHD.
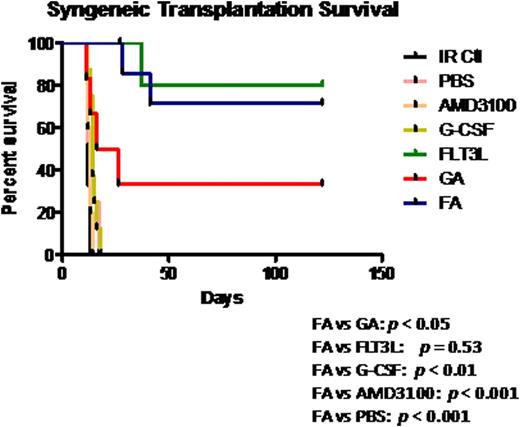
To test the clinical potential of FA mobilized grafts, cells mobilized by several different mobilizing regimens were transfused into lethally irradiated syngeneic mice. Mice receiving 2 × 105PB mobilized by PBS, AMD3100 alone or G-CSF alone failed to reconstitute hematopoietic cells, and died within 21 days (Fig. 2). In contrast, mice receiving FLT3L or FA-mobilized products survived 100% at day 21, and the survival rate maintained at about 70% as far out as 4 months after transplantation (Fig. 2). The engraftment of the cells mobilized by FA was significantly superior to that of the cells mobilized by GA, the latter of which had a survival rate of only 35% at 4 months post-transplant. An examination of BM in the surviving mice also showed that mice receiving FA grafts contained a higher frequency of LSK cells than those receiving GA grafts.
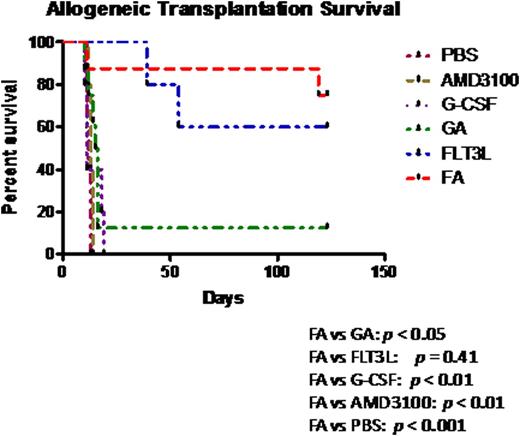
Next we explored the transplantation efficacy of the FA-mobilized cells in an MHC-mismatched allogeneic transplantation model. Lethally irradiated Balb/c mice were transplanted with 8 × 105 PB cells mobilized by the different regimens from C57BL/6 mice. All recipients of grafts mobilized by PBS, AMD3100 alone or G-CSF alone died within 3 weeks after transplantation (Fig. 3), with apparent clinical evidence of acute GVHD, while 80% and 66% of mice receiving grafts mobilized by FA or FLT3L alone survived to 4-months post-transplantation, respectively (Fig.3), showing no or modest acute GVHD symptoms. The survival rate of both the FA and the FLT3L groups was significantly higher than that observed in the GA group (12.5%). At 4-months post-transplantation, the survived mice in the FA and FLT3L groups were sacrificed and found to have both long-term (LT) HSCs (FLT3-CD34- LSK) and short-term (ST) HSCs (FLT3-CD34+LSK) originated from donor mice (H2Kb) dominating in BM, suggesting that their better transplantation outcome may be due not only to the suppression of GVHD but also to the better hematopoietic reconstitution.
Hofmeister:Celgene Corporation: Membership on an entity’s Board of Directors or advisory committees, Speakers Bureau. March:Celldex Therapeutics: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal