Key Points
The tyrosine kinase inhibitor crenolanib has type 1 inhibitor properties and has potent activity against FLT3-activating mutations.
Crenolanib is active in vitro and in vivo against FLT3 inhibitor-resistant FLT3-ITD/D835 mutations.
Abstract
FLT3 kinase internal tandem duplication (ITD) mutations are common in acute myeloid leukemia (AML) and are associated with poor clinical outcomes. Although initial responses to FLT3 tyrosine kinase inhibitors (TKIs) are observed in FLT3-ITD−positive patients, subsequent relapse often occurs upon acquisition of secondary FLT3 kinase domain (KD) mutations, primarily at residues D835 and F691. Using biochemical assays, we determined that crenolanib, a novel TKI, demonstrates type I properties and is active against FLT3 containing ITD and/or D835- or F691-activating mutations. Potent activity was observed in FLT3-ITD−positive AML cell lines. Crenolanib delayed the outgrowth of MV4-11 cells in a xenograft mouse model, whereas in combination with the type II TKI sorafenib, a significant decrease in leukemic burden (P < .001) and prolonged survival (P < .01) was observed compared with either type I or II TKI alone. Crenolanib was active against Ba/F3 cells harboring FLT3-ITD and secondary KD mutations and sorafenib-resistant MOLM-13 cells containing FLT3-ITD/D835Y both in vitro and in vivo. In addition, crenolanib inhibited drug-resistant AML primary blasts with FLT3-ITD and D835H/Y mutations. These preclinical data demonstrate that crenolanib is effective against FLT3-ITD containing secondary KD mutations, suggesting that crenolanib may be a useful therapeutic agent for TKI-naive and drug-resistant FLT3-ITD−positive AML.
Introduction
Overall survival in children with acute myeloid leukemia (AML) has improved to 60% to 70%, exceeding survival rates of approximately 30% to 40% in adults.1-6 However, after the recurrence of disease, the likelihood of long-term survival is poor. Patients with activating FLT3 internal tandem duplication (ITD) mutations, which occur in ∼15% of pediatric AML patients and ∼30% of adult AML patients, are at high risk for disease relapse.7-9 Although therapy with FLT3 tyrosine kinase inhibitors (TKIs), such as sorafenib and quizartinib, initially produces clinical responses, most patients develop drug-resistant disease within a few months to a year of treatment.10-14 Therefore, new therapies are needed for newly diagnosed and drug-resistant FLT3-ITD−positive AML.
Data from preclinical studies indicate that one mechanism of FLT3 TKI resistance is the acquisition of secondary point mutations in the FLT3 kinase domain (KD), which may alter drug binding and/or shift kinases to an autoactivated conformation.14 Recently, secondary FLT3 mutations have been observed in adults and children who developed resistance to sorafenib or quizartinib.10,12,13 Specifically, amino acid exchanges at residue D835 (D835F/H/V/Y) are the most commonly observed secondary FLT3 KD mutations, followed by the F691L mutation. In a recent report, Smith et al13 proposed that mutation of D835 destabilizes the inactive conformation of FLT3; therefore, targeting these variants with type I TKIs that bind the FLT3 active conformation may be necessary. To our knowledge, this approach has not yet been investigated.
Crenolanib (CP-868,596) is a novel TKI that was developed as a selective and potent inhibitor of PDGFR α and β but also has high affinity for other type III receptor tyrosine kinases (RTKs), such as FLT3.15,16 Preclinical and clinical data have shown crenolanib to be active in imatinib-resistant gastrointestinal stromal tumor with PDGFRα D842 mutations. Because D842 mutations are thought to stabilize PDGFRα in the active conformation, this finding suggests that crenolanib is a type I TKI.15 Most TKIs with activity against FLT3, such as quizartinib and sorafenib, are type II inhibitors that bind the inactive kinase conformation; these inhibitors show limited activity against the clinically relevant FLT3 D835 secondary mutations because kinase activity overcomes inhibitor capacity,12,13 which suggests that crenolanib may be active against mutations at the analogous FLT3 D835 residue, with the potential to benefit therapy for drug-resistant AML.
In this report, various in vitro and in vivo studies demonstrate that crenolanib is a potent FLT3 inhibitor with type I properties and activity against FLT3-ITD−positive AML. Furthermore, we determined that the combination of crenolanib and sorafenib, a type II inhibitor, potentiates antileukemic activity in a MV4-11 mouse xenograft model of AML. Finally, we show that crenolanib (1) decreases the viability of Ba/F3 cells expressing FLT3-ITD and TKI-resistant D835H/Y or F691L mutations and delays engraftment of FLT3-ITD/D835H cells in vivo; (2) decreases the viability of a TKI-resistant FLT3-ITD−positive MOLM-13 AML cell line harboring a D835Y mutation in vitro and prolongs survival in a mouse xenograft model; and (3) is active against TKI-resistant pediatric AML blast samples containing FLT3 ITD and D835H/Y mutations. Taken together, these results support clinical evaluation of crenolanib for treatment of AML patients harboring FLT3-activating mutations.
Materials and methods
Cell culture and reagents
Human AML MV4-11, THP-1, U937, and OCI-AML3 (ATCC, Manassas, VA) cell lines and MOLM-13 and PL21 (DSMZ, Brunswick, Germany) cell lines were maintained in RPMI 1640 media (Life Technologies, Carlsbad, CA) supplemented with 10% fetal bovine serum (Atlanta Biologicals, Lawrenceville, GA) at 37°C in a humidified atmosphere containing 5% CO2. Mouse Ba/F3 cells were derived and maintained as previously described (supplemental Materials; see the Blood Web site).10
Antibodies against FLT3 (C-20), β-actin (C4), and phospho-tyrosine (pY99) were obtained from Santa Cruz Biotechnology (Dallas, TX) and phospho-STAT5 (pY694), STAT5, phospho-extracellular signal-regulated kinase (pT202/pY204; 197G2), extracellular signal-regulated kinase (137F5), phospho-S6 (pS235/pS236; D57.2.2E), and S6 (5G10) antibodies were purchased from Cell Signaling Technology (Danvers, MA). Crenolanib (AROG Pharmaceuticals LLC, Dallas, TX) and sorafenib (Chemietek, Indianapolis, IN) were formulated in dimethyl sulfoxide (DMSO) for in vitro assays. Cytosine β-d-arabinofuranoside (AraC) was purchased from Sigma-Aldrich (St. Louis, MO) and reconstituted in water.
Western blot analysis
FLT3 immunoprecipitation and western blot analysis was performed as previously described10 ; details are provided in the supplemental Materials.
Cell viability assessment
The effects of drug treatment on the viability of AML cell lines, Ba/F3 cells, and primary leukemic blast samples were assessed by use of the WST-1 or 3-(4,5-dimethylthiazol-2-yl)-2,5-dimethyltetrazolium bromide; 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide cell proliferation reagents (Roche Applied Science, Indianapolis, IN) according to the manufacturer’s instructions; details are provided in the supplemental Materials.
Binding affinity for FLT3 and Abl variants
Binding of crenolanib and sorafenib to purified FLT3 and Abl kinase was performed by use of the commercially available KdELECT assay (DiscoveRx, Fremont, CA), as previously described.17 The apparent dissociation constant (Kd) was calculated for the following kinases: wild-type (WT) FLT3, FLT3 D835H, FLT3 D835Y, FLT3 D835V, FLT3-ITD, FLT3-ITD/D835V, FLT3-ITD/F691L, WT Abl, and Abl Q252H.
Crenolanib tolerability and pharmacokinetic studies
Crenolanib tolerability and pharmacokinetic studies were performed in nonleukemia-bearing and leukemia-bearing mice. Details of drug formulation and administration, blood sampling and processing, analytical methods, and pharmacokinetic data analysis are provided in the supplemental Materials. All animal studies were approved by the Animal Care and Use Committee at St. Jude Children’s Research Hospital.
In vivo xenograft mouse models
Details on the cell engraftment and dosing of xenograft mouse models are provided in the supplemental Materials.
Statistical analysis
Prism (Graphpad, La Jolla, CA) software was used for nonlinear regression analysis of cell viability dose−response data and Kaplan-Meier analysis of animal survival. WinNonlin (Pharsight, Cary, NC) and NONMEM (Icon, Dublin, Ireland) software were used for pharmacokinetic data analysis. Calcusyn (Biosoft, Cambridge, UK) software was used for the analysis of drug combination effects. Densitometry was measured with ImageJ software (National Institutes of Health, Bethesda, MD). Animal images were analyzed using Living Image version 4.2 software (Perkin Elmer). The statistical significance of data was determined by the Student t test with a P value < .05.
Results
Crenolanib is a FLT3 inhibitor with type I properties
Crenolanib was developed as a potent inhibitor of PDGFR and is highly selective for other class III RTKs.16 In agreement with Heinrich et al,15 crenolanib bound WT FLT3 with high affinity (Kd = 0.15 nM; Table 1; supplemental Figure 4). Crenolanib had similar affinity to FLT3 variants containing activating mutations (Kd range, 0.14-22 nM) compared with WT FLT3 (Table 1; supplemental Figure 4). For comparison, we tested the affinity of FLT3 variants to sorafenib, a TKI used to treat AML patients expressing FLT3-ITD.18,19 Of note, WT FLT3 as well as the FLT3 mutants had greater affinity for crenolanib compared with sorafenib, particularly for the dual ITD/835V and ITD/F691L mutants (ITD/D835V Kd = 3.6 vs 630 nM; ITD/F691L Kd = 22 vs 860 nM, crenolanib vs sorafenib, respectively; Table 1; supplemental Figure 4).
Crenolanib and sorafenib in vitro kinase binding*
| . | Crenolanib Kd, nM . | Sorafenib Kd, nM . |
|---|---|---|
| FLT3 variant | ||
| FLT3 | 0.15 | 13† |
| FLT3 (ITD) | 0.26 | 95 |
| FLT3 (D835H) | 0.16 | 11 |
| FLT3 (D835Y) | 0.14 | 24 |
| FLT3 (D835V) | 3.3 | 140 |
| FLT3 (ITD, D835V) | 3.6 | 630 |
| FLT3 (ITD, F691L) | 22 | 860 |
| Abl variant | Crenolanib active/inactive Kd ratio | |
| Active Abl | 140 | 0.33 |
| Inactive Abl | 430 | − |
| Active Abl (Q252H) | 36 | 0.36 |
| Inactive Abl (Q252H) | 100 | − |
| . | Crenolanib Kd, nM . | Sorafenib Kd, nM . |
|---|---|---|
| FLT3 variant | ||
| FLT3 | 0.15 | 13† |
| FLT3 (ITD) | 0.26 | 95 |
| FLT3 (D835H) | 0.16 | 11 |
| FLT3 (D835Y) | 0.14 | 24 |
| FLT3 (D835V) | 3.3 | 140 |
| FLT3 (ITD, D835V) | 3.6 | 630 |
| FLT3 (ITD, F691L) | 22 | 860 |
| Abl variant | Crenolanib active/inactive Kd ratio | |
| Active Abl | 140 | 0.33 |
| Inactive Abl | 430 | − |
| Active Abl (Q252H) | 36 | 0.36 |
| Inactive Abl (Q252H) | 100 | − |
Data obtained from KdELECT screening.
As reported in Davis et al.17
Type II inhibitors preferentially bind kinases in the inactive conformation, whereas type I inhibitors do not discriminate between active and inactive kinase conformations.20 To gain insight into the conformational preference for crenolanib binding, we compared the crenolanib Kd for nonphosphorylated (inactive) WT Abl kinase and phosphorylated (active) WT Abl; this method has been previously validated to predict kinase inhibitor−binding properties.21 Crenolanib had greater affinity for active WT Abl vs inactive WT Abl with an active/inactive ratio of 0.33 (Table 1; supplemental Figure 5). This finding was confirmed by measuring the Kd for active and inactive Abl Q252H (active/inactive Kd ratio: 0.36; Table 1; supplemental Figure 5). Unlike the type II inhibitors sorafenib and imatinib, which have active/inactive Kd ratios >1.0, the active/inactive Kd ratio for crenolanib was similar to the type I inhibitors staurosporine and PD-173955 (supplemental Figure 5).17 These data confirm preliminary work suggesting that crenolanib is a type I inhibitor.22 Overall, our data demonstrate that crenolanib is a TKI with type I properties and binds with high affinity to WT FLT3 and FLT3 mutants.
Crenolanib inhibits FLT3-ITD-positive AML cell viability in vitro
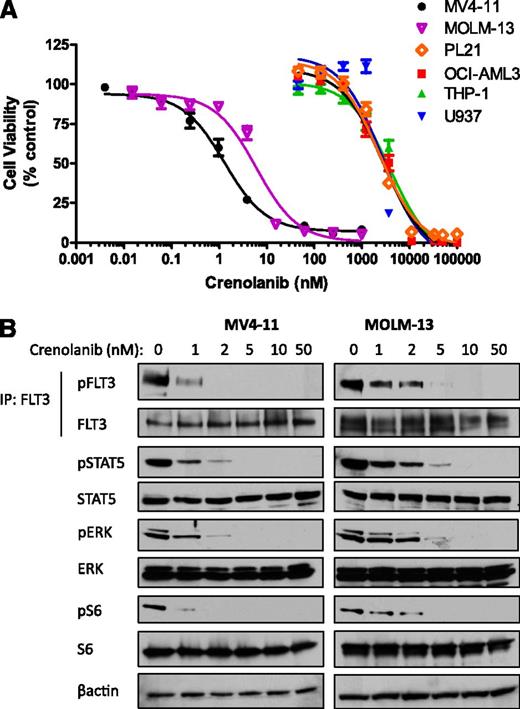
Gain-of-function FLT3-ITD mutations represent one of the most common gene alterations in AML and confer a poorer prognosis.7 We next tested whether crenolanib has activity against a panel of AML cell lines with FLT3-ITD mutations or WT FLT3. Crenolanib potently decreased the viability of FLT3-ITD−expressing MV4-11 and MOLM-13 cells, with a 50% inhibition/inhibitory concentration (IC50) of 1.3 and 4.9 nM, respectively (Figure 1; supplemental Table 1). The sensitivity to crenolanib was similar to that of sorafenib (IC50: 4.9 and 17 nM for MV4-11 and MOLM-13, respectively; supplemental Table 1). In contrast, cells expressing WT FLT3 were less sensitive to treatment with crenolanib and sorafenib (IC50 range: 2.8-3.5 µM and 3.9-13 µM, respectively; Figure 1; supplemental Table 1). The loss of FLT3-ITD cell viability correlated with a decrease in FLT3-ITD prosurvival signaling (Figure 1).
Crenolanib potently inhibits FLT3-ITD AML cell viability and FLT3 signaling. AML cells were treated with DMSO or increasing concentrations of crenolanib (A) for 72 hours and cell viability was measured or (B) for 1 hour and lysed. Western blot analysis was performed on FLT3 immunoprecipitation eluent or whole-cell lysate by use of the indicated antibodies . Cell viability measurements represent the mean ± SEM of 3 experiments with 6 replicates each (n = 18). IP, immunoprecipitation.
Crenolanib potently inhibits FLT3-ITD AML cell viability and FLT3 signaling. AML cells were treated with DMSO or increasing concentrations of crenolanib (A) for 72 hours and cell viability was measured or (B) for 1 hour and lysed. Western blot analysis was performed on FLT3 immunoprecipitation eluent or whole-cell lysate by use of the indicated antibodies . Cell viability measurements represent the mean ± SEM of 3 experiments with 6 replicates each (n = 18). IP, immunoprecipitation.
We next determined the combination effects of crenolanib with AraC, the most widely used chemotherapeutic agent for AML, in FLT3-ITD−positive AML cells by using a cell viability assay. After simultaneous treatment with both drugs for 72 hours, a synergistic loss in cell viability was observed in MV4-11 and MOLM-13 cells, with combination index (CI) values ranging from 0.3 to 0.7 (supplemental Figure 6). Synergy also was observed for additional sequences and schedules of drug exposure, including AraC before crenolanib, crenolanib before AraC, and simultaneous drug treatment of 4 hours followed by 68 hours’ treatment with crenolanib (supplemental Figure 6).
Crenolanib pharmacokinetics in mice
To test the in vivo activity of crenolanib, we first determined the pharmacokinetic properties of crenolanib in mice. Initial studies using nonleukemia-bearing FLT3-ITD transgenic C57Bl/6 mice demonstrated that intraperitoneal administration achieved greater crenolanib plasma concentrations compared with oral administration (supplemental Figure 7). NOD.Cg-Prkdcscid II2rgtm1Wjl/SzJ (NSG) mice engrafted with MV4-11-luc cells were administered crenolanib at a maximally tolerated dose of 15 mg/kg intraperitoneally (described in the supplemental Materials) and serial blood sampling was performed during a 24-hour period. A summary of crenolanib pharmacokinetic parameters is presented in supplemental Table 2, and population model-predicted plasma concentration-time plots are shown in supplemental Figure 7. The median area under the concentration-time curve from time zero to 24 hours after drug administration was approximately 2140 ng/mL*h, and the maximum plasma concentration was 1370 ng/mL (∼3.1 µM). The area under the curve achieved at 15 mg/kg intraperitoneally in mice is comparable with the exposure observed in adults receiving crenolanib 100 mg orally.23
Crenolanib has antileukemic activity in a FLT3-ITD−positive AML mouse model
The evaluation of crenolanib in biochemical assays and AML cell lines demonstrated activity against FLT3-ITD. Therefore, we determined the in vivo antileukemic effects of crenolanib using a FLT3-ITD−positive MV4-11 AML xenograft mouse model. Crenolanib 15 mg/kg intraperitoneally was administered to male NSG mice once daily (Monday to Friday) for 3 consecutive weeks beginning 10 days after cell injection. As depicted in supplemental Figure 8, treatment with crenolanib suppressed MV4-11-luc bone marrow infiltration compared with vehicle-treated animals. A significant prolongation of survival also was observed after treatment with crenolanib (median group survival: 55 and 71 days for vehicle and crenolanib, respectively; P = .0031; supplemental Figure 8).
Combination of type I and II FLT3 inhibitors potentiates antileukemic activity in vivo
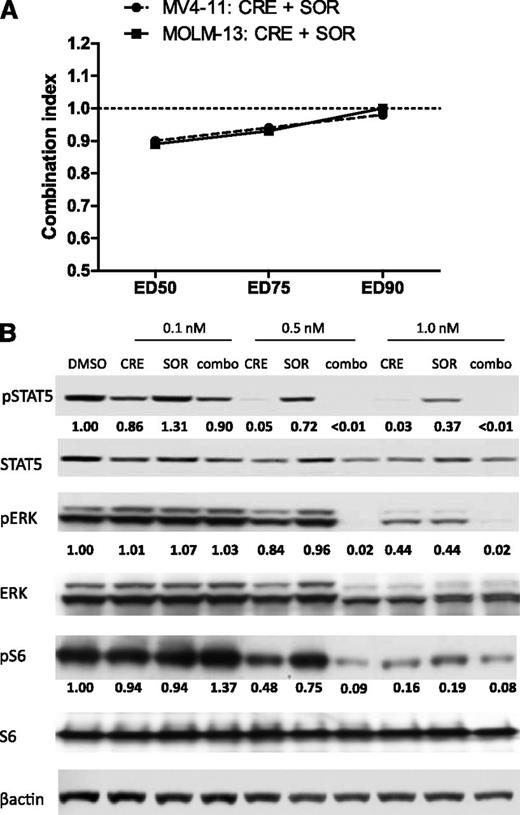
It has been hypothesized that the combination of crenolanib with a type II inhibitor, such as sorafenib, may provide superior FLT3 inhibition by concomitant targeting of multiple kinase conformations.24 Combination treatment with crenolanib and sorafenib for 72 hours had additive effects on inhibiting the in vitro viability of MV4-11 and MOLM-13 cells (CI range, 0.89-1.0; Figure 2). Profiling FLT3-ITD prosurvival signaling in MV4-11 cells, we found that crenolanib had more potent inhibitory effects compared with sorafenib, which is consistent with the cell viability data (supplemental Table 1), and the drug combination caused greater inhibition of signaling compared with each drug alone (Figure 2).
Drug combination effects on MV4-11 cell viability and FLT3 signaling. (A) MV4-11 and MOLM-13 cells were treated with DMSO or increasing concentrations of crenolanib (CRE), sorafenib (SOR), or both at a fixed concentration ratio for 72 hours and cell viability was measured. Calcusyn combination index (CI) values were generated for drug combination effects on cell viability. Cell viability experiments were performed in triplicate with 6 replicates each (n = 18; CI value > 1.0: antagonism; CI value = 1.0: additivity; CI value < 1.0: synergism). (B) MV4-11 cells were treated for 1 hour with DMSO or the indicated concentration of crenolanib (CRE), sorafenib (SOR), or both (combo) and lysed. Western blot analysis was performed on whole-cell lysate by use of the indicated antibodies. Densitometry measurements were normalized to total protein amount.
Drug combination effects on MV4-11 cell viability and FLT3 signaling. (A) MV4-11 and MOLM-13 cells were treated with DMSO or increasing concentrations of crenolanib (CRE), sorafenib (SOR), or both at a fixed concentration ratio for 72 hours and cell viability was measured. Calcusyn combination index (CI) values were generated for drug combination effects on cell viability. Cell viability experiments were performed in triplicate with 6 replicates each (n = 18; CI value > 1.0: antagonism; CI value = 1.0: additivity; CI value < 1.0: synergism). (B) MV4-11 cells were treated for 1 hour with DMSO or the indicated concentration of crenolanib (CRE), sorafenib (SOR), or both (combo) and lysed. Western blot analysis was performed on whole-cell lysate by use of the indicated antibodies. Densitometry measurements were normalized to total protein amount.
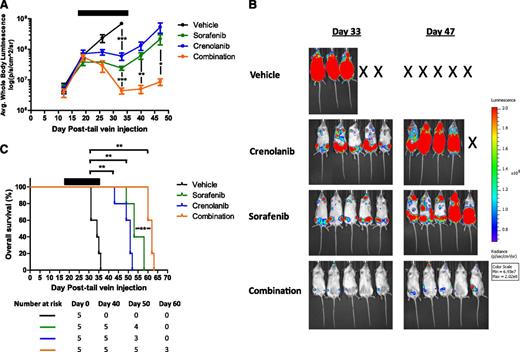
We next tested this hypothesis by determining the in vivo effects of crenolanib in combination with sorafenib in the MV4-11-luc xenograft mouse model. Leukemia-bearing female NSG mice were treated with crenolanib 15 mg/kg intraperitoneally once daily, sorafenib 15 mg/kg orally once daily, or the drug combination (crenolanib 15 mg/kg intraperitoneally and sorafenib 15 mg/kg orally) once daily (Monday to Friday) for 3 consecutive weeks. For this study, treatment began 17 days after cell injection to allow for considerable tumor engraftment (supplemental Figure 1). Both TKIs given alone significantly delayed the outgrowth of MV4-11-luc cells compared with vehicle-treated mice (P < .001), whereas the drug combination resulted in tumor regression and a significant decrease in leukemia burden compared with groups treated with vehicle or single-agent TKI (P < .001; Figure 3). Sorafenib or crenolanib monotherapy prolonged survival for more than 2 weeks beyond vehicle-treated animals (median survival: 34, 53, and 51 days for vehicle, sorafenib, and crenolanib, respectively; P = .0017 for crenolanib or sorafenib vs vehicle), whereas the drug combination conferred the greatest survival advantage (median survival: 62 days; P = .0017 for the drug combination vs vehicle; P = .0021 and P = .0026 for the drug combination vs crenolanib or sorafenib alone, respectively). Overall, these data suggest that inhibition of multiple FLT3 kinase conformations by simultaneous treatment with type I and type II kinase inhibitors results in greater antileukemic efficacy compared with either inhibitor alone and represents a novel treatment strategy for FLT3-ITD−positive AML.
Antileukemic effects of the combination of crenolanib and sorafenib in a MV4-11 xenograft AML mouse model. Female NSG mice engrafted with MV4-11-luc cells were treated with vehicle, crenolanib 15 mg/kg intraperitoneally, sorafenib 15 mg/kg orally, or crenolanib (15 mg/kg, intraperitoneally) plus sorafenib (15 mg/kg, orally) once daily (Monday to Friday) for 3 consecutive weeks beginning on day 17. (A-B) Leukemic cell bone marrow infiltration was monitored by noninvasive luciferase imaging (*P < .05; **P < .01; ***P < .001). (C) Kaplan-Meier analysis of animal survival (**P = .0017 for crenolanib vs vehicle; P = .0017 for sorafenib vs vehicle; P = .0017 for the drug combination versus vehicle; P = .0021 and P = .0026 for the drug combination vs crenolanib or sorafenib alone, respectively). Black bar denotes treatment period.
Antileukemic effects of the combination of crenolanib and sorafenib in a MV4-11 xenograft AML mouse model. Female NSG mice engrafted with MV4-11-luc cells were treated with vehicle, crenolanib 15 mg/kg intraperitoneally, sorafenib 15 mg/kg orally, or crenolanib (15 mg/kg, intraperitoneally) plus sorafenib (15 mg/kg, orally) once daily (Monday to Friday) for 3 consecutive weeks beginning on day 17. (A-B) Leukemic cell bone marrow infiltration was monitored by noninvasive luciferase imaging (*P < .05; **P < .01; ***P < .001). (C) Kaplan-Meier analysis of animal survival (**P = .0017 for crenolanib vs vehicle; P = .0017 for sorafenib vs vehicle; P = .0017 for the drug combination versus vehicle; P = .0021 and P = .0026 for the drug combination vs crenolanib or sorafenib alone, respectively). Black bar denotes treatment period.
Crenolanib is active against FLT3 secondary drug-resistant mutations in Ba/F3 cells in vitro and in vivo
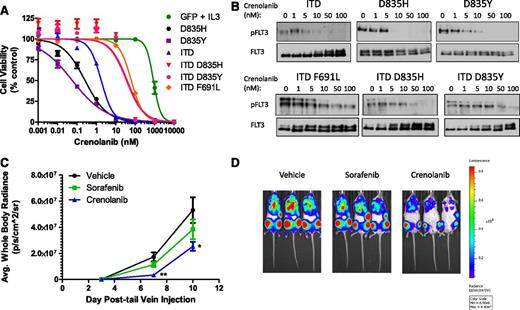
A commonly described mechanism of resistance to FLT3 inhibitors in preclinical models, and more recently in patients with FLT3-ITD−positive AML, is the acquisition of secondary KD point mutations primarily at residues D835 and F691.10,12-14 In addition, Ba/F3 cells expressing these mutations alone or in combination with an ITD mutation were resistant to sorafenib (supplemental Table 3).10,13 Using this same model, we determined that crenolanib potently inhibited the viability of cells expressing single FLT3 D835H or D835Y mutations (IC50, 0.3 and 0.06 nM, respectively) and moderately inhibited cells expressing double FLT3 mutants containing the ITD and D835H/Y or F691L (IC50 range, 35-62 nM; Figure 4; supplemental Table 3); reduction in Ba/F3 cell viability correlated with a decrease in FLT3 phosphorylation (Figure 4).
Crenolanib activity against Ba/F3 cells expressing various FLT3 mutants in vitro and in vivo. (A-B) Ba/F3 cells expressing FLT3 mutants were treated with DMSO or increasing concentrations of crenolanib (A) for 72 hours and viability was measured or (B) for 1 hour and lysed. Western blot analysis was performed on FLT3 immunoprecipitation eluent by use of the indicated antibodies. Cell viability measurements represent the mean ± SEM of 2 to 4 experiments with 6 replicates each (n = 12-24). (C-D) Male NSG mice engrafted with Ba/F3 FLT3-ITD/D835H-luc cells were treated with vehicle, crenolanib 15 mg/kg intraperitoneally twice daily, or sorafenib 15 mg/kg orally once daily for 10 consecutive days beginning on day 1 (5 mice per treatment group). (C) Ba/F3 FLT3-ITD/D835H-luc cell bone marrow infiltration was monitored by noninvasive luciferase imaging. Black bar denotes treatment period (*P = .0157; **P = .0013). (D) Representative whole-body luciferase images from each treatment group are shown.
Crenolanib activity against Ba/F3 cells expressing various FLT3 mutants in vitro and in vivo. (A-B) Ba/F3 cells expressing FLT3 mutants were treated with DMSO or increasing concentrations of crenolanib (A) for 72 hours and viability was measured or (B) for 1 hour and lysed. Western blot analysis was performed on FLT3 immunoprecipitation eluent by use of the indicated antibodies. Cell viability measurements represent the mean ± SEM of 2 to 4 experiments with 6 replicates each (n = 12-24). (C-D) Male NSG mice engrafted with Ba/F3 FLT3-ITD/D835H-luc cells were treated with vehicle, crenolanib 15 mg/kg intraperitoneally twice daily, or sorafenib 15 mg/kg orally once daily for 10 consecutive days beginning on day 1 (5 mice per treatment group). (C) Ba/F3 FLT3-ITD/D835H-luc cell bone marrow infiltration was monitored by noninvasive luciferase imaging. Black bar denotes treatment period (*P = .0157; **P = .0013). (D) Representative whole-body luciferase images from each treatment group are shown.
Cross-resistance to multiple FLT3 inhibitors, including sorafenib, quizartinib, and ponatinib, develops upon acquisition of a secondary FLT3 KD mutation at residue D835.10,13,25 Therefore, an inhibitor with activity against FLT3 D835 mutations might provide clinical benefit for patients with TKI-resistant FLT3-ITD−positive AML. We tested the in vivo activity of crenolanib in female NSG mice engrafted with Ba/F3 FLT3-ITD/D835H-luc cells. Because of the shorter half-life of crenolanib compared with sorafenib in mice (1.2 vs 3.4 hours, respectively),26 we administered crenolanib twice daily in this study involving highly aggressive and FLT3 inhibitor-resistant Ba/F3 cells. Mice received crenolanib 15 mg/kg intraperitoneally twice daily or sorafenib 15 mg/kg orally once daily for 10 consecutive days beginning on day 1. In contrast to sorafenib, treatment with crenolanib resulted in a significant decrease in Ba/F3 FLT3-ITD/D835H-luc bone marrow infiltration (P = .0013 and P = .0157 on days 7 and 10 after cell injection, respectively), as determined by noninvasive luciferase imaging (Figure 4).
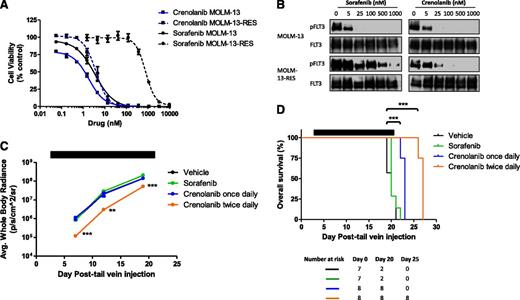
Crenolanib is active against drug-resistant MOLM-13 AML cells with a FLT3-ITD/D835Y mutation in vitro and in vivo
To test crenolanib efficacy in a model of drug-resistant FLT3-ITD−positive AML, we generated MOLM-13-RES-luc cells, which express FLT3-ITD/D835Y, by continuous treatment with the FLT3 inhibitor tandutinib. Similar to Moore et al,27 no alteration in FLT3 allele number was apparent in the MOLM-13-RES-luc cells (supplemental Figure 9). In addition, polymerase chain reaction−based phase analysis determined that the D835Y mutation was expressed on FLT3-ITD alleles (supplemental Table 4). In comparison with the parental MOLM-13-luc cell line, MOLM-13-RES-luc cells displayed increased FLT3-ITD phosphorylation and activation of downstream signaling (supplemental Figure 9). In vitro cell viability assays determined that the MOLM-13-RES-luc cells were resistant to sorafenib (IC50, 3.1 and 785 nM in MOLM-13-luc and MOLM-13-RES-luc cells, respectively; Figure 5). In contrast, MOLM-13-RES-luc cells were sensitive to crenolanib, with an IC50 value similar to the parental MOLM-13-luc cell line (IC50, 1.2 and 3.9 nM in MOLM-13-luc and MOLM-13-RES-luc cells, respectively). The relative sensitivity of the MOLM-13-luc and MOLM-RES-luc cells to crenolanib correlated with loss of FLT3-ITD phosphorylation (Figure 5). We also observed synergistic activity of crenolanib combined with AraC against MOLM-13-RES-luc cells after simultaneous drug exposure for 72 hours (supplemental Figure 6).
Crenolanib activity against MOLM-13-RES cells in vitro and in vivo. (A-B) MOLM-13-luc and MOLM-13-RES-luc cells were treated with DMSO or increasing concentrations of crenolanib or sorafenib (A) for 72 hours and viability was measured or (B) for 1 hour and lysed. Western blot analysis was performed on FLT3 immunoprecipitation eluent using the indicated antibodies. Cell viability measurements represent the mean ± SEM of 2 to 3 experiments with 6 replicates each (n = 12-18). (C-D) Female NSG mice engrafted with MOLM-13-RES-luc cells were treated with vehicle, sorafenib 15 mg/kg orally once daily, or crenolanib 15 mg/kg intraperitoneally once or twice daily (Monday to Friday) for 3 consecutive weeks beginning on day 3. (C) Leukemic cell bone marrow infiltration was monitored by noninvasive luciferase imaging (**P < .01; ***P < .001). (D) Kaplan-Meier analysis of animal survival (***P < .0001 for crenolanib once daily vs vehicle; ***P < .0001 for crenolanib twice daily vs vehicle). Black bar denotes treatment period.
Crenolanib activity against MOLM-13-RES cells in vitro and in vivo. (A-B) MOLM-13-luc and MOLM-13-RES-luc cells were treated with DMSO or increasing concentrations of crenolanib or sorafenib (A) for 72 hours and viability was measured or (B) for 1 hour and lysed. Western blot analysis was performed on FLT3 immunoprecipitation eluent using the indicated antibodies. Cell viability measurements represent the mean ± SEM of 2 to 3 experiments with 6 replicates each (n = 12-18). (C-D) Female NSG mice engrafted with MOLM-13-RES-luc cells were treated with vehicle, sorafenib 15 mg/kg orally once daily, or crenolanib 15 mg/kg intraperitoneally once or twice daily (Monday to Friday) for 3 consecutive weeks beginning on day 3. (C) Leukemic cell bone marrow infiltration was monitored by noninvasive luciferase imaging (**P < .01; ***P < .001). (D) Kaplan-Meier analysis of animal survival (***P < .0001 for crenolanib once daily vs vehicle; ***P < .0001 for crenolanib twice daily vs vehicle). Black bar denotes treatment period.
We next determined whether MOLM-13-RES-luc cells were sensitive to crenolanib treatment in vivo. NSG mice engrafted with MOLM-13-RES-luc cells received sorafenib 15 mg/kg orally once daily or crenolanib 15 mg/kg intraperitoneally once or twice daily (Monday to Friday) for 3 consecutive weeks beginning on day 3. MOLM-13-RES-luc leukemic infiltration was significantly reduced in mice treated with crenolanib twice daily compared with vehicle-treated animals (P < .0001 on day 7; P = .0028 and 0.0002 on days 12 and 19, respectively), whereas mice treated with sorafenib or crenolanib once daily showed infiltration comparable with the vehicle group (Figure 5; supplemental Figure 10). Notably, once- and twice-daily treatment with crenolanib produced a significant increase in survival compared with vehicle-treated mice (median survival: 27, 23, and 20 days for crenolanib twice daily, crenolanib once daily, and vehicle treatment groups, respectively; P < .0001; Figure 5). In contrast, sorafenib treatment produced no survival advantage over vehicle-treated controls (median survival = 20 days; Figure 5). Overall, these data suggest that crenolanib may be beneficial in the context of drug-resistant FLT3-ITD−positive AML, particularly after acquisition of a FLT3 D835 secondary mutation. This may have clinical implications for the use of crenolanib in drug-resistant FLT3-ITD−positive AML.
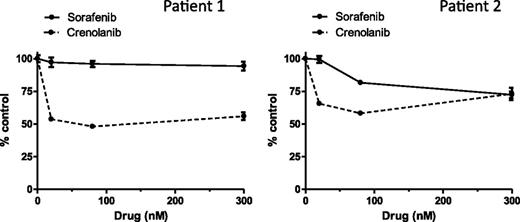
Activity of crenolanib against TKI-resistant FLT3-ITD−positive AML primary blast samples
We recently described the development of clinical TKI resistance after sequential therapy with sorafenib and sunitinib in FLT3-ITD−positive pediatric AML patients; the loss in TKI sensitivity was associated with acquisition of FLT3 D835H/Y mutations.10 We next determined the sensitivity of primary blast samples taken from 2 TKI-resistant patients to crenolanib and sorafenib ex vivo (supplemental Table 5) in a cell viability assay. Treatment with crenolanib produced moderate inhibition at low nanomolar concentrations (average inhibition range, 34%-46% at 20 nM crenolanib and 48%-52% at 80 nM crenolanib; Figure 6); in contrast, sorafenib produced minimal effects (average inhibition range, 1%-3% at 20 nM sorafenib and 4%-18% at 80 nM sorafenib; Figure 6). Two recent preliminary reports show similar effects of crenolanib and sorafenib on primary blast samples from 2 adult patients with AML harboring FLT3 D835V/F mutations.28,29 In agreement with our in vitro and in vivo studies, these data suggest that crenolanib has activity against leukemic cells with FLT3-ITD containing D835 mutations.
Crenolanib activity against TKI-resistant pediatric AML primary blasts ex vivo. Primary blast samples were treated with DMSO or increasing concentrations of crenolanib or sorafenib for 72 hours. Cell viability measurements represent the mean ± SEM of 4 replicates.
Crenolanib activity against TKI-resistant pediatric AML primary blasts ex vivo. Primary blast samples were treated with DMSO or increasing concentrations of crenolanib or sorafenib for 72 hours. Cell viability measurements represent the mean ± SEM of 4 replicates.
Discussion
We describe the biochemical and pharmacologic characterization of crenolanib, a selective inhibitor of type III RTKs, in preclinical models of FLT3-mutant AML. In binding assays, crenolanib demonstrated type I kinase inhibitor properties and potent activity against FLT3-ITD. In cellular assays, the administration of crenolanib inhibited the viability of FLT3-ITD−positive AML cell lines. In vivo, crenolanib delayed tumor infiltration and prolonged survival in a mouse xenograft model of FLT3-ITD−positive AML. Furthermore, the combination of crenolanib and the type II inhibitor sorafenib produced tumor regression and prolonged survival compared with either TKI administered alone. Crenolanib demonstrated potent inhibition of TKI-resistant single FLT3 D835 mutants and retained activity against double FLT3-ITD/D835 mutants in binding assays and Ba/F3 cells. Finally, crenolanib inhibited the viability of primary AML blast samples harboring FLT3-ITD and D835 mutations and demonstrated antileukemic activity against a sorafenib-resistant FLT3-ITD−positive AML cell line with a D835Y mutation in vitro and in vivo. Consistent with our findings, in a preliminary report, Smith et al22 showed that crenolanib binds FLT3-ITD with high affinity, displays potent activity against FLT3-ITD−positive AML cells, and exhibits type I properties in vitro.
When compared with other FLT3 inhibitors in development, crenolanib displays greater binding affinity to FLT3.30 Crenolanib is selective to type III RTKs and shows similar potency as the selective inhibitor quizartinib and the multikinase inhibitor sorafenib against FLT3-ITD−expressing MV4-11 cells.13,29,30 However, unlike crenolanib, quizartinib and sorafenib display type II inhibitor characteristics and are not active against FLT3-ITD/D835 mutations observed clinically.10,12,13 On the other hand, midostaurin shows type I properties but lacks selectivity and potency against FLT3-ITD in binding and cellular assays.30,31 Like most TKIs showing high binding in human plasma (range, 95 to >99.5%), crenolanib exhibits high protein binding (95.9%).16 However, crenolanib inhibited FLT3 phosphorylation at clinically achievable concentrations in a plasma inhibitory assay.28 Therefore, crenolanib as a type I inhibitor may be advantageous compared with other selective and potent FLT3-targeted TKIs in development, which are primarily type II inhibitors.
Enhanced antileukemic activity observed with the combination of crenolanib and sorafenib suggests that total inhibition of multiple FLT3-ITD conformations (eg, active and inactive) by simultaneous type I and type II inhibitor therapy may be an effective treatment strategy for FLT3-ITD−positive AML. In previous studies authors have evaluated the combination of type I and type II kinase inhibitors in other tyrosine kinase-mutant cancers. For example, the combination of the BCR-Abl/cKit inhibitors imatinib and dasatinib (type II and type I, respectively) resulted in greater antitumor effects compared with imatinib monotherapy in a mouse model of gastrointestinal stromal tumor containing a cKit V558 deletion mutation.32 In addition, the combination of ponatinib (type II) with midostaurin (type I) had synergistic effects on neoplastic mast cells containing the cKit D816V mutation.33 Furthermore, it has been shown that imatinib and dasatinib combination therapy suppresses the outgrowth of imatinib-resistant BCR-Abl mutations in vitro.34 Whether treatment with the combination of crenolanib and sorafenib similarly suppresses the development of sorafenib-resistant clones with FLT3 KD mutations is currently being tested. It also remains to be determined whether type I and II inhibitors can be combined safely clinically due to the potential for drug−drug interactions and increased toxicity.
Although some success has been observed with FLT3 inhibitor therapy in FLT3-ITD−positive AML, the acquisition of secondary FLT3 KD mutations often diminishes the response to TKIs.10,12,13 In particular, emergence of mutations at residues D835 and F691L is associated with clinical resistance to sorafenib and the second-generation FLT3 inhibitor quizartinib.10,12,13 Recent in vitro studies showed that substitutions at residue D835 also confer a high degree of resistance to the novel TKI ponatinib, whereas ponatinib retained modest activity against F691L mutants.25 The D835 mutation is hypothesized to indirectly change the conformation of the kinase activation loop and stabilize the active, DFG-in conformation, similar to the corresponding D186V mutant of cKit,35 whereas F691 is analogous to the “gatekeeper” residue T315 in BCR-Abl, the mutation of which is predicted to impair drug binding.10,13 Modeling these mutations in a Ba/F3 cell line, we demonstrated potent activity of crenolanib against single FLT3 D835H/Y mutants and modest activity against cells expressing double FLT3-ITD/D835H/Y or FLT3-ITD/F691L mutations. Similarly, recent preliminary reports describe crenolanib activity against quizartinib-resistant single FLT3 D835V/Y and double FLT3-ITD/D835V/Y/F mutants modeled in Ba/F3 or 32D cells.22,28 In addition, adult AML primary blast samples expressing a FLT3 D835V/F mutation were sensitive to crenolanib, whereas cross-resistance was observed to quizartinib and sorafenib.28,29 Our data are consistent with these reports and extend their findings by demonstrating that crenolanib has activity against (1) Ba/F3 cells harboring clinically relevant sorafenib-resistant FLT3-ITD/D835H mutations in vitro and in vivo; (2) a TKI-resistant MOLM-13 AML cell line containing double FLT3-ITD/D835Y mutations in vitro and in vivo; and (3) TKI-resistant pediatric AML primary blasts harboring double FLT3-ITD/D835H/Y mutations.
In conclusion, crenolanib is a novel FLT3 inhibitor with type I properties and potent activity against FLT3-ITD as well as FLT3 D835 mutations, which are resistant to currently available FLT3 inhibitors. Our results support the evaluation of crenolanib in patients with TKI-naive and TKI-resistant FLT3-ITD-positive AML. To this effect, several phase 2 studies of crenolanib for the treatment of adults with relapsed/refractory AML and FLT3 activating mutations are ongoing (NCT01522469 and NCT01657682).
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work is supported by the American Lebanese Syrian Associated Charities (ALSAC) and National Institutes of Health Cancer Center Support grants P30 CA021765 and R01 CA138744.
Authorship
Contribution: E.I.Z. and S.D.B. designed research; E.I.Z., J.B., S.H., and S.O. performed research; E.I.Z., D.C.T., M.S.R., L.J.J., C.F.S., and S.D.B. analyzed data; A.R. provided insightful discussion and essential reagents; H.I. provided insightful discussion; and E.I.Z. and S.D.B. wrote the paper.
Conflict-of-interest disclosure: A.R. is an employee of AROG Pharmaceuticals, LLC. The remaining authors declare no competing financial interests.
Correspondence: Sharyn D. Baker, Department of Pharmaceutical Sciences, St. Jude Children’s Research Hospital, 262 Danny Thomas Place, CCC, Room I5306, Memphis TN 38105; e-mail: sharyn.baker@stjude.org.