Abstract
Epstein-Barr virus (EBV) positive diffuse large B-cell lymphoma (DLBCL) of the elderly, initially described in 2003, is a provisional entity in the 2008 World Health Organization classification system and is defined as an EBV-positive monoclonal large B-cell proliferation that occurs in patients >50 years of age and in whom there is no known immunodeficiency or history of lymphoma. These tumors are more common in Asia but also occur in North America and Europe at a low frequency. These neoplasms exhibit a morphologic continuum, from polymorphous to monomorphous, but morphologic features do not correlate with prognosis as all patients have a clinically aggressive course. Most EBV-positive DLBCL of the elderly patients have an activated B-cell immunophenotype and are characterized by prominent nuclear factor-κB activation. Cytogenetic complexity is usually low. In this review, we comprehensively delineate the data emerging from analyses of EBV latency program, microRNA-mediated EBV viral oncogenesis, functional genomics of EBV and its biology, and differential diagnosis challenge for EBV-positive DLBCL of the elderly. It is hoped that the improved understanding of these tumors will lead to the development of novel therapeutic approaches, enhance the effectiveness of clinical trials, and improve prognosis.
Introduction
The 2008 World Health Organization (WHO) classification of tumors of hematopoietic and lymphoid tissues has recognized a new provisional entity designated as Epstein-Barr virus (EBV)–positive diffuse large B-cell lymphoma (DLBCL) of the elderly. This tumor is defined as an EBV-positive monoclonal large B-cell lymphoproliferative disorder arising in immunocompetent patients >50 years.1 To denote patient age in the definition was a means of emphasizing the tendency of these tumors to arise in patients of advanced age, but this age cutoff is clearly arbitrary, and EBV-positive DLBCL of the elderly has been reported rarely in younger patients.
Since the initial description of 22 patients with EBV-positive DLBCL of the elderly in 2003 by Oyama et al,2 knowledge of EBV-mediated mechanisms of oncogenesis, microRNA (miRNA) profiles in EBV-positive lymphomas, and novel efficient therapeutics for patients with these neoplasms have been developed. In this review, we discuss the epidemiology, clinicopathologic features, and differential diagnoses of EBV-positive DLBCL of the elderly. We also review novel molecular findings including host and virus-induced miRNAs and some novel therapeutic approaches.
Epidemiology
The median age of patients with EBV-positive DLBCL of the elderly is 71 years (range, 50-91 years); however, younger patients can be affected.3 There is a slight male predominance, with a male to female ratio of 1.4 to 1.4-10 There is a higher prevalence of EBV-positive DLBCL among East Asians (8.7%-11.4%)11-13 compared with <5% in Western countries5,6 (Table 1). Meanwhile, Beltran et al4 has reported the highest frequency of EBV-positive DLBCL in a Peruvian population, 14.9%, using a criterion of ≥20% cells with positive EBV-encoded RNA (EBER) expression. Of note, there are no uniform criteria for the percentage of EBV-positive cells in EBV-positive DLBCL, and this is a limitation when assessing disease prevalence. The fact that differences in cutoffs for EBER positivity clearly impact the frequency of EBV-positive DLBCL was shown by Wada et al and others.13-15 These authors emphasized the need for establishing uniform criteria for EBV positivity, either >20%, >50%, or almost all tumor cells. Hofscheier et al7 interpreted EBER-positive DLBCL with <20% EBER as clonally unrelated EBV-transformed B cells or secondary EBV infection in an established B-cell clone. The definitive criterion for EBV positivity in EBV-positive DLBCL remains under discussion.
Frequency of EBV positivity with different cutoffs in B-cell LPD among different countries
| Country . | EBER+ cases . | Percent . | EBER cutoff 20% . | EBER cutoff 50% . | Diagnosis . | Reference . |
|---|---|---|---|---|---|---|
| Japan | 13/114 | 11.4 | Not specified | Not specified | DLBCL | 11 |
| Japan | 156/1792 | 8.7 | n/a | 156/1,792 (8.7%) | B-LPD* | 12 |
| Japan | 5/460* | 1.1 | 11/460 (2.4%) | 5/460 (1.1%) | DLBCL | 14 |
| Korea | 34/380 | 8.9 | 34/380 (8.9%) | n/a | DLBCL | 13 |
| Korea | 14/468 | 3.0 | n/a | 14/468 (3.0%) | DLBCL | 15 |
| China | 10/212 | 4.7 | n/a | 10/212 (4.7%) | DLBCL | 9 |
| Turkey | 12/178* | 6.7 | Not specified | Not specified | DLBCL | 10 |
| Peru | 28/188* | 14.9 | 28/188 (14.9%) | 17/188 (9.0%) | DLBCL | 4 |
| Mexico | 9/134 | 6.7 | 9/134 (6.7%) | 9/134 (6.7%) | DLBCL | 7 |
| Germany | 4/167 | 2.4 | 4/167 (2.4%) | 4/167 (2.4%) | DLBCL | 7 |
| S/I/A | 8/258 | 3.1 | 7/258 (2.7%) | 5/258 (1.9%) | DLBCL | 6 |
| United States | 5 patients (2002-2007) | n/a | Not specified | Not specified | DLBCL | 5 |
| Saudi Arabia | 16/217 | 7.4 | Not specified | Not specified | DLBCL | 25 |
| Country . | EBER+ cases . | Percent . | EBER cutoff 20% . | EBER cutoff 50% . | Diagnosis . | Reference . |
|---|---|---|---|---|---|---|
| Japan | 13/114 | 11.4 | Not specified | Not specified | DLBCL | 11 |
| Japan | 156/1792 | 8.7 | n/a | 156/1,792 (8.7%) | B-LPD* | 12 |
| Japan | 5/460* | 1.1 | 11/460 (2.4%) | 5/460 (1.1%) | DLBCL | 14 |
| Korea | 34/380 | 8.9 | 34/380 (8.9%) | n/a | DLBCL | 13 |
| Korea | 14/468 | 3.0 | n/a | 14/468 (3.0%) | DLBCL | 15 |
| China | 10/212 | 4.7 | n/a | 10/212 (4.7%) | DLBCL | 9 |
| Turkey | 12/178* | 6.7 | Not specified | Not specified | DLBCL | 10 |
| Peru | 28/188* | 14.9 | 28/188 (14.9%) | 17/188 (9.0%) | DLBCL | 4 |
| Mexico | 9/134 | 6.7 | 9/134 (6.7%) | 9/134 (6.7%) | DLBCL | 7 |
| Germany | 4/167 | 2.4 | 4/167 (2.4%) | 4/167 (2.4%) | DLBCL | 7 |
| S/I/A | 8/258 | 3.1 | 7/258 (2.7%) | 5/258 (1.9%) | DLBCL | 6 |
| United States | 5 patients (2002-2007) | n/a | Not specified | Not specified | DLBCL | 5 |
| Saudi Arabia | 16/217 | 7.4 | Not specified | Not specified | DLBCL | 25 |
n/a, not applicable; S/I/A, Switzerland, Italy, and Austria.
EBER-positive rate was recalculated in these cases after excluding immunodeficiency-associated LPD (including PTLD and HIV-associated lymphoma), autoimmune-associated LPD, and non-DLBCL patients from each study.
Epidemiologic features correlate with geographical differences in the literature. In contrast with East Asian series, cases in Mexico display a relatively younger age at presentation, with predominantly nodal disease and rare expression of EBV nuclear antigen (EBNA)2.7 Recent evidence casts a doubt on geographic and/or ethnic variation in the prevalence of EBV-positive DLBCL of the elderly. Contrary to earlier reports from the Far East, a report from northern China showed a frequency of EBV positivity in only 8 of 212 (3.8%) in patients >50 years old with DLBCL (using a cutoff of >50% EBER positivity).9
Pathogenetic aspects
From an immunologic viewpoint, aging can be characterized as a dysregulated relationship between inflammatory and inflammation-neutralizing processes, resulting in a low-grade chronic proinflammatory state.16 This imbalance might exert other potential effects promoting lymphomagenesis. Inflammation induces radical oxygen species that can cause dysregulation of critical oncogenic pathways, such as p53, retinoblastoma (Rb), nuclear factor (NF)-κB, and mitogen-activated protein kinases (MAPKs).17
It has been postulated that EBV-positive DLBCL of the elderly might be caused by the senescence of the immune system as a part of the normal aging process, based largely on shared features with immunodeficiency-associated lymphoproliferative disorders (LPDs). These shared features include EBV infection, similar EBV latency pattern, morphologic similarities, and presence of monoclonal T-cell populations.8,12,18,19 Of note, immunosenescence is not simply the result of a progressive decline of all immune functions but rather refers to a continuous remodeling process, whereby the adaptive immunity is preferentially affected compared with innate immunity.20,21 In this context, B-cell diversity decreases with age and is characterized by clonal expansions of B cells in vivo.22 Concurrently, the T-cell compartment shows characteristic modifications, such as a reduction of the absolute number of total T lymphocytes, including helper/inducer (CD4+) and suppressor/cytotoxic (CD8+) cell counts; a progressive decline of the näive T-cell repertoire with a parallel decline in T-cell receptor repertoire diversity; and a progressive expansion of oligoclonal CD28− T cells, particularly among the CD8+ T-cell subset.20,21
Particularly, Ouyang et al23 showed a significantly greater frequency of EBV-specific receptor–carrying cells within the CD8+ T-cell subset and a lower frequency of EBV antigen-specific interferon-γ–producing T cells in the elderly, suggesting clonal expansion of dysfunctional EBV-specific cells to age. Whether oligoclonal or monoclonal T-cell populations in patients with EBV-positive DLBCL of the elderly reflects a physiological T-cell response to EBV infection8 or antedate the development of the LPD stemming from an underlying deficiency in T-cell function18 is unknown.
EBV is a γ-herpes virus that has a tropism for lymphocytes. The virus infects and replicates within epithelial cells, and most viral particles are cleared by cytotoxic T cells. However, EBV survives in a host cell by establishing latency as an episome (a double-stranded circular form of the genome) in memory B cells. In latent infection, EBV uses only a limited number of genes to maintain its genome and evade the host immune reaction. Based on different patterns of viral gene use, latency can be classified as type III, type II, or type I. Type III is characterized by the expression of all 6 EBV nuclear antigens (EBNA1, 2, 3A, 3B, 3C, and LP), 3 latent membrane proteins (LMP1, 2A, and 2B), and EBER (Figure 1A-B). Type III latency is observed in patients with infectious mononucleosis or a subset of posttransplant lymphoproliferative disorders (PTLDs). Type II latency is associated with expression of EBNA1, LMP1, LMP2A, and EBER and is commonly seen in classical Hodgkin lymphoma and a subset of PTLD. In type I latency, only EBNA1 and EBER are expressed; this pattern is characteristic of Burkitt lymphoma.
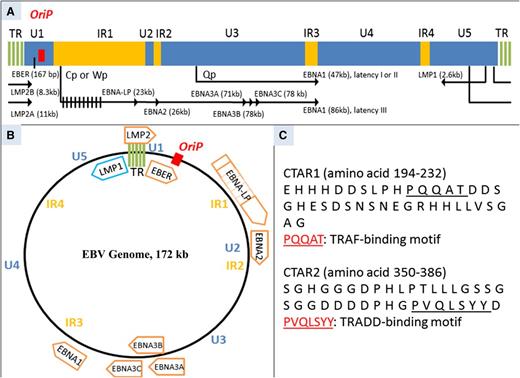
Schematic diagram of linear EBV genome (∼172 kb). (A) In proportion to their size, largely unique (U1-U5), internal repeat (IR1-IR4), and terminal repeat (TR) sequence domains are demonstrated. Ori P (indicated in red), the origin for latent infection EBV episome replication, has plasmid maintenance and DNA replication activity. Location of exons for latent EBV-induced membrane proteins and nuclear proteins and the size of each gene are shown. Of note, LMP1 transcription has an opposite direction to the other gene transcripts. LMP2 can only be transcribed when EBV exists as episome because the gene spans across the TR. LMP2A is transcribed from exon 1, whereas LMP2B is transcribed from exon 2. EBNAs are transcribed from differently spliced primary EBNA transcript. In latency III, EBNA1 transcription begins from Cp or Wp, whereas it starts from Qp in latency I or II. EBNA-LP has variable sizes due to variable numbers of repetitive exons.98 (B) Schematic diagram of circular EBV genome. The U1-U5, IR1-IR4, TR, and Ori P sequence domains are indicated. Open reading frames for latent proteins are shown. Of note, the size of each open reading frame does not illustrate the size of each latent protein.98 (C) Amino acid sequences of CTAR1 and CTAR2. TRAF-binding motif in CTAR1 and TRADD-binding motif are indicated with an underbar. Numbers on the right denote the order of the amino acid sequence.99 Cp, C promoter; EBNA-LP, EBNA leader protein; LMP, latent membrane protein; TRAF, tumor necrosis factor receptor-associated factor; TRADD, tumor necrosis factor receptor type 1–associated DEATH domain; Qp, Q promoter; Wp, W promoter.
Schematic diagram of linear EBV genome (∼172 kb). (A) In proportion to their size, largely unique (U1-U5), internal repeat (IR1-IR4), and terminal repeat (TR) sequence domains are demonstrated. Ori P (indicated in red), the origin for latent infection EBV episome replication, has plasmid maintenance and DNA replication activity. Location of exons for latent EBV-induced membrane proteins and nuclear proteins and the size of each gene are shown. Of note, LMP1 transcription has an opposite direction to the other gene transcripts. LMP2 can only be transcribed when EBV exists as episome because the gene spans across the TR. LMP2A is transcribed from exon 1, whereas LMP2B is transcribed from exon 2. EBNAs are transcribed from differently spliced primary EBNA transcript. In latency III, EBNA1 transcription begins from Cp or Wp, whereas it starts from Qp in latency I or II. EBNA-LP has variable sizes due to variable numbers of repetitive exons.98 (B) Schematic diagram of circular EBV genome. The U1-U5, IR1-IR4, TR, and Ori P sequence domains are indicated. Open reading frames for latent proteins are shown. Of note, the size of each open reading frame does not illustrate the size of each latent protein.98 (C) Amino acid sequences of CTAR1 and CTAR2. TRAF-binding motif in CTAR1 and TRADD-binding motif are indicated with an underbar. Numbers on the right denote the order of the amino acid sequence.99 Cp, C promoter; EBNA-LP, EBNA leader protein; LMP, latent membrane protein; TRAF, tumor necrosis factor receptor-associated factor; TRADD, tumor necrosis factor receptor type 1–associated DEATH domain; Qp, Q promoter; Wp, W promoter.
EBV-positive DLBCL of the elderly shares a similar EBV latency patterns (type II or III) with PTLD.24 Using a reverse transcription-polymerase chain reaction method, Nguyen-Van et al19 showed that many EBV-positive DLBCL of the elderly and EBV-positive PTLD resembling DLBCL shared features of EBV latency type III. A relatively high frequency of EBNA2 expression (28%-32%) has been observed in several studies.2,12 However, Hofscheier et al7 found history of immunosuppression or immunodysfunction in 4 of 17 cases with EBNA2 expression, emphasizing that care should be taken in the diagnosis of EBNA2-positive DLBCL cases.
EBV-positive DLBCL of the elderly is characterized by prominent classical and alternative NF-κB pathway activation, with most cases displaying an activated B-cell (ABC) immunophenotype.8 Whereas NF-κB activation has been known in DLBCL, mostly in the ABC subtype,25 the association between NF-κB activation and the presence of EBV infection in DLBCL is much stronger.8 Interestingly, Montes-Moreno et al8 found NF-κB activation not only in the ABC but also in the germinal center B-cell (GCB) subtype of EBV-positive DLBCL, suggesting an additional role for EBV in NF-κB activation independent of the cell of origin. This finding could be related to c-Rel (a subunit of NF-κB transcription factor) amplification, which has been observed exclusively in the GCB immunophenotype of DLBCL on the basis of gene expression profiling.
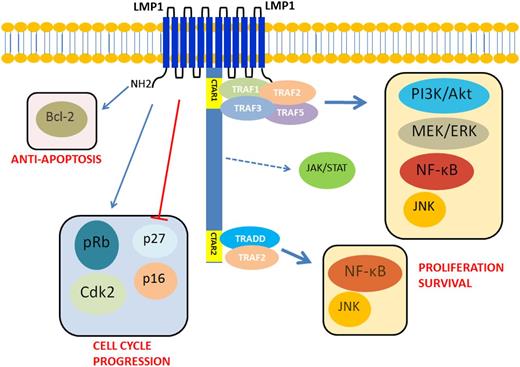
The oncogenic mechanisms of EBV are thought to be attributable predominantly to LMP1 (Figure 2). LMP1 (molecular weight, 63 kDa) is an integral membrane protein that consists of 3 different domains. The N-terminal cytoplasmic domain orientates LMP1 protein and binds to the plasma membrane. The long C-terminal cytoplasmic tail contains signaling activity. A 6-transmembrane loop between the C- and N-terminal domains provides a platform for self-aggregation and oligomerization. LMP1 functionally mimics CD40, which is expressed constitutively on the B-cell membrane involving in B-cell activation and proliferation. Of note, the function of LMP1 is ligand independent and is constitutively activated as a result of self-aggregation and oligomerization.26 The C-terminal tail has 2 distinct domains: C-terminal activation regions 1 and 2 (CTAR1 and 2), which activate the NF-κB pathway.27 LMP1 aggregates provide a platform for CTAR1 and CTAR2 to interact with downstream molecules (Figure 1C).28-30 LMP1 can also activate the c-Jun N-terminal kinase (JNK)/AP-1, MAPK, and phosphatidylinositol 3-kinase (PI3K)-Akt pathways.31-33
Schematic diagram of EBV-mediated oncogenic signaling pathway activation in EBV-positive diffuse large B-cell lymphoma of the elderly. LMP1 has a 6-transmembrane domain with a long cytoplasmic C-terminal chain. It can self-aggregate to activate itself constitutively and provide a platform to interact with downstream molecules. LMP1 provides a proliferation signal via activating NF-κB, PI3K/Akt, MEK-ERK, and JNK–AP-1 MAPK pathways. It also gives signal for cell cycle progression by enhancing cyclin-dependent kinase 2 and phosphorylation of Rb protein and by inhibiting p16 and p27. LMP1 can also activate bcl-2 to provide an antiapoptotic signal. CTAR1 can directly interact with TRAF1, 2, 3, and 5 to activate NF-κB, PI3K/Akt, MEK-ERK, and JNK–AP-1 MAPK pathways. CTAR2 needs TRADD to interact with TRAF2 to activate NF-κB. Both canonical and noncanonical pathways of NF-κB are activated to give proliferation signal to the nucleus. There are 3 putative JAK3-binding motifs between CTAR1 and CTAR2. However, this finding was not reproduced by others.26-36 AP-1, activator protein 1; ERK, extracellular signal-regulated kinases; JAK3, Janus kinase 3; JNK, Jun amino-terminal kinases; MEK, MAPK/ERK kinase; PI3K, phosphatidylinositol 3-kinase; TRADD, tumor necrosis factor receptor type 1–associated DEATH domain; TRAF, tumor necrosis factor receptor–associated factor.
Schematic diagram of EBV-mediated oncogenic signaling pathway activation in EBV-positive diffuse large B-cell lymphoma of the elderly. LMP1 has a 6-transmembrane domain with a long cytoplasmic C-terminal chain. It can self-aggregate to activate itself constitutively and provide a platform to interact with downstream molecules. LMP1 provides a proliferation signal via activating NF-κB, PI3K/Akt, MEK-ERK, and JNK–AP-1 MAPK pathways. It also gives signal for cell cycle progression by enhancing cyclin-dependent kinase 2 and phosphorylation of Rb protein and by inhibiting p16 and p27. LMP1 can also activate bcl-2 to provide an antiapoptotic signal. CTAR1 can directly interact with TRAF1, 2, 3, and 5 to activate NF-κB, PI3K/Akt, MEK-ERK, and JNK–AP-1 MAPK pathways. CTAR2 needs TRADD to interact with TRAF2 to activate NF-κB. Both canonical and noncanonical pathways of NF-κB are activated to give proliferation signal to the nucleus. There are 3 putative JAK3-binding motifs between CTAR1 and CTAR2. However, this finding was not reproduced by others.26-36 AP-1, activator protein 1; ERK, extracellular signal-regulated kinases; JAK3, Janus kinase 3; JNK, Jun amino-terminal kinases; MEK, MAPK/ERK kinase; PI3K, phosphatidylinositol 3-kinase; TRADD, tumor necrosis factor receptor type 1–associated DEATH domain; TRAF, tumor necrosis factor receptor–associated factor.
LMP1 also deregulates the cell cycle. Everly et al34 showed that LMP1 increases expression of cyclin-dependent kinase 2 (Cdk2), phosphorylates Rb protein, and inhibits expression of p27Kip1. The p16INK4a-Rb pathway has been shown to be hindered by LMP1.35 LMP1 also increases expression of Bcl-2 to prevent latently EBV-infected cells from undergoing apoptosis.36 Of note, virus and host transcriptome analyses have revealed an unexpected coordination between viral lytic reactivation and cellular pathways involved in B-cell expansion and tumor promotion,37 supporting a role for the lytic cycle in virus-mediated oncogenesis while further highlighting the complexity of interactions between EBV and host.38
Clinical features
EBV-positive DLBCL of the elderly is characterized by higher age distribution and an aggressive clinical course with a median survival of 2 years in Asian patients (Figure 3A-B).4,7,8,10,12,13,18 Initial reports emphasized that EBV-positive DLBCL of the elderly commonly involved extranodal sites.2,13 Site of primary extranodal involvement include the skin, soft tissue, bones, nasal cavity, pharynx/hypopharynx, tonsils, tongue, lung, pleura, stomach, liver, spleen, peritoneum, cecum, and bone marrow.4-8,10,39 Subsequently, several studies showed that lymph node involvement is very common, in up to 70% of patients.4-8,10,39
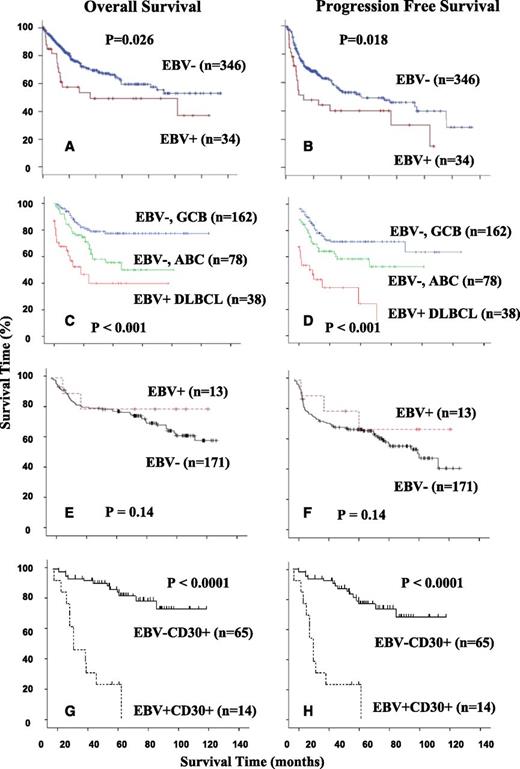
Geographical variations of overall survival and progression-free survival in EBV-positive diffuse large B-cell lymphoma of the elderly. (A-B) EBV-positive DLBCLs of the elderly showed worse overall survival and progression-free survival in Korean patients.13 (C-D) EBV positive DLBCLs of the elderly were more aggressive compared with >60-year-old patients with ABC subtype and GCB subtype in European patients.8 (E-F) In North America, EBV-positive DLBCLs of the elderly showed no difference in overall survival and progression-free survival in comparison with EBV-negative DLBCL patients.100 (G-H) In North America, EBV+CD30+ DLBCL of the elderly showed significant difference in overall survival and progression-free survival in comparison with either EBV+CD30− or EBV−D30+ DLBCL patients.
Geographical variations of overall survival and progression-free survival in EBV-positive diffuse large B-cell lymphoma of the elderly. (A-B) EBV-positive DLBCLs of the elderly showed worse overall survival and progression-free survival in Korean patients.13 (C-D) EBV positive DLBCLs of the elderly were more aggressive compared with >60-year-old patients with ABC subtype and GCB subtype in European patients.8 (E-F) In North America, EBV-positive DLBCLs of the elderly showed no difference in overall survival and progression-free survival in comparison with EBV-negative DLBCL patients.100 (G-H) In North America, EBV+CD30+ DLBCL of the elderly showed significant difference in overall survival and progression-free survival in comparison with either EBV+CD30− or EBV−D30+ DLBCL patients.
EBV-positive DLBCLs, including EBV-positive DLBCL of the elderly, respond more poorly to treatment with a poorer outcome compared with patients who have EBV-negative DLBCL. Therefore, novel therapeutic agents are needed to treat patients with EBV-positive DLBCL of the elderly.13 Montes-Moreno et al8 compared the survival distributions of age-related EBV-positive DLBCL cases and a control cohort either selecting ABC-type DLBCL cases or patients >60 years of age and subsequently confirmed that patients with EBV-positive DLBCL of the elderly have a poorer overall survival and progression-free survival than patients with ABC-type EBV-negative DLBCL in older European patients (Figure 3C-D). In comparison, a negative impact of EBV infection in DLBCL survival was not observed in the North American patients. There was a trend toward a higher percentage of stage III/IV disease and bone marrow involvement (85% vs 57% and 46% vs 16%, P = .052), but no unique clinical features were found in EBV-positive DLBCL patients (Figure 3E-F). However, significantly worse survival is observed in EBV-positive DLBCL patients if CD30 expression is also present (Figure 3G-H).
Recently, Dojcinov et al18 expanded the spectrum of EBV-positive DLBCL. They analyzed 122 cases of EBV-positive B-cell LPDs in adults with no other identifiable cause of immunosuppression and classified them into 4 diagnostic categories: (1) reactive lymphoid hyperplasia; (2) polymorphic extranodal lymphoproliferative disease; (3) polymorphic nodal lymphoproliferative disease; and (4) DLBCL. In these 4 groups, the 5-year disease-specific survival decreases progressively (100%, 93%, 57%, and 25%, respectively), whereas the frequency of monoclonality increases (16%, 33%, 63%, and 56%, respectively). It needs to be emphasized that these categories are not fixed, as patients can progress from reactive hyperplasia to polymorphic or monomorphic EBV-positive LPD.40 With respect to morphology, some EBV-positive DLBCL of the elderly display a mixed pattern with polymorphic and monomorphic areas,8 or polymorphic cases eventually progress to the monomorphic subtype of EBV-positive DLBCL of the elderly.5,41
Two additional entities have been described that further add to the spectrum of EBV-positive LPDs. EBV-positive mucocutaneous ulcer is a term used recently to designate small-volume extranodal polymorphic lymphoproliferative disease, particularly involving mucosal sites and skin, and characterized by an indolent clinical course and good prognosis.18,42 Plasmablastic lymphoma of the elderly is another newly recognized entity with a relatively indolent clinical course and better prognosis compared with other age-related EBV-positive B-cell LPDs.24 Plasmablastic lymphoma of the elderly shares features with age-related EBV-positive B-cell LPDs, including older age, immunosenescence, EBV infection, HIV negativity, and an ABC immunophenotype, although contrasting features include more frequent extranodal involvement and MYC translocations and EBV type I latency pattern.24
Histopathologic features
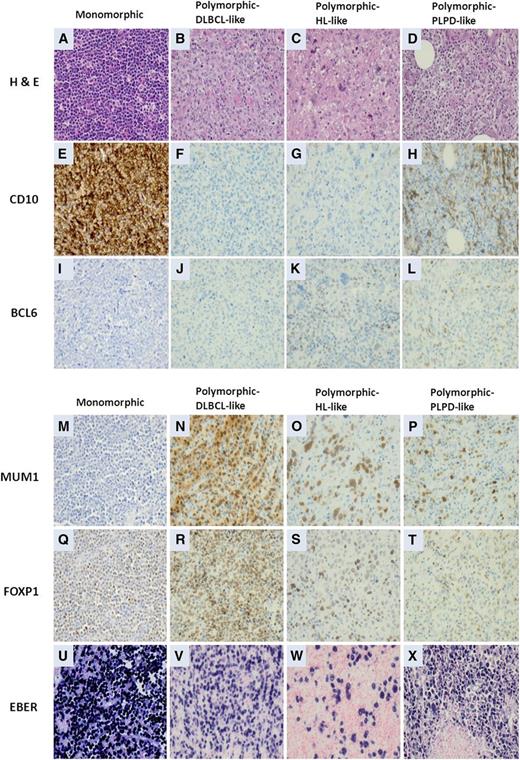
Two morphologic subtypes of EBV-positive DLBCL of the elderly have been recognized: polymorphic and monomorphic. Both subtypes may include large transformed cells or immunoblasts, as well as Hodgkin and Reed-Sternberg (HRS)-like giant cells and may demonstrate increased mitotic activity and areas of geographic necrosis.1 The polymorphic subtype displays a broad range of B-cell maturation, and lesions are composed of centroblasts, immunoblasts, and plasmablasts with a variable component of admixed reactive cells, including small lymphocytes, plasma cells, histiocytes, and epithelioid histiocytes.1 Montes-Moreno et al8 subdivided the polymorphic EBV-positive DLBCL of the elderly into 3 subgroups based on the relative proportion of large neoplastic cells and presence of HRS-like cells: (1) canonical large B-cell neoplasm (high density of large neoplastic cells and scattered cells with HRS-like features); (2) DLBCL with Hodgkin lymphoma-like features (lower density of neoplastic cells with HRS-like features); and (3) DLBCL with polymorphic LPD-like features (low density of neoplastic cells without HL-like features). The monomorphic subtype of EBV-positive DLBCL of the elderly is composed of monotonous sheets of large transformed B cells (Figure 4).1 Although this distinction is helpful for recognizing the spectrum, these morphologic variants do not impart clinical or prognostic significance.1 Cases of EBV-positive DLBCL of the elderly also can have a mixed pattern with intermingled polymorphic/and monomorphic areas,8 suggesting that the subtypes represent 2 ends of a morphologic spectrum.
Morphologic variants and immunophenotypic profiling in EBV-positive diffuse large B-cell lymphomas of the elderly. (A,E,I,M,Q,U) The monomorphic subtype is characterized by monotonous sheets of large transformed B cells. (B,F,J,N,R,V) Polymorphic DLBCL-like subtype shows canonical large B-cell neoplasm morphology, characterized by a high density of large neoplastic cells and scattered cells with Reed-Sternberg (RS)-like and Hodgkin-like features. (C,G,K,O,S,W) The polymorphic HL-like subtype shows a lower density of neoplastic cells with RS-like and Hodgkin-like features. (D,H,L,P,T,X) The polymorphic PLPD-like subtype is DLBCL with polymorphic LPD-like features. It is characterized by a low density of neoplastic cells without HL-like features. EBV-positive DLBCL of the elderly is predominantly the ABC subtype. (A,E,I,M,Q,U) Interestingly, the monomorphic case presented in this figure shows the GCB subtype. All polymorphic subtypes show the ABC-DLBCL molecular phenotype.
Morphologic variants and immunophenotypic profiling in EBV-positive diffuse large B-cell lymphomas of the elderly. (A,E,I,M,Q,U) The monomorphic subtype is characterized by monotonous sheets of large transformed B cells. (B,F,J,N,R,V) Polymorphic DLBCL-like subtype shows canonical large B-cell neoplasm morphology, characterized by a high density of large neoplastic cells and scattered cells with Reed-Sternberg (RS)-like and Hodgkin-like features. (C,G,K,O,S,W) The polymorphic HL-like subtype shows a lower density of neoplastic cells with RS-like and Hodgkin-like features. (D,H,L,P,T,X) The polymorphic PLPD-like subtype is DLBCL with polymorphic LPD-like features. It is characterized by a low density of neoplastic cells without HL-like features. EBV-positive DLBCL of the elderly is predominantly the ABC subtype. (A,E,I,M,Q,U) Interestingly, the monomorphic case presented in this figure shows the GCB subtype. All polymorphic subtypes show the ABC-DLBCL molecular phenotype.
Immunophenotype
The neoplastic cells are of B-cell lineage; they express the pan B-cell antigens CD19, CD20, CD22, CD79a, and PAX5 and are negative for pan T-cell antigents. Immunoglobulin light chain restriction may be difficult to demonstrate, except in cases with immunoblastic or plasmablastic features in which cytoplasmic Ig can be assessed. Plasmacytoid cases can be weakly positive or negative CD20.1 EBV-positive DLBCL of the elderly usually has an ABC immunophenotype being MUM1/IRF4+ and CD10− and usually BCL6−. BCL-2 and CD30 are usually positive; CD15 is negative.1,8 Ki-67 generally shows a high proliferation index.8 By definition, the neoplastic cells are EBV positive and accordingly display EBER, and LMP1 and EBNA2 are expressed in >90% and 15∼30% of cases, respectively.1,43 The concordance rate between EBER and LMP1 can reach 80% to 90% but is not uniformly consistent.
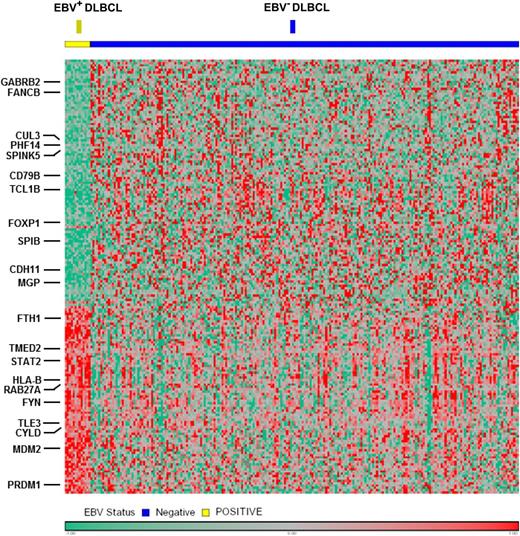
The proportion of EBV-positive DLBCL cases among all DLBCL patients increases with age,12,44 which is analogous to the ABC subtype that also increases with age.45 These findings are in agreement with recent data indicating that aging is an important determinant of lymphoma biology.46 In an effort to explain the proportional increase of ABC DLBCL with age, Mareschal et al45 speculated that either there is a change in B-cell population during aging or there is putative pathological specificity of EBV in elderly patients with DLBCL. Not surprisingly, most cases of EBV-positive DLBCL of the elderly displayed a striking shift to an ABC immunophenotype with prominent activation of NF-κB pathway (Figure 5).8
Gene expression profiling in the DLBCL ABC subtype was performed according to EBV status. The EBV-positive case shows a distinct gene expression pattern compared with cases with EBV negativity, with overexpression of multiple components of the NF-κB pathway.
Gene expression profiling in the DLBCL ABC subtype was performed according to EBV status. The EBV-positive case shows a distinct gene expression pattern compared with cases with EBV negativity, with overexpression of multiple components of the NF-κB pathway.
Differential diagnosis
The major entities in the differential diagnosis of EBV-positive DLBCL of the elderly are summarized in Table 2. In part, EBV-positive DLBCL of the elderly is a diagnosis of exclusion. If there is a known cause of immunodeficiency, these tumors are better classified as posttransplant LPDs, other iatrogenic immunodeficiency-associated LPDs, etc. Other forms of EBV-positive DLBCL such as lymphomatoid granulomatosis, DLBCL associated with chronic inflammation, primary effusion lymphoma, and plasmablastic lymphoma are also considered in the differential diagnosis. Reactive diseases such as infectious mononucleosis of the elderly and chronic active EBV infection may also be considered based on the morphologic findings, but clinical history and serologic testing are helpful.
Differential diagnostic considerations of EBV-positive DLBCL of the elderly
| Entity . | Site . | Architecture . | Immunophenotype . | Specific findings . | Light chain . | Clonality . |
|---|---|---|---|---|---|---|
| EBV-positive DLBCL, elderly | N (70%) | Effaced | Pan B markers+ | Geographic necrosis | Monotypic | Monoclonal |
| E (30%) | CD10−/BCL6−/MUM1+, EBER+,CD30+/− | |||||
| EBV-associated reactive atypical lymphoproliferations/infectious mononucleosis in elderly | N | Not effaced | Mixed B- and T-cell nature of HRS-like cells (CD3+/20+/30+) | n/a | Polytypic | Polyclonal |
| EBER+ | ||||||
| EBV-positive classical Hodgkin lymphoma | N | Effaced | CD20-/CD79a-/Pax5w+ | HRS cells in reactive background | Polytypic | Polyclonal |
| CD30+/CD15+, EBER+/− | ||||||
| DLBCL, NOS, anaplastic variant | N | Effaced | Pan B markers+ | Sinusodal or cohesive pattern | Monotypic | Monoclonal |
| EBER-, CD30+ | ||||||
| T-cell/histiocyte-rich large B-cell lymphoma | N | Effaced | Pan B markers+ | Scattered B cells in benign T cells/histiocytes background | Monotypic | Monoclonal |
| EBER−, CD30+/−, CD15- | ||||||
| Primary cutaneous DLBCL, leg type | E (skin) | Dermal infiltration | Pan B-cell markers+, EBER- | Diffuse sheets of centroblasts and immunoblasts | Monotypic | Monoclonal |
| BCL2+/MUM1+/FOXP1+ | ||||||
| Lymphomatoid granulomatosis | E | polymorphic infiltration | EBER+, LMP1− | Angioinvasion, HRS-like cells in reactive background | Noninformative | Monoclonal |
| Plasmablastic lymphoma of the elderly | N (50%) | Effaced | CD38+, CD138+, MUM1+, CD20− | Plasmablastic morphology | Monotypic | n/a |
| E (50%) | EBER+, LMP1−, EBNA2- | EBV latency I | ||||
| Angioimmunoblastic T-cell lymphoma | N | Effaced | CD10+, CXCL13+, PD1+ | FDC expansion | Polytypic | Monoclonal in T cells |
| Peripheral T-cell lymphoma, not otherwise specified | N | Effaced | CD3+/− with partial loss of T-markers, CD30+/−, CD15−/+ | Large atypical cells | Polytypic | Monoclonal in T cells |
| EBER−/+ | Clear cytoplasm+/− |
| Entity . | Site . | Architecture . | Immunophenotype . | Specific findings . | Light chain . | Clonality . |
|---|---|---|---|---|---|---|
| EBV-positive DLBCL, elderly | N (70%) | Effaced | Pan B markers+ | Geographic necrosis | Monotypic | Monoclonal |
| E (30%) | CD10−/BCL6−/MUM1+, EBER+,CD30+/− | |||||
| EBV-associated reactive atypical lymphoproliferations/infectious mononucleosis in elderly | N | Not effaced | Mixed B- and T-cell nature of HRS-like cells (CD3+/20+/30+) | n/a | Polytypic | Polyclonal |
| EBER+ | ||||||
| EBV-positive classical Hodgkin lymphoma | N | Effaced | CD20-/CD79a-/Pax5w+ | HRS cells in reactive background | Polytypic | Polyclonal |
| CD30+/CD15+, EBER+/− | ||||||
| DLBCL, NOS, anaplastic variant | N | Effaced | Pan B markers+ | Sinusodal or cohesive pattern | Monotypic | Monoclonal |
| EBER-, CD30+ | ||||||
| T-cell/histiocyte-rich large B-cell lymphoma | N | Effaced | Pan B markers+ | Scattered B cells in benign T cells/histiocytes background | Monotypic | Monoclonal |
| EBER−, CD30+/−, CD15- | ||||||
| Primary cutaneous DLBCL, leg type | E (skin) | Dermal infiltration | Pan B-cell markers+, EBER- | Diffuse sheets of centroblasts and immunoblasts | Monotypic | Monoclonal |
| BCL2+/MUM1+/FOXP1+ | ||||||
| Lymphomatoid granulomatosis | E | polymorphic infiltration | EBER+, LMP1− | Angioinvasion, HRS-like cells in reactive background | Noninformative | Monoclonal |
| Plasmablastic lymphoma of the elderly | N (50%) | Effaced | CD38+, CD138+, MUM1+, CD20− | Plasmablastic morphology | Monotypic | n/a |
| E (50%) | EBER+, LMP1−, EBNA2- | EBV latency I | ||||
| Angioimmunoblastic T-cell lymphoma | N | Effaced | CD10+, CXCL13+, PD1+ | FDC expansion | Polytypic | Monoclonal in T cells |
| Peripheral T-cell lymphoma, not otherwise specified | N | Effaced | CD3+/− with partial loss of T-markers, CD30+/−, CD15−/+ | Large atypical cells | Polytypic | Monoclonal in T cells |
| EBER−/+ | Clear cytoplasm+/− |
Genetic and miRNA profile
Only a few genetic studies on cases of EBV-positive DLBCL of the elderly have been performed.8,18 The immunoglobulin genes are monoclonally rearranged in most cases, with clonality of EBV also usually detectable using EBV terminal repeat regions probes and molecular techniques.1,5,7,8,18,47 IgH-mediated translocations are uncommon (∼15%).8,18 However, these analyses were restricted to single loci (IGH, IGK, IGL, PAX5, C-MYC, BCL2, and BCL6). A single case with a t(9;14)(p13;q32) translocation involving the PAX5 gene has been reported.48 Given the presence of a low number of genomic aberrations in EBV-positive DLBCL of the elderly, it has been suggested that immunosenescence coupled with the EBV oncogenic properties is sufficient, and additional chromosomal alterations are therefore usually not needed for lymphomagenesis.8
EBV was the first virus in which miRNAs were identified, and 44 mature EBV-miRNAs are currently known.49 A detailed list of EBV miRNAs is summarized in Table 3. EBV-miRNAs can regulate viral and cellular targets, and they generally target host transcripts during latent infection (Table 3).50,51 Although viral miRNA constitutes only ∼2% of all miRNA in EBV-positive DLBCL, the viral miRNAs share seed sequence homology with cellular miRNA. For example, EBV-miR-Bam HI-A region rightward transcript (BART)5 and EBV-miR-BART1-3p share similarity with cellular miR-18 (miR-17-92 cluster) and miR-29a/b, respectively.52,53 Furthermore, viral miRNAs share seed sequence homology among themselves.50 Moreover, individual EBV miRNAs can target multiple mRNAs, suggesting that many other targets are actually affected and await further identification.54
Viral and cellular targets and functions of EBV miRNAs
| Targets . | EBV-miRNA . | Function of EBV-miRNA . | Reference . |
|---|---|---|---|
| Viral targets | |||
| BALF5 | EBV-miR-BART2 | Inhibition of lytic cycle | 89 |
| EBNA2 | EBV-miR-BART6 | Maintenance of viral latency | 90 |
| LMP1 | EBV-miR-BART-Cluster1 | Growth control or escape from immunosurveillance | 59,91 |
| EBV-miR-BART19-5p | Prevent apoptosis | 59 | |
| LMP2A | EBV-miR-BART22 | Growth control or escape from immunosurveillance | 92 |
| Cellular targets | |||
| Dicer | EBV-miR-BART6 | Promote latency | 90 |
| Bim | EBV-miR-BART-Cluster 1 | Anti-apoptosis | 93 |
| EBV-miR-BART-Cluster 2 | Anti-apoptosis | 93 | |
| CXCL-11/I-TAC | EBV-miR-BHRF1-3 | Anti-apoptosis | 94 |
| PUMA | EBV-miR-BART5 | Anti-apoptosis | 55,93 |
| IPO7 | EBV-miR-BART3-3p | Anti-inflammation | 59,95 |
| TOMM22 | EBV-miR-BART16-5p | Anti-apoptosis | 59,95 |
| MICB | EBV-miR-BART2-5p | Immune evasion | 96 |
| BCLAF1 | EBV-miR-BART17-5p | Anti-apoptosis | 59 |
| CLEC2D (LLT1) | EBV-miR-BART3-3p | Immune evasion | 52 |
| EBV-miR-BART1-3p | Immune evasion | 52 | |
| LY75 (DEC205) | EBV-miR-BART1-5p | Immune evasion | 52 |
| CLEC7A (Dectin-1) | EBV-miR-BART4 | Immune evasion | 52 |
| CLEC2B (AICL) | EBV-miR-BART2-5p | Immune evasion | 52 |
| NLRP3 | EBV-miR-BART15 | Immune dysregulation | 97 |
| Targets . | EBV-miRNA . | Function of EBV-miRNA . | Reference . |
|---|---|---|---|
| Viral targets | |||
| BALF5 | EBV-miR-BART2 | Inhibition of lytic cycle | 89 |
| EBNA2 | EBV-miR-BART6 | Maintenance of viral latency | 90 |
| LMP1 | EBV-miR-BART-Cluster1 | Growth control or escape from immunosurveillance | 59,91 |
| EBV-miR-BART19-5p | Prevent apoptosis | 59 | |
| LMP2A | EBV-miR-BART22 | Growth control or escape from immunosurveillance | 92 |
| Cellular targets | |||
| Dicer | EBV-miR-BART6 | Promote latency | 90 |
| Bim | EBV-miR-BART-Cluster 1 | Anti-apoptosis | 93 |
| EBV-miR-BART-Cluster 2 | Anti-apoptosis | 93 | |
| CXCL-11/I-TAC | EBV-miR-BHRF1-3 | Anti-apoptosis | 94 |
| PUMA | EBV-miR-BART5 | Anti-apoptosis | 55,93 |
| IPO7 | EBV-miR-BART3-3p | Anti-inflammation | 59,95 |
| TOMM22 | EBV-miR-BART16-5p | Anti-apoptosis | 59,95 |
| MICB | EBV-miR-BART2-5p | Immune evasion | 96 |
| BCLAF1 | EBV-miR-BART17-5p | Anti-apoptosis | 59 |
| CLEC2D (LLT1) | EBV-miR-BART3-3p | Immune evasion | 52 |
| EBV-miR-BART1-3p | Immune evasion | 52 | |
| LY75 (DEC205) | EBV-miR-BART1-5p | Immune evasion | 52 |
| CLEC7A (Dectin-1) | EBV-miR-BART4 | Immune evasion | 52 |
| CLEC2B (AICL) | EBV-miR-BART2-5p | Immune evasion | 52 |
| NLRP3 | EBV-miR-BART15 | Immune dysregulation | 97 |
Several studies have shown that EBV miRNAs contribute to EBV-induced oncogenesis. EBV-miR-BART5 targets and degrades p53-up-regulated modulator of apoptosis, a proapoptotic protein.55 EBV-miR-BHRF1 promotes B-cell transformation and proliferation and prevents apoptosis.56 EBV-miR-BART9 and BART17-5p can down-regulate BCL6 expression.57 Considering the fact that BCL6 represses NF-κB, down-regulation of BCL6 by EBV-miRNA might indirectly contribute to NF-κB activation.57,58 Riley et al59 showed that numerous EBV-miR-BARTs likely target at least 132 apoptotic mRNAs in latency type III infection, highlighting their collective influence on apoptosis regulation.
It also becomes evident that EBV miRNAs have evolved to target multiple cellular pathways rather than a single pathway. Pathway analysis has predicted that there are several EBV miRNA targeted genes in ≥20 specific pathways, including p53 feedback loops, B-cell activation, oxidative stress response, inflammation mediated by chemokines or the cytokine signaling pathway, the PI3K pathway, and apoptosis (Figure 6).52
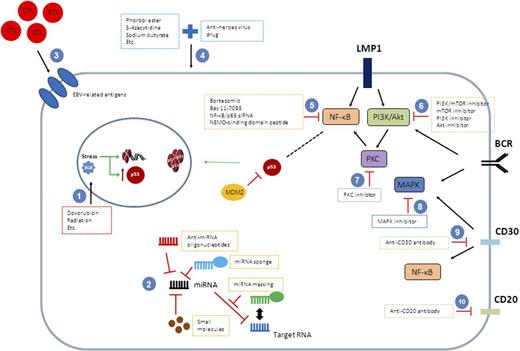
Therapeutic modulation of EBV infection and associated signaling pathways. Strategies to inactivate EBV infection or EBV-associated oncogenic pathways have been explored in lymphoma cells. Many therapeutic targets have been identified. (1) Conventional chemotherapeutic agents or radiation target DNA. (2) Different strategies disrupting miRNAs can be offered. (3) EBV-specific cytotoxic T cells are engineered and reinfused to patients as immunoregulatory therapy approach. (4) Lytic cycle of EBV is induced by a couple of agents, and antiherpetic agents targeting virus in lytic cycle are added. Agents targeting the (5) NF-κB pathway, (6), PI3K/Akt pathway, (7) PKC pathway, or (8) MAPK pathway can be tried. Monoclonal antibodies, Brentuximab vedotin and Rituximab, are available targeting for (9) CD30 and (10) CD20, respectively.
Therapeutic modulation of EBV infection and associated signaling pathways. Strategies to inactivate EBV infection or EBV-associated oncogenic pathways have been explored in lymphoma cells. Many therapeutic targets have been identified. (1) Conventional chemotherapeutic agents or radiation target DNA. (2) Different strategies disrupting miRNAs can be offered. (3) EBV-specific cytotoxic T cells are engineered and reinfused to patients as immunoregulatory therapy approach. (4) Lytic cycle of EBV is induced by a couple of agents, and antiherpetic agents targeting virus in lytic cycle are added. Agents targeting the (5) NF-κB pathway, (6), PI3K/Akt pathway, (7) PKC pathway, or (8) MAPK pathway can be tried. Monoclonal antibodies, Brentuximab vedotin and Rituximab, are available targeting for (9) CD30 and (10) CD20, respectively.
Interestingly, cellular miRNAs are modulated by viral proteins. Mir-155 has been shown in DLBCL, especially in the ABC subtype, and can be induced by LMP1 via the NF-κB pathway.60 Of note, miR-155 has been shown to cotarget INPP5D (SHIP1), a key target implicated in lymphomagenesis, with EBV miRNAs.59,61 Mir-34a is induced by LMP1 via the NF-κB pathway and promotes the growth of EBV-transformed cells.62 EBNA2 has been shown to induce miR-21 and down-regulate miR-146a.63 Overexpression of miR-21 has been found to promote tumor progression and invasion in solid tumors via targeting PTEN.64,65 Mir-146a has ben shown to inhibit the NF-κB pathway in the NK/T-cell lymphoma cell lines.66 Therefore, EBNA2 can provide extra signals via miRNAs to enhance the NF-κB and Akt pathways, thereby providing survival/proliferation benefits.
Novel therapeutic approaches
Novel therapeutic approaches need to be considered for patients with EBV-positive DLBCL of the elderly. Possible therapeutic approaches include (1) EBV-specific adoptive immunotherapy; (2) miRNA-targeted therapy; (3) combination therapy based on EBV lytic phase induction followed by exposure of the tumor cells to anti-herpesvirus drugs; and (4) targeting specific signaling pathways, including the NF-κB pathway (Table 4; Figure 6).
Differentially expressed genes in EBV-positive ABC DLBCL vs EBV-negative ABC DLBCL
| Gene functional categories . | No. of genes . | Representative genes . |
|---|---|---|
| Genes upregulated in EBV-positive ABC-DLBCL | ||
| NF-κB target | 11 | PRDM1, CYLD, FYN, TNFAIP3, FTH1, LITAF |
| Cell cycle regulation | 4 | CYTIP, PPM1A, TMEM30A, MRPL41 |
| Antiapoptosis | 4 | MDM2, DNAJC5, SERPINB9, GSTO1 |
| Tumor progression | 5 | LAMP1, PTP4A2, DYNLRB1, ASAP1, ERLEC1 |
| Cell proliferation | 3 | TXN, PDXK, GLUL |
| Cell adhesion and cytoskeleton | 6 | GABARAP, WIPF, AOC3, DST, SLAMF7, C1orf38 |
| Intracellular trafficking | 4 | TMED2, RAB27A, M6PR, FLOT1 |
| Immune response | 2 | SAMHD1, GBP2 |
| Cell metabolism | 12 | GPD2, P4HB, GNS, RPN2, APOL6, LASS6 |
| Transcription factors | 2 | ARNTL, TLE3 |
| Others and unknown | 17 | RPN2, SLC31A1, M6PR, SETD3, KIAA2013, TPCN2 |
| Genes downregulated in EBV-positive ABC-DLBCL | ||
| Proapopototic | 2 | TBX5, CUL3 |
| Immune response | 2 | ENPP3, CD79B |
| Cell metabolism | 3 | GATM, MOCOS, GALNT6 |
| Extracellular matrix, adhesion, cytoskeleton | 10 | CDHR1, CDHR3, CDH11, TRIP6, CLDN10, SMAGP |
| Signaling and transcription factors | 3 | CHN2, STYXL1, SPIB |
| Others and unknown | 49 | SPINK5, MYO3A, PHF14, LRRC39, MAGEB3, MGP |
| Gene functional categories . | No. of genes . | Representative genes . |
|---|---|---|
| Genes upregulated in EBV-positive ABC-DLBCL | ||
| NF-κB target | 11 | PRDM1, CYLD, FYN, TNFAIP3, FTH1, LITAF |
| Cell cycle regulation | 4 | CYTIP, PPM1A, TMEM30A, MRPL41 |
| Antiapoptosis | 4 | MDM2, DNAJC5, SERPINB9, GSTO1 |
| Tumor progression | 5 | LAMP1, PTP4A2, DYNLRB1, ASAP1, ERLEC1 |
| Cell proliferation | 3 | TXN, PDXK, GLUL |
| Cell adhesion and cytoskeleton | 6 | GABARAP, WIPF, AOC3, DST, SLAMF7, C1orf38 |
| Intracellular trafficking | 4 | TMED2, RAB27A, M6PR, FLOT1 |
| Immune response | 2 | SAMHD1, GBP2 |
| Cell metabolism | 12 | GPD2, P4HB, GNS, RPN2, APOL6, LASS6 |
| Transcription factors | 2 | ARNTL, TLE3 |
| Others and unknown | 17 | RPN2, SLC31A1, M6PR, SETD3, KIAA2013, TPCN2 |
| Genes downregulated in EBV-positive ABC-DLBCL | ||
| Proapopototic | 2 | TBX5, CUL3 |
| Immune response | 2 | ENPP3, CD79B |
| Cell metabolism | 3 | GATM, MOCOS, GALNT6 |
| Extracellular matrix, adhesion, cytoskeleton | 10 | CDHR1, CDHR3, CDH11, TRIP6, CLDN10, SMAGP |
| Signaling and transcription factors | 3 | CHN2, STYXL1, SPIB |
| Others and unknown | 49 | SPINK5, MYO3A, PHF14, LRRC39, MAGEB3, MGP |
EBV-specific adoptive immunotherapy, in which EBV-specific cytotoxic T-lymphocytes (CTLs) are ex vivo activated and expanded and then reinfused seem promising.67 Using this approach, EBV-specific CTLs could be engineered to recognize and lyse tumor cell targets via chimeric receptors while maintaining their ability to proliferate in response to EBV target antigens and accordingly destroy virus-infected cells.68 Optimal tumor eradication appears to require proper target antigen selection, costimulatory signaling, and the ability of chimeric antigen receptor-modified T cells to traffic, persist, and retain function following adoptive transfer.69 Recent evidence has suggested that both EBV-positive monomorphic PTLD that resembles DLBCL and EBV-positive DLBCL of the elderly share features consistent with type III EBV latency, including immunodominant EBNA3A protein expression, indicating that such an approach may be feasible.19
Given the aforementioned complex interplay between host and viral-encoded miRNAs in EBV-mediated oncogenesis, targeting miRNA as a therapeutic approach appears to be promising. Various approaches have been proposed: (1) anti-miRNA oligonucleotides are inhibitory molecules blocking the interactions between miRNAs and their target mRNAs by interacting directly to the target miRNA; (2) miRNA mask is a sequence with perfect complementarity to the binding site for a miRNA in the target mRNA, which can form a duplex with the target mRNA with higher affinity and block the access of the miRNA; (3) miRNA sponges are synthetic decoys that contain multiple binding sites for a miRNA of interest, preventing the interaction between miRNA and its targets; (4) small molecule inhibitors against specific miRNAs can specifically inhibit miRNA synthesis; (5) restoring activity of miRNAs with oncosuppressive functions; and (6) using miRNA to sensitize tumors to known and efficient cancer therapeutic modalities.70
Another potential approach could be combining induction of EBV lytic phase with subsequent exposure to anti-herpesvirus drugs.71 Lytic phase inducers may refer to phorbol esters, DNA methylase transferase inhibitors, histone deacetylase inhibitors, proteasome inhibitors, B-cell receptor-blocking antibodies, chemotherapeutic drugs, and cellular miRNAs.71,72 Although proof of principle of a combination therapy approach has been documented in a clinical trial,73 the optimal pharmacological lytic phase inducer remains to be determined. It has been recently shown that other histone deacetylase inhibitors such as panobinostat appear to be efficient and potent for the treatment of EBV-associated lymphomas.74
Given that the EBV-positive DLBCL of the elderly is an aggressive postgerminal center B-cell neoplasm characterized by prominent NF-κB activation,8 targeting the NF-κB pathway constitutes a rational therapeutic approach (Table 4). Bortezomib, a proteasome inhibitor, has been found to induce apoptosis in EBV lymphoblastoid cell lines and to reduce canonical and noncanonical activities of the NF-κB pathway.75 A recent phase I/II clinical trial with bortezomib plus Rituximab-Cyclophosphamide, Doxorubicin Hydrochloride, Vincristine Sulphate, and Prednisone (R-CHOP) in DLBCL abrogated the adverse outcome in non-GCB and showed similar survival between GCB and non-GCB patients.76 Bay 11-7085, a pharmacological inhibitor of NF-κB activity, or NF-κB/p65 small interfering RNA, can inhibit DLBCL growth in vitro via induction of apoptosis.25 In contrast with bortezomib, the latter strategies of NF-κB inhibition down-regulate expression of key NF-κB regulated gene products, including Bcl-2 and Bcl-XL.25,75 NF-κB essential modulator-binding domain peptide has been shown to inhibit NF-κB target gene expression and reduce tumor burden in an in vivo model as well.77
Recent evidence indicates a critical function of PI3K-PDK1 signaling upstream of NF-κB in ABC-DLBCL cells.78 PI3K inhibition reduced NF-κB activity and decreased the expression of NF-κB target genes in ABC-DLBCL cell lines, providing a rationale for the pharmacological use of PI3K inhibitors for treating patients with ABC-type DLBCL.78 In addition, LMP1 has been implicated in the activation of the PI3K/Akt signaling pathway, which lies upstream of mTOR.79 Experimental evidence stemming either from dual PI3K/mTOR inhibitors or a single mTOR inhibitor further reinforces the putative exploitation of LMP1/LMP2A-activated PI3K/Akt/mTOR signaling pathway.80 Another attractive target for developing antiviral or antitumor strategies is EBNA1 owing to its essential role in maintaining the EBV genome and the consistent expression of EBNA1 in all proliferating EBV-positive cells.81 Sun et al showed that Hsp90 inhibitors block the outgrowth of EBV-infected malignant cells in vitro and in vivo through an EBNA1-dependent mechanism.81 Notably, Hsp90 inhibitors have been found to suppress NF-κB activity.82 A CD30-directed antibody-drug conjugate could be a good option given frequent CD30 expression in EBV-positive DLBCL patients. A recent trial of Brentuximab Vedotin in 2 cases of EBV-positive DLBCL did not show promising results, but the small size trial might not be representative.83
Conclusions
We provided a comprehensive review of EBV-positive DLBCL of the elderly, discussing its epidemiologic, pathogenetic, and clinicopathologic features; discussing the differential diagnosis; and proposing some comments about potential novel therapeutic approaches for patients with this disease. Currently, an arbitrary cutoff of 50 years is used to define this entity in the current WHO classification, but patients <50 years can also be affected. Although these tumors were originally reported as usually being extranodal, it is clear that nodal presentation is also seen. Most cases of EBV-positive DLBCL of the elderly have an ABC immunophenotype. Assessment for EBV infection in tissue biopsy specimen can be easily performed by in situ hybridization analysis for EBER, but other methods of EBV detection including polymerase chain reaction can be used. Caution should be exercised before rendering this particular diagnosis because other causes of EBV-positive DLBCL must be excluded, and there can be no known explanation for immunodeficiency. Clearly, a better understanding of the molecular genetic basis of EBV-positive DLBCL of the elderly is needed. Further studies are required to address the heterogeneous EBV latency status and miRNA expression profile of EBV-positive DLBCL of the elderly, as well as the role of the NF-κB pathway.
Acknowledgments
This work was supported by an Anderson Pathology Fellowship Award (C.Y.O.), the University of Texas MD Anderson Cancer Center Institutional R & D Fund, an Institutional Research Grant award, an Anderson Lymphoma Specialized Programs of Research Excellence (SPORE) Research Development Program award, an Anderson Myeloma SPORE Research Development Program award, and Anderson Collaborative Research Funds with Daiichi Sankyo Pharmaceuticals, High-Throughput Molecular Diagnostics and Roche Molecular System (K.H.Y.). This work was also partially supported by the National Cancer Institute and National Institutes of Health grants R01CA138688, 1RC1CA146299, P50CA136411, and P50CA142509.
Authorship
Contribution: C.Y.O., T.G.P., L.J.M., and K.H.Y. designed the study, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ken H. Young, University of Texas MD Anderson Cancer Center, Department of Hematopathology, 1515 Holcombe Blvd, Houston, TX 77030-4009; e-mail: khyoung@mdanderson.org.