Key Points
JAK2V617F causes intrinsic changes in the process of platelet formation from megakaryocytes.
JAK2V617F platelets are prothrombotic and demonstrate increased reactivity to different agonists.
Abstract
The principal morbidity and mortality in patients with essential thrombocythemia (ET) and polycythemia rubra vera (PV) stems from thrombotic events. Most patients with ET/PV harbor a JAK2V617F mutation, but its role in the thrombotic diathesis remains obscure. Platelet function studies in patients are difficult to interpret because of interindividual heterogeneity, reflecting variations in the proportion of platelets derived from the malignant clone, differences in the presence of additional mutations, and the effects of medical treatments. To circumvent these issues, we have studied a JAK2V617F knock-in mouse model of ET in which all megakaryocytes and platelets express JAK2V617F at a physiological level, equivalent to that present in human ET patients. We show that, in addition to increased differentiation, JAK2V617F-positive megakaryocytes display greater migratory ability and proplatelet formation. We demonstrate in a range of assays that platelet reactivity to agonists is enhanced, with a concomitant increase in platelet aggregation in vitro and a reduced duration of bleeding in vivo. These data suggest that JAK2V617F leads to intrinsic changes in both megakaryocyte and platelet biology beyond an increase in cell number. In support of this hypothesis, we identify multiple differentially expressed genes in JAK2V617F megakaryocytes that may underlie the observed biological differences.
Introduction
The myeloproliferative neoplasms (MPNs) polycythemia rubra vera (PV) and essential thrombocythemia (ET) are characterized by an increase in red cell mass and platelet count, respectively, although both entities overlap phenotypically. This overlap was confirmed at a genetic level in 2005 when several groups reported the presence of a point mutation in the Janus kinase 2 (JAK2V617F) in 50% of ET and more than 90% of PV cases.1-4
Arterial and venous thromboses are the major causes of morbidity and mortality in MPN patients. In a prospective study with a well-defined end point of 1638 patients with PV, 38% of patients had experienced a previous thrombosis at the time of diagnosis, of which two-thirds were arterial events.5 Thromboses were also seen in ET6-9 and are particularly frequent in JAK2V617F-positive patients.10-13 Crucially, the thrombotic risk in PV and ET is in excess of that seen in patients who have a secondary erythocytosis or thrombocytosis, indicating that intrinsic factors over and above the increase in platelet number and blood viscosity contribute to thrombotic events. In keeping with this observation, an excessive number of patients with splanchnic vein thrombosis but otherwise normal platelet count (ie, no clinical MPN) carry the JAK2V167F mutation.14,15 A variety of structural and functional abnormalities of platelets have been reported in patients with MPNs, including abnormal expression of platelet membrane glycoproteins (GPs), most notably integrin αIIbβ3 and GPIbα; reduced responses to adenosine 5′-diphosphate (ADP), epinephrine, and collagen, in particular in aggregation studies; and storage pool deficiency.16-18 Paradoxically, there is also in vivo evidence of augmented platelet reactivity, including elevated numbers of circulating platelet aggregates and platelet microparticles, increased expression of platelet P-selectin, and raised platelet-mediated thrombin production.16,19-21 This dichotomy is also found in studies of intracellular signaling pathways relevant to platelet function: one group has described increased activation of Src kinases in ET platelets,22 and another recently reported a dysfunction of the phosphatidylinositol 3-kinase (PI3K) pathway.23
This bewildering variation in results is potentially due to the fact that the study of platelet function using samples from patients with PV and ET has been hampered by a number factors, including the following: (1) Clonality: Peripheral blood cells from patients with MPN have been shown to originate from an abnormal hemopoietic clone,24 but in a significant proportion of patients, normal polyclonal hemopoiesis coexists alongside the abnormal clone.25 This means that studies of peripheral blood platelets investigate the compound activities of platelets originating from both normal and malignant clones in varying ratios between patients. (2) Molecular mechanism of the disease: the JAK2V617F mutation is only found in 50% of all ET patients, and for most of the studies reported previously, the JAK2V617F carrier status was not known. (3) Platelet function varies widely between healthy individuals.26 (4) Different antiplatelet therapies have been used for the patients.
The study reported here addresses whether the human JAK2V617F causes a difference in cellular biology in megakaryocytes (MKs) and platelets beyond a simple increase in numbers. To this end, we have taken advantage of a conditional knock-in mouse model of ET where the human JAK2V617F has been knocked into the endogenous mouse Jak2 locus.27 This previously published model presents a stable ET phenotype after induction and is ideally suited to the problem at hand because (1) it provides access to a population with a genetically consistent background, eliminating interindividual variation in platelet reactivity; and, (2) following induction, 100% of the bone marrow (BM) stem cells (and therefore MKs and platelets) are heterozygous for JAK2V617F,27 eliminating the variable introduced by different clonality levels between each individual human patient. We show that JAK2V617F increases proplatelet formation and the migratory ability of MKs, adding to their increased number in the BM in terms of overall excessive platelet production. We also demonstrate in a wide variety of assays that JAK2V617F platelets have a heightened response to agonists that translates into increased aggregation at arterial shear in vitro and increased hemostasis in vivo independently of the increase in platelet count.
Material and methods
Chemicals
Detailed description of all chemicals can be found in the supplemental Methods (available on the Blood Web site).
Mice
The knock-in inducible JAK2V617F mouse model has been previously described.27 Heterozygous Jak2Floxed/+Mx1Cre+ (hereafter described as JAK2V617F mice) were induced with polyinosinic-polycytidylic acid (pIpC) in the first 10 days after birth, and the mice were used from 8 to 16 weeks. Control animals were Jak2+/+Mx1Cre+. All mice were kept in specific pathogen-free conditions, and all procedures were performed according to the United Kingdom Home Office regulations.
MK cultures
Mouse BM cells were prepared using the methodology previously described28-30 and cultured in CellGro (Cellgenix, Freiburg, Germany) with 20 ng/mL murine stem cell factor and 50 ng/mL murine thrombopoietin (TPO) at 37°C under 5% CO2 for 5 days. Mature MKs were purified using a 1.5%/3% bovine serum albumin (BSA) gradient as described.30 Differentiation and ploidy of cultured MKs were analyzed as described using fluorescein isothiocyanate–labeled anti-CD41 and DNA staining with 4,6-diamidino-2-phenylindole. Samples were acquired using a Beckman Coulter Cyan flow cytometer and analyzed using Summit v4.3 software (DAKO, Cambridge, United Kingdom).
Expression of mouse and human JAK2V617F in MKs
DNA was extracted from cultured MKs using Purelink genomic DNA mini kit (Invitrogen). RNA was extracted using the RNeasy kit (Qiagen, Manchester, United Kingdom), and cDNA made using Superscript III (First Strand kit; Invitrogen). The primers for amplification of the floxed and recombined JAK2V617F alleles have been published previously.27 Polymerase chain reaction (PCR) products were run on a 1.5% agarose gel. Quantitative PCR for the mouse endogenous Jak2 and the human JAK2V617F genes was carried out with QPCR SYBR Green (Agilent Technologies, Wokingham, United Kingdom) and Mx3000P Real-Time PCR system (Stratagene, Santa Clara, CA) as described.27
MK biochemistry
MK response to TPO was assessed as described, using mature cultured MKs starved for 4 hours at 37°C in cytokine-free medium prior to the addition of TPO and analyzing subsequent cell lysates by western blotting for phosphorylation of Stat3 and Erk.31 Outside-in signaling was assessed by adhering mature MKs to a fibrinogen-coated surface and analyzing phosphorylation of Plcγ2 and Syk by western blotting as described.30
MK migration assay
Chemotaxis was assessed using the Dunn chamber (Weber Scientific International, Teddington, United Kingdom) as described previously using a stromal-derived factor 1 alpha (aka CXCL12) gradient.32 Time-lapse images were captured using a Zeiss 20× 1.40 NA plan-apochromat lens on a Zeiss Axiovert 200 inverted high-end microscope (Welwyn Garden City, United Kingdom) and a Hamamatsu Orca 285 cooled digital camera. Slidebook (3I; http://www.intelligent-imaging.com/) and Image J (http://rsb.info.nih.gov/ij/) software programs were used to acquire and process images.
Proplatelet formation
Proplatelet formation was analyzed as described.32 Mature MKs resuspended in Tyrode’s buffer (134 mM NaCl, 2.9 mM KCl, 0.34 mM Na2HPO4.12H2O, 12 mM NaHCO3, 20 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, 1 mM MgCl2, 5 mM glucose) supplemented with 100 U/mL heparin were plated on fibrinogen-coated coverslips for 5 hours at 37°C, and the percentage of proplatelet-forming MKs counted against the total number of MKs adhering to the surface.
Tail-bleeding assay and thrombus formation under flow
Tail-bleeding was carried out as previously described, and blood volume loss assessed after removal of a 2-mm section of the tip of the tail.33 Thrombus formation in laminar flow was carried out as described in glass capillary tubes coated with collagen, and perfusion for 4 minutes at 1000 s−1 of heparinized whole blood taken from the inferior vena cava.34 Platelet thrombi volume was assessed after lysis with 50 μL Nonidet P-40 lysis buffer (300 mM NaCl2, 20 mM tris(hydroxymethyl)aminomethane-HCl, 2 mM EGTA, 2 mM EDTA, 2% Nonidet P-40, 2 mM Na3VO4, 1 mM 4-(2-aminoethyl) benzenesulfonyl fluoride hydrochloride, 1 μg/mL leupeptin, 10 μg/mL aprotinin, 1 μg/mL pepstatin), and protein quantification was carried out by western blot using a tubulin antibody. In a separate set of experiments, the platelet count in the blood sample of JAK2V617F mice was adjusted to the same level as in the control sample by addition of mouse platelet-poor plasma (PPP) prior to assessing thrombus formation. Mouse plasma was generated using heparinized mouse whole blood centrifuged at 1000 rpm (150g) to generate platelet-rich plasma (PRP), followed by a further centrifugation step at 3000 rpm (1000g) to generate PPP. The complete blood count was carried out using an Scil Vet ABC animal blood analyzer (Woodley, United Kingdom).
Platelet response to agonists
Whole blood was taken from the inferior vena cava and placed into acid citrate dextrose (111 mM glucose, 71 mM citric acid, 116 mM sodium citrate), and flow cytometry was used to look for P-selectin exposure on and fibrinogen binding to platelets following stimulation with the following agonists: collagen-related peptide (CRP), thrombin, and ADP as described.26 Platelet aggregation in PRP was assessed by light transmission using a Born aggregometer (PAP-4D; Bio/Data Corporation, Alpha Labs Limited, Horsham, PA).
Platelet spreading and outside-in signaling experiments
Platelet spreading on fibrinogen-coated coverslips was performed as previously described35 using washed platelets isolated from acid citrate dextrose–anticoagulated whole blood resuspended in Tyrode’s buffer, and acquisition of images was performed after 45 minutes at 37°C using a Zeiss Axiovert differential interference contrast (DIC) microscope. In some experiments, adherent platelets were lysed, and outside-in signaling was assessed looking for phosphorylation of Plcγ2 and Syk by western blotting.
Expression arrays
MK isolation and RNA and cDNA preparations were performed as described previously. Expression levels were assayed using Illumina MouseWG-6 v2.0 expression beadchip. Data processing and analysis are described fully in the supplemental Methods. Confirmation of differential expression was done by quantitative PCR as described previously for selected genes using the primers presented in the supplemental Methods.
Statistical analysis
Each experiment was carried out using paired JAK2V617F and control animals. In flow cytometry experiments, each individual point was performed in duplicate. Unless stated otherwise, statistical analysis was carried out using the Student t test to assess the significance of the results. The analysis of data from the expression arrays is described in the supplemental Methods.
Results
In vitro cultured MKs express physiological levels of human JAK2V617F
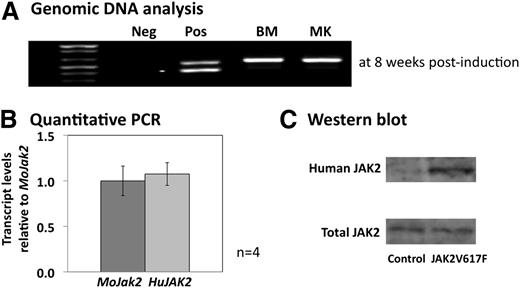
In the first instance, we ascertained the proportion of JAK2V617F mouse MKs that had undergone recombination of the floxed human JAK2V617F allele following pIpC injection. PCR analysis of genomic DNA from BM cells and cultured MKs showed 100% recombined DNA (Figure 1A) and confirmed, therefore, that analysis of platelet function in the JAK2V617F mouse peripheral blood would be carried out in a homogenous population emerging from MKs expressing the mutated JAK2 kinase. Quantitative PCR analysis on RNA extracted from cultured MKs showed equivalent levels of expression of the wild-type endogenous mouse Jak2 and knocked-in human JAK2V617F, similar to the situation in patients heterozygous for the JAK2V617F mutation (Figure 1B). Western blot analysis of MK protein lysates using an antibody that recognizes both human and mouse JAK2 confirmed equivalent expression of total JAK2 protein between control and JAK2V617F mice, whereas only the human JAK2 was detected in the JAK2V617F animals using a species-specific antibody (Figure 1C).
Molecular and protein analysis of murine JAK2V617F MKs. (A) Gel analysis of the PCR products from genomic DNA extracted from fresh BM cells and cultured MKs from JAK2V617F animals 8 weeks after pIpC induction. The upper band in the positive control (pos) corresponds to the recombined human JAK2V617F, and the lower band to the floxed allele. BM and MK samples only show the upper band confirming 100% recombination within these 2 cell populations. (B) Quantitative PCR looking at the relative level of the human JAK2V617F (HuJAK2) transcripts compared with the endogenous mouse Jak2 (MoJak2) in cultured MKs at 8 weeks postinduction. Both alleles are expressed at similar levels. Error bars represent standard deviation (SD) for 4 separate MK cultures. (C) Western blots of MK protein lysates from control and JAK2V617F animals. Total levels of JAK2 protein are similar between control and JAK2V617F MKs, but only the JAK2V617F MKs show the presence of the human JAK2 protein. The blot is representative of 4 different cultures.
Molecular and protein analysis of murine JAK2V617F MKs. (A) Gel analysis of the PCR products from genomic DNA extracted from fresh BM cells and cultured MKs from JAK2V617F animals 8 weeks after pIpC induction. The upper band in the positive control (pos) corresponds to the recombined human JAK2V617F, and the lower band to the floxed allele. BM and MK samples only show the upper band confirming 100% recombination within these 2 cell populations. (B) Quantitative PCR looking at the relative level of the human JAK2V617F (HuJAK2) transcripts compared with the endogenous mouse Jak2 (MoJak2) in cultured MKs at 8 weeks postinduction. Both alleles are expressed at similar levels. Error bars represent standard deviation (SD) for 4 separate MK cultures. (C) Western blots of MK protein lysates from control and JAK2V617F animals. Total levels of JAK2 protein are similar between control and JAK2V617F MKs, but only the JAK2V617F MKs show the presence of the human JAK2 protein. The blot is representative of 4 different cultures.
JAK2V617F increases MK maturation at a low concentration of TPO and correlates to increased TPO signaling in JAK2V617F MKs
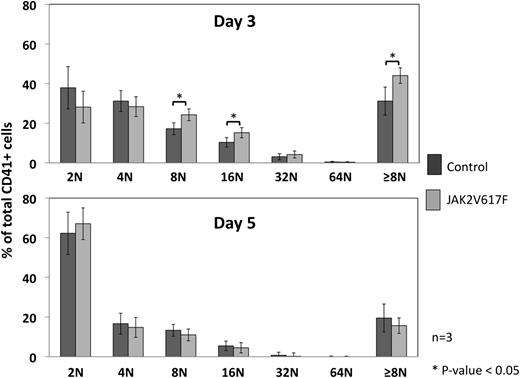
The hallmark histologic finding on BM examination in patients with ET is an increase in polylobulated MKs with typical “clustering” of cells. We have previously reported the same finding in this mouse model.27 The increase in BM MK content is presumed to be through a differentiation/growth advantage resulting from an increase in signaling downstream of the TPO receptor via the mutated JAK2V617F kinase. To test this hypothesis, we cultured BM from JAK2V617F and control mice with increasing concentrations of TPO and examined MK differentiation daily by flow cytometry, including ploidy analysis. No differences were observed in the presence of a high concentration of TPO (50 ng/mL) in terms of the percentage of CD41-positive cells and ploidy (data not shown), whereas at a lower concentration (1 ng/mL) and an intermediate time point (day 3), higher ploidy MKs were present in the JAK2V617F BM culture compared with control (42% ± 2% vs 36% ± 4% of cells ≥8N, mean ± SD, and P < .05), although the percentage of CD41-positive cells was slightly lower in JAK2V617F culture vs control (27% ± 6% vs 35% ± 8%, mean ± SD, and P > .1). These differences were not observed after 5 days in culture (Figure 2).
Ploidy analysis of cultured MKs. BM from control and JAK2V617F animals was cultured with different concentrations of TPO (1 and 50 ng/mL), and ploidy measured in the cells positive for CD41 (a surface marker for MKs) at days 3 and 5 of culture. No difference was observed using 50 ng/mL of TPO (not shown), but the ploidy profile at day 3 with 1 ng/mL of TPO (upper graph) showed a significant increase in high ploidy MKs (≥8N) when compared with control in the CD41+ population. This difference was lost by day 5 of culture (lower graph). Error bars represent SD for 3 different MK cultures.
Ploidy analysis of cultured MKs. BM from control and JAK2V617F animals was cultured with different concentrations of TPO (1 and 50 ng/mL), and ploidy measured in the cells positive for CD41 (a surface marker for MKs) at days 3 and 5 of culture. No difference was observed using 50 ng/mL of TPO (not shown), but the ploidy profile at day 3 with 1 ng/mL of TPO (upper graph) showed a significant increase in high ploidy MKs (≥8N) when compared with control in the CD41+ population. This difference was lost by day 5 of culture (lower graph). Error bars represent SD for 3 different MK cultures.
To determine whether this biological effect on MK differentiation correlates with an increase in intracellular downstream signaling of the TPO receptor (c-MPL), BM-derived MKs from JAK2V617F and control animals were cultured and purified by means of a BSA gradient. These cells were starved of cytokines for 4 hours postpurification and then stimulated with TPO. Cell lysates were generated and analyzed by western blots to determine changes in the phosphorylation of downstream effectors of JAK2, namely, signal transducer and activator of transcription (STAT) 3 and extracellular signal-regulated kinase (ERK). Phosphorylation of both STAT3 and ERK was increased at low and intermediate concentrations of TPO in JAK2V617F mice compared with control (Figure 3A). In addition, time-course experiments over 1 hour showed a significant degree of dephosphorylation of both STAT3 and ERK in the later time points, indicating that the negative feedback loop is functional in primary JAK2V617F MKs (Figure 3B).
Dose response and time course analysis of MK signaling after TPO stimulation. Dose response (A) and time-course (B) analysis of MK signaling in response to TPO. Mature MKs derived from BM cultures of control and JAK2V617F animals were purified by means of a BSA gradient and starved of cytokines for 4 hours. They were then stimulated with the dose of TPO indicated and for various lengths of time. Cell lysates were subsequently analyzed by western blotting for evidence of signaling downstream of JAK2. Densitometry analysis confirmed a significant increase in phosphorylation of both ERK and STAT3 in response to TPO in JAK2V617F MKs. Error bars represent SD.
Dose response and time course analysis of MK signaling after TPO stimulation. Dose response (A) and time-course (B) analysis of MK signaling in response to TPO. Mature MKs derived from BM cultures of control and JAK2V617F animals were purified by means of a BSA gradient and starved of cytokines for 4 hours. They were then stimulated with the dose of TPO indicated and for various lengths of time. Cell lysates were subsequently analyzed by western blotting for evidence of signaling downstream of JAK2. Densitometry analysis confirmed a significant increase in phosphorylation of both ERK and STAT3 in response to TPO in JAK2V617F MKs. Error bars represent SD.
JAK2V617F MKs show increased migration and proplatelet formation
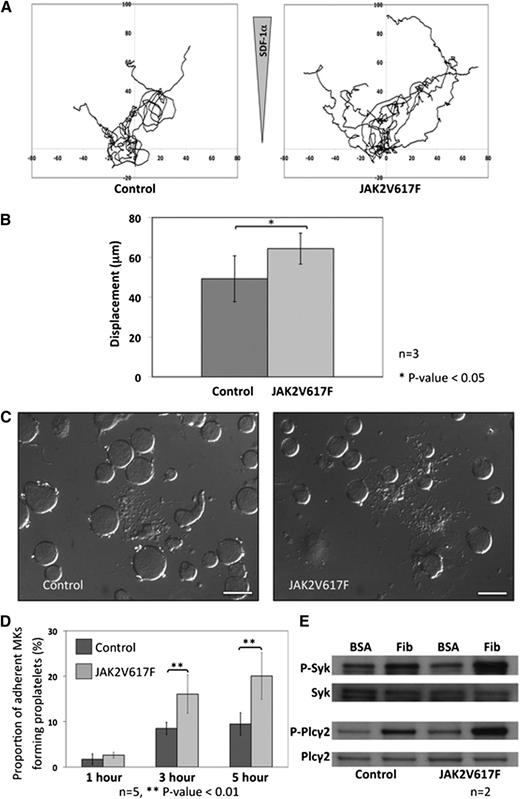
During maturation, MKs migrate from the “bony niche” where the most immature cells reside to the “vascular niche” where mature MKs release their platelets by a tightly regulated process of proplatelet formation. To ascertain the influence of JAK2V617F in these 2 biological processes, control and JAK2V617F MKs were cultured and purified over a BSA gradient. In the first instance, the migratory ability of the MKs was assessed in a Dunn chamber coated with fibronectin in response to an SDF1α gradient. JAK2V617F MKs showed increased motility over a period of 4 hours when compared with control MKs (displacement 65 ± 8 μm vs 48 ± 10 μm, mean ± SD, and P < .05) (Figure 4A-B). When JAK2V617F and control MKs were plated on a fibrinogen surface, the proportion of JAK2V617F MKs forming proplatelets was increased after 3 hours (16.3% ± 5.2% vs 7.1% ± 3.4%, mean ± SD, and P < .01) and 5 hours (20.3% ± 8.1% vs 10.5% ± 4.2%, mean ± SD, and P < .01) (Figure 4C-D). As outside-in signaling downstream of the αIIbβ3 integrin has been shown to play a crucial role in the regulation of proplatelet formation, we went on to look at phosphorylation of Syk and Plcγ2 in adherent MKs and found a clear increase in the JAK2V617F MKs compared with control cells (Figure 4E).
MK migration and proplatelet formation. (A) Cultured MKs from control and JAK2V617F mice were isolated by BSA gradient, and migration was assessed in a fibronectin-coated Dunn chamber under an SDF1α gradient. Each track represents an individual MK movement over a period of 4 hours. (B) Analysis of the data showed that JAK2V617F MKs migrated farther over the 4-hour time period compared with control MKs (n = 7 control MKs and 9 Jak2V617F MKs from 3 different cultures; error bars represent SD). (C) Proplatelet formation was assessed by plating culture-derived MKs from JAK2V617F and control animals onto fibrinogen-coated coverslips and fixing the cells at various time points before analysis using DIC microscopy (scale bars represent 50 μm). (D) The percentage of JAK2V617F MKs forming proplatelet at different time points was significantly increased compared with control MKs. Results are shown for 5 different MK cultures, with a minimum of 100 MKs analyzed in 5 different high-power fields for each individual experiment. Error bars represent SD. (E) Outside-in signaling downstream of integrin αIIbβ3 was studied in MKs adhered to fibrinogen (compared with unstimulated MKs plated onto BSA). The western blots show increased phosphorylation of Plcγ2 and Syk in JAK2V617F MKs adherent to fibrinogen compared with control MKs.
MK migration and proplatelet formation. (A) Cultured MKs from control and JAK2V617F mice were isolated by BSA gradient, and migration was assessed in a fibronectin-coated Dunn chamber under an SDF1α gradient. Each track represents an individual MK movement over a period of 4 hours. (B) Analysis of the data showed that JAK2V617F MKs migrated farther over the 4-hour time period compared with control MKs (n = 7 control MKs and 9 Jak2V617F MKs from 3 different cultures; error bars represent SD). (C) Proplatelet formation was assessed by plating culture-derived MKs from JAK2V617F and control animals onto fibrinogen-coated coverslips and fixing the cells at various time points before analysis using DIC microscopy (scale bars represent 50 μm). (D) The percentage of JAK2V617F MKs forming proplatelet at different time points was significantly increased compared with control MKs. Results are shown for 5 different MK cultures, with a minimum of 100 MKs analyzed in 5 different high-power fields for each individual experiment. Error bars represent SD. (E) Outside-in signaling downstream of integrin αIIbβ3 was studied in MKs adhered to fibrinogen (compared with unstimulated MKs plated onto BSA). The western blots show increased phosphorylation of Plcγ2 and Syk in JAK2V617F MKs adherent to fibrinogen compared with control MKs.
Whole blood from JAK2V617F mice shows increased thrombus formation in vivo and platelet aggregation in vitro
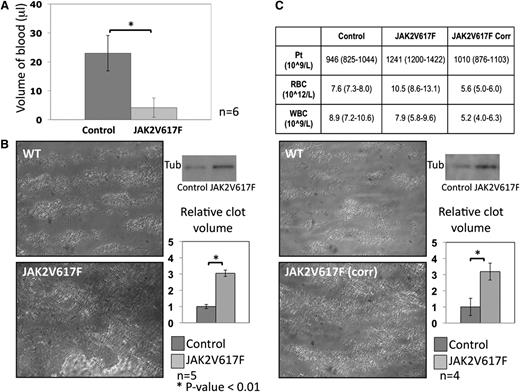
Hemostasis in JAK2V617F and control animals was initially assessed by tail-bleeding time. Following a 2-mm cut from the tip of the tail, blood volume loss was assessed and showed a marked decrease in the JAK2V617F animals in comparison with control mice (4 ± 3 μL vs 22 ± 7 μL, mean ± SD, and P < .01; Figure 5A). Platelet thrombus formation under flow was analyzed using an in vitro laminar flow chamber where whole blood was flowed at high shear rate (1000 s−1) over a collagen-coated surface for 4 minutes. Surface coverage was increased in the JAK2V617F blood as well as total thrombus volume (Figure 5B). In a further set of experiments, the platelet count in JAK2V617F whole blood was adjusted to the same level as in control blood by the addition of plasma. The red cell count in the adjusted JAK2V617F blood was lower than in control animals (5.6 × 1012/L vs 7.6 × 1012/L), but remarkably, surface coverage and total thrombus volume was still raised in these experiments (Figure 5C) in keeping with an intrinsic increased platelet reactivity.
In vivo hemostasis and in vitro platelet aggregate formation. (A) Tail-bleeding assay measuring blood volume loss following removal of 2 mm from the tip of the tail in both control and JAK2V617F animals. Blood volume loss was significantly less in the JAK2V617F animals (n = 6; error bars represent SD). (B) Platelet aggregate formation was assessed in a collagen-coated flow chamber at arterial shear rate (1000 s−1) using whole blood anticoagulated with heparin and D-Phe-Pro-Arg-CMK. Surface coverage was increased in JAK2V617F animals as illustrated in the micrographs taken after 4 minutes of flow. Thrombi volume was assessed by protein quantification by western blot using a tubulin antibody. Densitometry results, expressed relative to control, show a statistically significant increase in thrombus formation in JAK2V617F animals (n = 5; error bars represent SD). (C) To determine whether this increase was a reflection of the raised platelet count in the JAK2V617F mice or attributable to an increase in platelet reactivity, the same experiments were performed after correcting the platelet count in JAK2V617F samples to control level by addition of mouse plasma. Mouse plasma was generated from heparinized mouse blood centrifuged at 150g to generate PRP and then 1000g to generate PPP. Blood counts pre- and postcorrection are presented in the top panel (mean and range). Surface coverage was again increased, and thrombi volume remained higher in the Jak2V617F samples compared with control (n = 4; error bars represent SD).
In vivo hemostasis and in vitro platelet aggregate formation. (A) Tail-bleeding assay measuring blood volume loss following removal of 2 mm from the tip of the tail in both control and JAK2V617F animals. Blood volume loss was significantly less in the JAK2V617F animals (n = 6; error bars represent SD). (B) Platelet aggregate formation was assessed in a collagen-coated flow chamber at arterial shear rate (1000 s−1) using whole blood anticoagulated with heparin and D-Phe-Pro-Arg-CMK. Surface coverage was increased in JAK2V617F animals as illustrated in the micrographs taken after 4 minutes of flow. Thrombi volume was assessed by protein quantification by western blot using a tubulin antibody. Densitometry results, expressed relative to control, show a statistically significant increase in thrombus formation in JAK2V617F animals (n = 5; error bars represent SD). (C) To determine whether this increase was a reflection of the raised platelet count in the JAK2V617F mice or attributable to an increase in platelet reactivity, the same experiments were performed after correcting the platelet count in JAK2V617F samples to control level by addition of mouse plasma. Mouse plasma was generated from heparinized mouse blood centrifuged at 150g to generate PRP and then 1000g to generate PPP. Blood counts pre- and postcorrection are presented in the top panel (mean and range). Surface coverage was again increased, and thrombi volume remained higher in the Jak2V617F samples compared with control (n = 4; error bars represent SD).
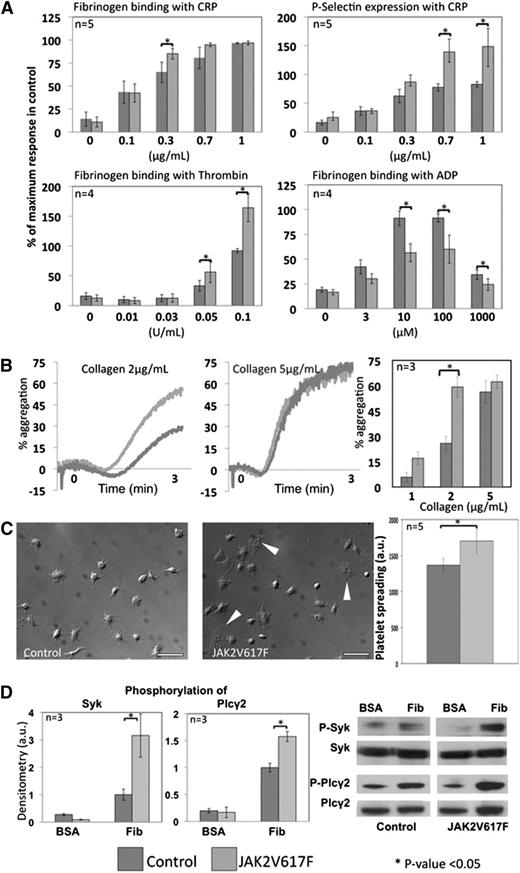
JAK2V617F platelets show differential response to a range of agonists
Initially, surface expression of the major platelet surface receptors was assessed using fluorescent-labeled antibodies against αIIb (GPIIb, CD41), GP1bβ (CD42b, part of the von Willebrand factor receptor complex GP1b-V-IX), GPVI (collagen receptor), and α2 (CD49, part of the α2β1 collagen receptor). No significant difference was observed between control and JAK2V617F platelets (data not shown). In a further set of experiments, platelet response in whole blood to a range of concentrations of CRP, thrombin, and ADP was assessed by means of P-selectin expression and fibrinogen binding measured by flow cytometry. Responses to CRP and thrombin were significantly increased, whereas the response to ADP was decreased in the JAK2V617F platelets (Figure 6A). Flow cytometry is a static assay and thus unsuitable to assess platelet response to collagen. This was therefore assessed in classical light transmission aggregometry using PRP. The results showed significantly increased aggregation to intermediate concentrations of collagen (2 μg/mL), which was not seen at higher concentrations (5 μg/mL and above) (Figure 6B). The decreased response to ADP documented in the flow cytometry assay was confirmed in aggregometry (data not shown).
Platelet response to agonists and spreading. (A) Platelet response to CRP, thrombin, and ADP was assessed by flow cytometry in whole blood, measuring fibrinogen binding and P-selectin expression. Although the response to CRP and thrombin was significantly increased in JAK2V617F platelets, the reverse was observed in response to ADP. For each experiment, blood from control and JAK2V617F mice was tested in duplicate at each agonist concentration. P-selectin expression and fibrinogen binding are presented as a percentage of the maximal response obtained in control blood for each agonist. Error bars represent SD. (B) Platelet stimulation in response to collagen relies on the existence of shear. This was therefore measured in classical light transmission aggregometry at 3 different concentrations of collagen in at least 3 experiments comparing a control and JAK2V617F sample. Aggregation in response to collagen was significantly increased at a medium concentration (2 μg/mL) but not at higher concentrations. Error bars represent SD. (C) Platelet spreading was assessed by adhering control and JAK2V617F platelets onto a fibrinogen-coated surface in static conditions for 45 minutes at 37°C in 5 different experiments. Pictures were taken with a DIC microscope (scale bars represent 10 μm), and spreading measured using ImageJ software, counting at least 50 platelets for each sample. JAK2V617F platelets showed significantly greater spreading, including some platelets displaying full lamellipodia formation (white arrowheads) even in the absence of exogenous agonists. Error bars represent SD. (D) Outside-in signaling downstream of integrin αIIbβ3 was studied in platelets adhered to fibrinogen (compared with unstimulated platelets plated onto BSA-coated surface). The western blots show increased phosphorylation of Plcγ2 and Syk in JAK2V617F platelets adherent to fibrinogen compared with control platelets. Densitometry analysis confirmed that the difference observed was statistically significant (P < .05).
Platelet response to agonists and spreading. (A) Platelet response to CRP, thrombin, and ADP was assessed by flow cytometry in whole blood, measuring fibrinogen binding and P-selectin expression. Although the response to CRP and thrombin was significantly increased in JAK2V617F platelets, the reverse was observed in response to ADP. For each experiment, blood from control and JAK2V617F mice was tested in duplicate at each agonist concentration. P-selectin expression and fibrinogen binding are presented as a percentage of the maximal response obtained in control blood for each agonist. Error bars represent SD. (B) Platelet stimulation in response to collagen relies on the existence of shear. This was therefore measured in classical light transmission aggregometry at 3 different concentrations of collagen in at least 3 experiments comparing a control and JAK2V617F sample. Aggregation in response to collagen was significantly increased at a medium concentration (2 μg/mL) but not at higher concentrations. Error bars represent SD. (C) Platelet spreading was assessed by adhering control and JAK2V617F platelets onto a fibrinogen-coated surface in static conditions for 45 minutes at 37°C in 5 different experiments. Pictures were taken with a DIC microscope (scale bars represent 10 μm), and spreading measured using ImageJ software, counting at least 50 platelets for each sample. JAK2V617F platelets showed significantly greater spreading, including some platelets displaying full lamellipodia formation (white arrowheads) even in the absence of exogenous agonists. Error bars represent SD. (D) Outside-in signaling downstream of integrin αIIbβ3 was studied in platelets adhered to fibrinogen (compared with unstimulated platelets plated onto BSA-coated surface). The western blots show increased phosphorylation of Plcγ2 and Syk in JAK2V617F platelets adherent to fibrinogen compared with control platelets. Densitometry analysis confirmed that the difference observed was statistically significant (P < .05).
Platelet spreading on a fibrinogen-coated surface is increased in JAK2V617F platelets
During thrombus formation, platelets change shape and spread by means of a reorganization of their cytoskeleton leading to the formation of filipodia and lamellipodia. The ability of JAK2V617F platelets to do this was assessed by quantifying platelet spreading on a fibrinogen-coated surface under static conditions for 45 minutes. JAK2V617F platelets showed increased spreading, and in particular, a significant number of JAK2V617F platelets demonstrated full lamellipodia spreading in the absence of exogenous agonists, which was not seen in the control platelets (Figure 6C). Similar to the observation made in MKs, downstream signaling from αIIbβ3 was shown to be increased in adherent JAK2V617F platelets as evidenced by an increase in phosphorylation of Syk and Plcγ2 (Figure 6D).
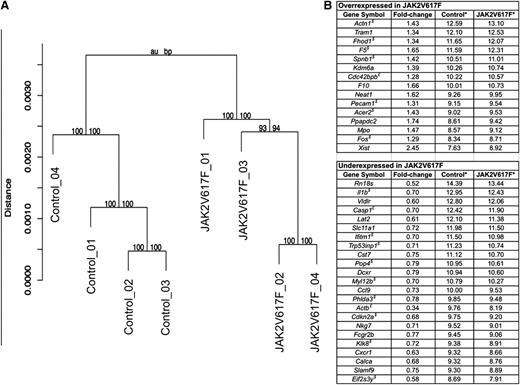
Differential gene expression in JAK2V617F MKs
One has to consider the possibility that the difference in phenotype between JAK2V617F MKs and platelets could be the result of an effect on gene expression during megakaryopoiesis. Signaling downstream of MPL and JAK2 triggers a series of pathways (notably through the STAT proteins, the mitogen-activated protein kinases, and the PI3K–protein kinase B [AKT] pathway) that will, ultimately, affect the cells through modification of transcriptional regulation. Indeed, JAK2 itself has been shown to affect transcriptional regulation by a direct connection with histone H3.36 To look into this particular hypothesis, 4 separate paired MK cultures were performed from control and JAK2V617F BM. After RNA extraction, whole genome expression arrays were performed to identify genes that were over- or underexpressed in the JAK2V617F cells. Analysis showed that control and JAK2V617F cells belonged to 2 separate clusters (Figure 7A). Genes were considered differentially expressed with a fold change >1.25, P < .05, and a false discovery rate <0.25. Sixteen genes were overexpressed in the JAK2V617F MKs, and a further 24 genes were underexpressed (Figure 7B). Functional analysis of the differentially expressed genes showed enrichment for genes involved in cytoskeleton remodeling (Fhod1, Cdc42bpb, Actn1, Spnb1, and Myl12b), genes involved in regulation of apoptosis and/or cell cycle progression (Casp1, Cdkn2a, Phlda3, Trp53inp1, and Ifitm1), and genes involved in regulation of transcription (Fos and Kdm6a) or translation (Eif2s3y and Pop4). Pecam1, whose role is well documented in MK maturation and particularly migration, was overexpressed in Jak2V617F MKs.
Differential gene expression in JAK2V617F MKs. MKs from 4 control and 4 JAK2V617F mice were cultured and purified through a BSA gradient. RNA was extracted, and expression arrays performed. (A) Hierarchical clustering of control and JAK2V617F gene expression values separates the samples into 2 different populations. Shown above each cluster are the corresponding approximately unbiased and bootstrap probability values, which indicate the strength by which the cluster is supported by the data. (B) Genes that were over- or underexpressed by a factor of 1.25 with P < .05 and false discovery rate <0.25 are presented in the table. * denotes average signal for all 4 control and JAK2V617F samples; $, genes for which the differential expression was confirmed by quantitative PCR; and £, genes for which the PCR results did not confirm the array findings. Genes with no indication were not analyzed by PCR.
Differential gene expression in JAK2V617F MKs. MKs from 4 control and 4 JAK2V617F mice were cultured and purified through a BSA gradient. RNA was extracted, and expression arrays performed. (A) Hierarchical clustering of control and JAK2V617F gene expression values separates the samples into 2 different populations. Shown above each cluster are the corresponding approximately unbiased and bootstrap probability values, which indicate the strength by which the cluster is supported by the data. (B) Genes that were over- or underexpressed by a factor of 1.25 with P < .05 and false discovery rate <0.25 are presented in the table. * denotes average signal for all 4 control and JAK2V617F samples; $, genes for which the differential expression was confirmed by quantitative PCR; and £, genes for which the PCR results did not confirm the array findings. Genes with no indication were not analyzed by PCR.
Discussion
This study demonstrates that the JAK2V617F mutation has an intrinsic effect on MK and platelet biology beyond a simple increase in numbers. To this end, we used a mouse model of ET where the human JAK2V617F gene has been knocked into the endogenous mouse Jak2 allele.27 In the first part of the study, we demonstrated that this provided us with an ideal model to study the effect of the JAK2V617F mutation in a homogeneous cell population where 100% of BM-derived MKs and peripheral blood platelets express the JAK2V617F protein, removing the distortion attributable to different clonality levels encountered in patient studies. In addition, we showed that the levels of expression of human JAK2V617F and mouse Jak2 were equivalent in the primary BM-derived MKs, reflecting what is seen in heterozygous patients, and the total level of JAK2 protein was comparable between control and JAK2V617F MKs.
We initially confirmed that JAK2V617F confers a differentiation advantage to MKs, with a higher level of ploidy reached earlier in the culture system than for control BM cultures. Interestingly, this was observed only when TPO concentration in the culture was closer to the reported in vivo plasma levels rather than the supraphysiological levels usually used in vitro. This effect was also observed at an intermediate time point of the culture (3 days), whereas at the end point (5 days) this difference was not seen. This probably relates to the fact that high ploidy JAK2V617F MKs had an increased propensity to form platelets in the culture dish (as quantified in the proplatelet assay) and therefore disappeared between day 3 and day 5 of culture. This increase in differentiation of JAK2V617F MKs correlated with the observation that signaling in response to TPO was increased in the JAK2V617F MKs using STAT3 and ERK as exemplar downstream targets of JAK2.
Platelet production not only requires sufficient numbers of MKs, but the MKs are also required to migrate toward the vascular niche and to form proplatelets. A previous study using human BM samples has shown that proplatelet formation is increased in patients with ET and PV,37 although in that particular study no clear distinction was made between patients who were JAK2V617F positive or negative. In our model, we demonstrate a similar increase in proplatelet formation in JAK2V617F MKs. In addition, we also show that JAK2V617F MKs migrate quicker and further in response to an SDF1α gradient, supporting the fact that the raised platelet number in ET is a consequence of not only a larger number of MKs but also the basic cellular biology of MKs. Compounds that inhibit proplatelet formation and MK migration are already in clinical practice, such as the Src inhibitor dasatinib used in patients with chronic myeloid leukemia.38 Targeting this particular mechanism may very well play a role in the pharmacologic control of the platelet count in patients with ET.
We next looked at whether the presence of the JAK2V617F mutation also caused a change in intrinsic platelet reactivity. We demonstrated, through a range of assays, that the response to different agonists was increased, with the notable exception of the response to ADP, which was reduced. Platelet spreading was also shown to be affected. This translated in whole blood flow assays and in vivo tail-bleeding assays to an increased ability to form platelet aggregates and increased hemostasis, respectively. Crucially, the increased aggregate formation was still present in experiments where platelets counts between JAK2V617F and control blood were equalized. It would be of interest to use this model to assess how JAK2V617F influences in vivo thrombus formation using the ferric chloride or the laser injury intravital model because this could explain the reduction in bleeding time in the tail assay.
The main therapeutic approach to prevent thrombotic events in patients with ET relies on reducing the platelet count to within the normal range. Our findings suggest that simply decreasing the platelet count may not completely reduce the thrombotic risk. It would therefore be extremely relevant to confirm these findings in human samples, especially in patients who have a very high allele burden, using a control cohort of a significant size to eliminate interpersonal variations.39,40
How JAK2V617F influences platelet function and the pathways by which this is achieved are currently unknown. Although TPO is unable to cause platelet aggregation by itself, it has been known for some time that TPO potentiates platelet activation by the majority of platelet agonists.41-44 This TPO-driven effect has been shown to contribute to the platelet hyperreactivity observed in patients with unstable angina.45 Some authors have proposed activation of PI3K as a potential pathway by which TPO may prime platelets.46-48 In keeping with these studies, a recent publication confirmed that TPO potentiation of platelet response to SFLLRN (a PAR-1 thrombin receptor agonist) was mediated by PI3K, leading to an increase in fibrinogen binding to integrin αIIbβ3 but without an effect on P-selectin expression (ie, α granule secretion).23 Interestingly, when looking at samples from patients with ET (with or without JAK2V617F), the same group made the exact reverse observation (ie, a decrease in the function of the PI3K/Rap1/integrin αIIbβ3 pathway, which could be secondary to medication). In contrast, our findings in this mouse model show an increase in fibrinogen binding as well as P-selectin expression in response to various agonists, in keeping with the increased hemostasis observed in the functional assays.
The model described here gave us an opportunity to isolate homogeneous primary MKs for detailed transcription profiling and characterization of transcriptional dysregulation that may elucidate how JAK2V617F exerts a biological effect on MKs and, potentially, platelets. Perhaps expectedly, given the subtle phenotype in the clinical features of the mice and cellular assays, the changes observed were small, and the list of differentially expressed genes was short. These changes, however, affected several key biological processes, which in combination could potentially lead to the biological observations made, in particular those related to the process of MK migration and platelet production/release from mature MKs. Cytoskeletal remodeling is essential to both platelet function as well as MK migration and proplatelet formation.49,50 Regulators of actin fiber assembly (Fhod1, Cdc42bpb, and Actn1), as well as myosin light chain function (Myl12b) and spectrin (Spb1), were identified among the list of differentially expressed genes. In addition, Acer2, a gene encoding for a protein that regulates sphingosine production, was overexpressed. Sphingosine-1-phosphate plays a key role in platelet release from the proplatelet buds.51,52 Pecam1 was overexpressed in JAK2V617F MKs, and there is ample evidence that Pecam1 plays a role in fine-tuning megakaryopoiesis, in particular regulating MK migration.32,53 Programmed cell death, or apoptosis, is a regulator of proplatelet formation (reviewed by Geddis54 ) as well as platelet count by influencing platelet survival in circulation.55 Several apoptosis-related genes were found to be less expressed in JAK2V617F MKs, among them Phlda3, a negative regulator of AKT signaling, a pathway now recognized as a therapeutic target in myeloproliferative disorders.56 Transforming growth factor β (TGF-β) signaling is known to effect MK differentiation and, crucially, myelofibrosis in a TPO overexpression murine model.57,58 TGFB2 was 1 of 11 genes identified in a study that looked at potential biomarkers using the platelet transcript profile to differentiate reactive thrombocytosis from ET.59 Fos was overexpressed in JAK2V617F MKs and is a known regulator of TGF-β signaling with the Smad3/Smad4/Jun complex.
In conclusion, using a JAK2V617F mouse model that phenotypically mimics human ET, we have been able to demonstrate a clear effect of JAK2V617F in both MK biology and platelet reactivity and to identify pathways and biological processes by which JAK2V617F may exert its effect.
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The funding for this project came from an Intermediate Clinical Fellowship from the British Heart Foundation (FS/09/039) (C.G.) and from the National Health Service Blood and Transplant, United Kingdom.
Authorship
Contribution: C.M.H., H.M., C.B., L.V., S.S., L.B., A.M., J.A.G., and J.L. performed experiments for this project and edited the manuscript; N.S., A.R.G., and S.P.W. took part in the experimental design and wrote the manuscript; and C.G. performed experiments, oversaw the work, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr C. Ghevaert, Division of Transfusion Medicine, Department of Haematology, University of Cambridge/NHS Blood and Transplant, Long Rd, Cambridge CB2 2PT, United Kindgom; e-mail: cg348@cam.ac.uk.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal