Key Points
CD1c+ DC but not BDCA-3+ DC or other antigen-presenting cells secrete high amounts of bioactive IL-12.
CD1c+ DC efficiently cross-present antigens, prime CD8+ T cells, and induce the highest levels of cytotoxic molecules.
Abstract
Dendritic cells (DC) have the unique capacities to induce primary T-cell responses. In mice, CD8α+DC are specialized to cross-prime CD8+ T cells and produce interleukin-12 (IL-12) that promotes cytotoxicity. Human BDCA-3+DC share several relevant characteristics with CD8α+DC, but the capacities of human DC subsets to induce CD8+ T-cell responses are incompletely understood. Here we compared CD1c+ myeloid DC (mDC)1, BDCA-3+mDC2, and plasmacytoid DC (pDC) in peripheral blood and lymphoid tissues for phenotype, cytokine production, and their capacities to prime cytotoxic T cells. mDC1 were surprisingly the only human DC that secreted high amounts of IL-12p70, but they required combinational Toll-like receptor (TLR) stimulation. mDC2 and pDC produced interferon-λ and interferon-α, respectively. Importantly, mDC1 and mDC2 required different combinations of TLR ligands to cross-present protein antigens to CD8+ T cells. pDC were inefficient and also expressed lower levels of major histocompatibility complex and co-stimulatory molecules. Nevertheless, all DC induced CD8+ memory T-cell expansions upon licensing by CD4+ T cells, and primed naive CD8+ T cells following appropriate TLR stimulation. However, because mDC1 produced IL-12, they induced the highest levels of cytotoxic molecules. In conclusion, CD1c+mDC1 are the relevant source of IL-12 for naive T cells and are fully equipped to cross-prime cytotoxic T-cell responses.
Introduction
Dendritic cells (DC) are professional antigen-presenting cells (APC) that possess the unique capacity to trigger primary adaptive immune responses through the antigen-specific activation of naive CD4+ and CD8+ T cells.1 DC are derived from proliferating precursors in the bone marrow that migrate via the blood to lymphoid and nonlymphoid tissues.2 Immature DC efficiently sample antigenic material, but upon encounter of a pathogen, they undergo a complex maturation process that leads to migration to secondary lymphoid organs, cytokine production, and enhanced antigen presentation and T-cell stimulatory capacities.1 In particular, the ability to “cross”-present extracellular antigens on major histocompatibility complex (MHC) class I to CD8+ T cells is important for the priming of cytotoxic T-cell responses,3 and this capacity is acquired by DC upon maturation, cytokine signaling, and CD40 stimulation by CD4+ helper T cells.4,5
Two broad subsets of plasmacytoid DC (pDC) and myeloid DC (mDC) with different phenotypes and functions have been identified both in mice and men. mDC respond to bacteria and other pathogens, can secrete interleukin-12 (IL-12) and induce Th1 responses.6 pDC respond to viruses with high interferon-α (IFN-α) production,7 can induce Th1 and Th2 responses,8,9 and also cross-present antigens to cytotoxic T lymphocyte (CTL).10-12 In the human system, pDC and mDC show complementarities in pathogen recognition13 and have different migratory behaviors.14 In particular, pDC respond selectively to Toll-like receptor 9 (TLR9) agonist with IFN-α production, might enter inflamed secondary lymphoid organs via CXCR3,7,15 and induce IL-10 production by T cells via costimulation through the inducible costimulator ligand (ICOSL).16 In mice, mDC that express CD8α have superior capacities to cross-present antigens to CD8+ T cells in vivo as compared with CD8α−DC17,18 and they secrete very high levels of IL-12.19,20 In humans, BDCA-3+mDC21,22 share relevant characteristics with CD8α+DC. Thus, these DC subsets selectively express CLEC9A and XCR1.23-27 In addition, both mouse CD8α+DC and human BDCA-3+DC share the dependency on the BATF3 transcription factor for their generation28 and produce IFN-λ.29 BDCA-3+DC also express higher levels of TLR3 than CD1c+DC, but lack TLR4.26 Because BDCA-3+DC had superior capacities to cross-present antigens and to produce IL-12, it was proposed that BDCA-3+ DCs are also functionally equivalent of mouse CD8α+DC.30 However, conflicting results have been published on IL-12 production and cross-presentation of soluble antigens by mDC2 as compared with other DC subsets,23,26,31-34 possibly because of different experimental conditions.3 Thus, the relative capacities of different human DC subsets to produce IL-12 and to induce CD8+ T-cell responses remain a highly relevant, open question.
Here we phenotypically and functionally characterized human CD1c+mDC, BDCA-3+mDC, and pDC in peripheral blood as well as in the bone marrow and tonsils, and lymphoid tissues where DC are respectively generated and present antigens to T cells. We show that CD1c+mDC1, but surprisingly not BDCA-3+mDC2, can secrete high levels of IL-12. In addition, we demonstrate that both mDC subsets can efficiently cross-present soluble antigens and prime cytotoxic T cells, indicating that cross-priming is not an exclusive feature of BDCA-3+mDC2 in humans.
Methods
Mononuclear cells from human tissues
Human bone marrow samples (kindly provided by M. Lösch, Department of Anaesthesiology, Intensive Care and Analgesia, and H. Kienapfel, Department of Orthopedics, Auguste-Viktoria-Klinikum, Charité, Berlin, Germany) were obtained from patients undergoing hip arthroplasty, tonsil specimens surgically removed from pediatric patients, and buffy-coat blood of healthy donors provided by Istituto Di Ricovero e Cura a Carattere Scientifico Policlinico Ospedale Maggiore, Milan, Italy, and by Charité Hospital, Berlin, Germany. Bone marrow mononuclear cells (BMMC), peripheral blood mononuclear cells (PBMC), and tonsillar mononuclear cells (TMC) were isolated by Ficoll-Hypaque gradient (Sigma-Aldrich), according to standard methods.
The ethical committee approved the use of PBMC, BMMC, and TMC for research purposes (permission for bone marrow [EK-No 208-13] and for TMC [EA1/107/10]) and informed consent was obtained from the subjects involved in this study in accordance with the Declaration of Helsinki. Cells were cultured in complete RPMI1640 (EuroClone) containing 10% fetal calf serum (EuroClone) or 5% Human Serum (EuroClone), 0.1% penicillin/streptomycin (EuroClone), 0.1% nonessential amino acids (Lonza), and 0.1% sodium pyruvate (Lonza) at 37°C and 5% CO2.
DC isolation
DC subsets were isolated from BMMC, PBMC, and TMC by magnetic enrichment followed by cell sorting. Briefly, cells were incubated with anti-CD1c-fluorescein isothiocyanate (AD5 8E7), anti-BDCA-3-APC (AD5 14H12), and anti-BDCA-4-PE (AD5 17F6; Miltenyi Biotech) antibodies, nonspecific binding to Fc receptors was prevented using FcR blocking reagent (Miltenyi Biotech), and DC were magnetically labeled with anti-fluorescein isothiocyanate, anti-phycoerythrin, and anti-APC beads and enriched on columns. The positive fraction was incubated with the lineage markers anti-CD3 (SK7; BioLegend), anti-CD14 (61D3), anti-CD16 (CD16), anti-CD19 (HIB19), anti-CD56 (B159; e-Bioscience), and anti-CD11c (3.9; BioLegend). mDC1 were sorted as lineage CD11c+ CD1c+, mDC2 as lineage CD11c+BDCA-3hi, and pDC as lineage CD11c−BDCA-4+; in some experiments, DC were sorted in addition as HLA-DR+ cells. Purity of all DC subset was >95%. Monocyte subsets were sorted directly from peripheral blood as lymphocyte lineage cells (CD19−, CD56−, and CD3−) according to CD14 and CD16 or SLAN (DD-1; Miltenyi Biotech) expression.
Flow cytometry
Phenotypical analysis was performed by gating DC as lineage HLA-DR+ cells and DC subsets according to CD1c, BDCA-3, or BDCA-4 expression. DC were then analyzed for HLA-ABC (B9.12.1, IOTest), HLA-DR (G46-6; BD), CCR5 (3A9; BD), CXCR3 (1C6; BD), ICOS-L (MIH12; eBioscience), CD40 (5C3; BD), and CD86 (2331; BD) expression. Production of IFN-γ or cytotoxic molecules in CD8+ T cells was assessed by intracellular staining with antibodies specific for IFN-γ (F4S.B3; eBioscience), Granzyme-B (GB11; BioLegend), and Granzyme-K (GM6C3; Santa Cruz) according to a standard protocol. Samples were analyzed on a FACSCanto flow cytometer (BD) using FlowJo software (Tristar).
DC cytokine production
Sorted DC or monocyte subsets were seeded at 104 cells/well in a 96-well plate for 24 hours with either 100 ng/mL lipopolysaccharide (LPS; Sigma-Aldrich), 100 ng/mL polyinosine-polycytidylic acid (polyI:C; Alexis), 10 μg/mL CpGA (Alexis), 2.5 μg/mL R848, (Alexis), 10 ng/mL IFN-γ (R&D) or 1 ng/mL IL-4 (R&D), or a CD40L-transfected murine cell line (ratio 1:1, JSSB, kindly provided by A. Lanzavecchia, Institute for Research in Biomedicine, Bellinzona, Switzerland). Cell-free supernatants were analyzed by enzyme-linked immunosorbent assay, according to the manufacturer’s guidelines. Enzyme-linked immunosorbent assays for IL-12p70 and IFN-λ were from R&D, with IFN-α2a from eBioscience.
CD8+ T-cell stimulation
A total of 5 × 104 carboxyfluorescein diacetate succinimidyl ester (CFSE)-labeled naive CD8+ T cells with or without 5 × 104 autologous naive CD4+ T cells were cocultured with 104 allogeneic purified DC or monocyte subsets for 7 days. DC were stimulated with 100 ng/mL LPS (mDC1), 100 ng/mL polyI:C (mDC1 and mDC2), and 10 μg/mL CpGB (pDC; Alexis) with or without 2.5 μg/mL R848. T-cell priming was assessed by CFSE dilution and calculated as the percentage of divided CD4+ or CD8+ T cells. IFN-γ production was detected by intracellular staining after 4 hours of phorbol 12-myristate 13-acetate/ionomycin stimulation; 10 μg/mL Brefeldin A (Sigma) was added for the last 3 hours of stimulation. Cells were stained with labeled antibodies for CD8 and the percentage of CFSElo CD8+ T cells producing IFN-γ or expressing cytotoxic molecules was analyzed. In some experiments, either 1 ng/mL recombinant IL-12 or 5 μg/mL neutralizing anti–IL-12 (R&D Systems) was added.
Cross-presentation
Cytomegalovirus (CMV)-pp65495-503–specific CD8+ T-cell lines were generated from HLA-A2+ CMV+ donors by sorting HLA-A2 CMV-pp65495-503 dextramer+ (Immudex) CD8+ T cells and expansion with allogenic-irradiated PBMC, an Epstein-Barr virus cell line, soluble anti-CD3 antibodies (OKT3 30 ng/mL), and 100 U/mL of IL-2. CMV-specific T-cell lines were obtained from 2 different HLA-A2+ donors and maintained in 100 U/mL IL-2. Purified DC subsets from HLA-A2+ donors were cocultured with CMV-pp65495-503–specific CD8+ T cells (ratio DC:T = 1:2) in 96-well round-bottom plate in RPMI medium supplemented with 5% human serum, with or without CMVpp65 protein (40 μg/mL; Miltenyi Biotech) for 20 hours. IFN-γ production by pp65-specific CD8+ T cells was measured as the readout of antigen cross-presentation. Unspecific IFN-γ production induced by DC in the absence of pp65 was subtracted, and IFN-γ production induced with 1 µg/mL CMVpp65495-503 peptide was set to 100% for each condition. For the stimulation of CMV-specific memory T cells, we adapted a previously published protocol.35 CFSE-labeled CD8+ T cells from HCMV+ donors were stimulated with autologous DC subsets and a lysate of CMV-infected cells or in some experiments with recombinant pp65 with or without autologous CD4+ T cells. Proliferation of CD8+ T cells was assessed by CFSE dilution.
Statistics
Statistical significance was calculated using two-tailed Student t test in case of Gaussian distribution, otherwise Mann-Whitney U test for unmatched or Wilcoxon for paired groups. P < .05 (*), P < .005 (**) P < .0005 (***) were regarded as statistically significant. One-way analysis of variance test was used to compare statistical significance among 3 different groups.
Results
Frequency and proliferation of human DC subsets in different tissues
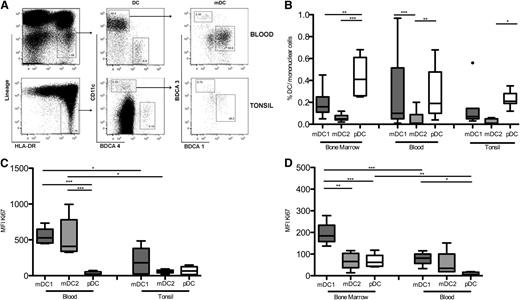
Proliferating murine DC precursors migrate from the bone marrow via the blood to seed secondary lymphoid organs, where they continue to divide.2 To analyze human DC subsets in relevant human tissues (bone marrow, blood, and tonsils), we identified DC as MHC class II+ (HLA-DR) cells that lacked lineage markers. DC were further subdivided into subsets of “conventional” CD11c+CD1c+ myeloid DC (“mDC1”), CD11c+BDCA-3+ myeloid DC (“mDC2”), and CD11c-BDCA-4+ plasmacytoid DC (“pDC”) (Figure 1A).
Frequency and proliferation of human DC subsets in bone marrow, peripheral blood, and tonsils. (A) Gating strategy for human DC subsets in peripheral blood and tonsils. (B) Frequency of mDC1 (dark gray boxes), mDC2 (light gray boxes), and pDC (white boxes) in total mononuclear cells isolated from bone marrow, blood, and tonsils. (C) Mean fluorescence intensity (MFI) of the Ki67 proliferation marker in DC subsets from peripheral blood of healthy donors and tonsils. (D) Ki67 expression of DC subsets from bone marrow and peripheral blood of patients that were undergoing hip arthroplasty. Shown are results of 6 donors in at least 2 experiments. *P < .05; **P < .005; ***P < .0005.
Frequency and proliferation of human DC subsets in bone marrow, peripheral blood, and tonsils. (A) Gating strategy for human DC subsets in peripheral blood and tonsils. (B) Frequency of mDC1 (dark gray boxes), mDC2 (light gray boxes), and pDC (white boxes) in total mononuclear cells isolated from bone marrow, blood, and tonsils. (C) Mean fluorescence intensity (MFI) of the Ki67 proliferation marker in DC subsets from peripheral blood of healthy donors and tonsils. (D) Ki67 expression of DC subsets from bone marrow and peripheral blood of patients that were undergoing hip arthroplasty. Shown are results of 6 donors in at least 2 experiments. *P < .05; **P < .005; ***P < .0005.
The 3 DC subsets had different frequencies in the analyzed tissues, but in all 3 tissues mDC2 was consistently the rarest population (Figure 1B).
In mice, DC subsets have different turnover rates.2,36 We analyzed the in vivo proliferation of human DC subsets by staining for the proliferation marker Ki-67. pDC were largely Ki-67− in peripheral blood of healthy donors and in tonsils, indicating that they proliferated poorly or not at all. In contrast, mDC1 and mDC2 proliferated in peripheral blood, but not in tonsils (Figure 1C).27 In hip arthroplasty patients, all 3 DC subsets proliferated in the bone marrow, but only mDC proliferated in the patient’s blood (Figure 1D). In summary, mDC2 represent the rarest DC subset and have a similar in vivo turnover as conventional mDC1 in blood and lymphoid tissues.
Surface receptor expression of DC subsets in blood and lymphoid tissues
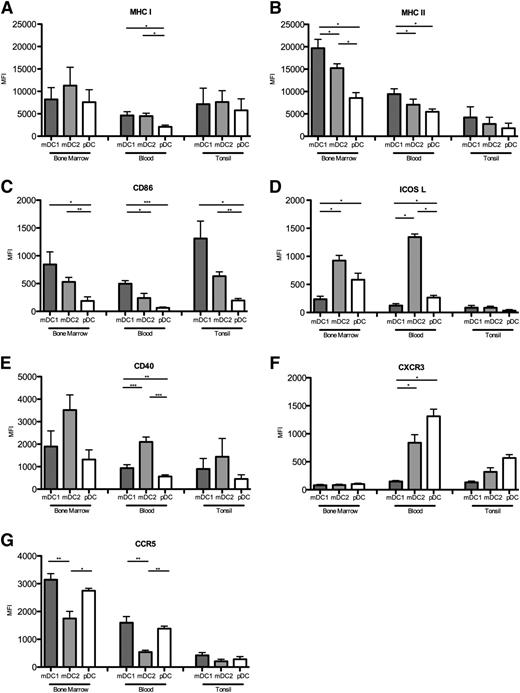
Expression of MHC and co-stimulatory molecules determines the capacities of DC to stimulate T cells, whereas chemokine receptors regulate their migration and positioning in tissues. We compared the expression of relevant surface receptors of DC subsets in bone marrow, peripheral blood, and tonsils (Figure 2). Although MHC class I was expressed at comparable levels on mDC subsets, pDC expressed lower levels (Figure 2A). MHC class II was expressed at the highest levels by mDC1, at intermediate levels on mDC2, and at the lowest levels by pDC in all tissues (Figure 2B). MHC class II expression was unexpectedly low in tonsils, possibly because of the degradation of surface MHC upon DC contact with activated CD4+ T cells.37 Conversely, the co-stimulatory molecule CD86, which was also highest on mDC1 and lowest on pDC, was higher in tonsils than in blood (Figure 2C). ICOSL was expressed on pDC but not on mDC1 as expected,16 but the highest levels were expressed on mDC2 in blood and bone marrow, whereas it was undetectable in tonsils (Figure 2D). Notably, mDC2 also expressed the highest levels of CD40 (Figure 2E). Among chemokine receptors, we found that CXCR3 was undetectable in the bone marrow but was selectively expressed on pDC and mDC2 in blood and tonsils (Figure 2F). CCR5 expression was highest in the bone marrow, intermediate in peripheral blood, and low in tonsils. Moreover, mDC2 expressed the lowest levels of CCR5 in all 3 tissues (Figure 2G). In summary, each human DC subset has a characteristic, but partially tissue-dependent expression of surface receptors. In particular, pDC express consistently lower levels of MHC molecules and CD86 compared with mDC, indicating that they are less potent to stimulate T cells than mDC.
Surface receptor expression on DC subsets in different tissues. Expression of (A) MHC class I, (B) MHC class II, (C) CD86, (D) ICOSL, (E) CD40, (F) CXCR3, and (G) CCR5 on mDC1, mDC2, and pDC in bone marrow, peripheral blood, and tonsils. Mean values for at least 7 donors in at least 3 experiments are shown. *P < .05; **P < .005; ***P < .0005.
Surface receptor expression on DC subsets in different tissues. Expression of (A) MHC class I, (B) MHC class II, (C) CD86, (D) ICOSL, (E) CD40, (F) CXCR3, and (G) CCR5 on mDC1, mDC2, and pDC in bone marrow, peripheral blood, and tonsils. Mean values for at least 7 donors in at least 3 experiments are shown. *P < .05; **P < .005; ***P < .0005.
mDC1 possess high IL-12–producing capacities
Production of bioactive IL-12p70 by DC drives IFN-γ production and promotes cytotoxicity in primed naive T cells and has thus been extensively studied in mouse DCs and in human in vitro differentiated monocyte-derived DC. In these DC, IL-12 production has a complex regulation and synergistic TLR stimulation, CD40L, IFN-γ, and IL-4 have been identified as critical factors.38 However, conflicting results have been published regarding the IL-12–producing capacities of in vivo–occurring human DC subsets,23,26,31,33,34 possibly because of the different stimulation conditions that were tested.
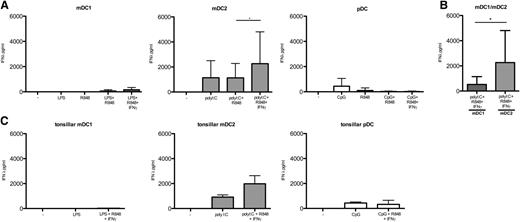
We found that mDC1 from peripheral blood could produce very high levels of IL-12p70, but required synergistic TLR stimulation by LPS and R848 (Figure 3A). In marked contrast, no IL-12 production was detected by mDC2 stimulated with polyI:C or by pDC stimulated with CpG DNA in the absence or presence of R848 (Figure 3A). IL-12 production by mDC1 was further enhanced by stimulation with IFN-γ (Figure 3B) or CD40L (Figure 3C), whereas IL-4 had a surprisingly inhibitory effect (Figure 3D). Importantly, mDC1 also secreted considerable amounts of IL-12 in response to polyI:C, R848, and IFN-γ, whereas mDC2 produced only very low levels of IL-12 under the same conditions (Figure 3E). CD40 stimulation of TLR-activated mDC2 did not increase IL-12 production (data not shown). Notably, IL-12 production by CD16+ and CD14+ monocytes was hardly detectable under the same conditions where mDC1 secreted high levels (supplemental Figure 1, available on the Blood Web site). Finally, mDC1 in tonsils also produced detectable IL-12 upon optimal stimulation, whereas tonsillar mDC2 and pDC did not (Figure 3F). We conclude that mDC1, but not mDC2, are the principal IL-12 producing APC in humans.
Regulation of IL-12 production by mDC1. (A) IL-12p70 production by peripheral blood DC subsets after a 24-hour culture in medium alone (−) or in the presence of the indicated TLR agonists. (B) Effects of IFN-γ, (C) CD40L, and (D) IL-4 on IL12p70 produced by mDC1. (E) IL-12 production by mDC1 (left column) and mDC2 (right column) in response to polyI:C, R848, and IFN-γ. Values show the mean of 16 donors in 8 different experiments. (F) IL-12p70 production by DC subsets in tonsils in response to the indicated stimuli. Results from 7 donors in 5 experiments are shown. nd, not determined. *P < .05; **P < .005.
Regulation of IL-12 production by mDC1. (A) IL-12p70 production by peripheral blood DC subsets after a 24-hour culture in medium alone (−) or in the presence of the indicated TLR agonists. (B) Effects of IFN-γ, (C) CD40L, and (D) IL-4 on IL12p70 produced by mDC1. (E) IL-12 production by mDC1 (left column) and mDC2 (right column) in response to polyI:C, R848, and IFN-γ. Values show the mean of 16 donors in 8 different experiments. (F) IL-12p70 production by DC subsets in tonsils in response to the indicated stimuli. Results from 7 donors in 5 experiments are shown. nd, not determined. *P < .05; **P < .005.
mDC2 secrete high levels of IFN-λ
Although mDC2 and pDC produced little or no IL-12p70, they could secrete high amounts of IFN-λ (Figure 4) and IFN-α (supplemental Figure 2), respectively. Thus, IFN-λ was secreted at the highest levels by mDC2 (Figure 4A-B) in response to polyI:C. Additional stimulation with R848 and IFN-γ further enhanced IFN-λ production by mDC2, whereas CD40L (data not shown) had no effect. In contrast, pDC secreted only low levels of IFN-λ in response to TLR-9 stimulation alone. mDC1 secreted some IFN-λ upon stimulation with polyI:C, R848, and IFN-γ, but mDC2 secreted much higher levels under the same conditions (Figure 4B). Also in tonsils, the highest levels of IFN-λ were produced by mDC2 (Figure 4C). Finally, IFN-α was as expected exclusively and abundantly produced by pDC both in peripheral blood and tonsils upon TLR-9 stimulation, and it was boosted by CD40 co-engagement (supplemental Figure 2). We conclude that human DC subsets have a specific cytokine profile, and that IFN-λ and IFN-α are predominantly or exclusively produced by mDC2 and pDC, respectively.
mDC2 secrete high amounts of IFN-λ. (A) IFN-λ production by peripheral blood DC subsets after a 24-hour culture in medium alone (−) or in the presence of the indicated stimuli. (B) IFN-λ production by mDC1 (left column) and mDC2 (right column) in response to polyI:C, R848, and IFN-γ. (C) IFN-λ production by DC subsets in tonsils. Values represent the mean of 16 PBMC donors in 8 different experiments and 7 TMC donors in 5 experiments. *P < .05.
mDC2 secrete high amounts of IFN-λ. (A) IFN-λ production by peripheral blood DC subsets after a 24-hour culture in medium alone (−) or in the presence of the indicated stimuli. (B) IFN-λ production by mDC1 (left column) and mDC2 (right column) in response to polyI:C, R848, and IFN-γ. (C) IFN-λ production by DC subsets in tonsils. Values represent the mean of 16 PBMC donors in 8 different experiments and 7 TMC donors in 5 experiments. *P < .05.
mDC subsets efficiently cross-present soluble antigens
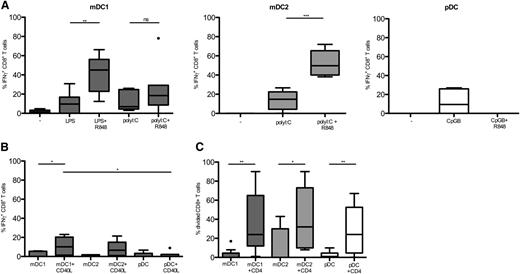
mDC2 have been shown to possess superior cross-presenting capacities, but recent reports have questioned this notion. We wondered if these inconsistent results might be due to the different stimulation conditions used.3,39 To test the cross-presentation capacities of human DC subsets, we used the CMV pp65 protein as a model antigen. We stimulated HLA-A2-pp65495-503–specific CD8+ T cells with DC subsets from HLA-A2+ donors with soluble pp65 protein. IFN-γ produced by the antigen-specific CD8+ T cells was used as a readout of cross-presentation, and DC loaded with the relevant pp65-derived peptide represented the positive control. As shown in Figure 5A and supplemental Figure 3, cross-presentation by immature DC was hardly detectable, but DC acquired cross-presenting capacities upon TLR stimulation. Thus, low cross-presentation could be detected by mDC1, mDC2, and pDC upon stimulation with, respectively, LPS, polyI:C, or CpG alone. Importantly, mDC2 stimulated with polyI:C and R848 acquired high cross-presentation capacities, whereas mDC1 were less efficient under the same condition. However, upon stimulation with LPS and R848, mDC1 also cross-presented efficiently. Conversely, cross-presentation by pDC stimulated with R848 in the absence or presence of CpG was undetectable (Figure 5A and data not shown). Moreover, CD40 stimulation of immature DC induced some cross-presentation by mDC1 and mDC2, but not by pDC (Figure 5B). CD40 co-stimulation also increased the cross-presentation capacities of TLR-stimulated DC, but the effect was weak and did not reach statistical significance (data not shown).
Efficient antigen cross-presentation by TLR-stimulated mDC. DC subsets obtained from HLA-A2+ donors were cocultured for 20 hours with HLA-A2 pp65-specific CD8+ T cells with recombinant soluble CMV pp65 protein in the absence (−) or presence of TLR agonists (A) or CD40L transfectants (B). Shown is the percentage of IFN-γ production by CD8+ T cells normalized on IFN-γ production in response to pp65495-503 peptide. Data are from 7 donors that were analyzed in 4 experiments. Primary data of 1 representative experiment is shown in supplemental Figure 3. (C) A total of 5 × 104 CFSE-labeled CD8+ T cells with or without 5 × 104 autologous CD4+ T cells were incubated with autologous purified DC subsets in the presence of CMV-derived proteins and the fraction of proliferating CD8+ T cells analyzed. Shown are results of 10 HCMV+ donors in different experiments. ns, not significant. *P < .05; **P < .005; ***P < .0005.
Efficient antigen cross-presentation by TLR-stimulated mDC. DC subsets obtained from HLA-A2+ donors were cocultured for 20 hours with HLA-A2 pp65-specific CD8+ T cells with recombinant soluble CMV pp65 protein in the absence (−) or presence of TLR agonists (A) or CD40L transfectants (B). Shown is the percentage of IFN-γ production by CD8+ T cells normalized on IFN-γ production in response to pp65495-503 peptide. Data are from 7 donors that were analyzed in 4 experiments. Primary data of 1 representative experiment is shown in supplemental Figure 3. (C) A total of 5 × 104 CFSE-labeled CD8+ T cells with or without 5 × 104 autologous CD4+ T cells were incubated with autologous purified DC subsets in the presence of CMV-derived proteins and the fraction of proliferating CD8+ T cells analyzed. Shown are results of 10 HCMV+ donors in different experiments. ns, not significant. *P < .05; **P < .005; ***P < .0005.
To address the role of CD4 help in cross-presentation and CD8+ T-cell activation in a more physiological system, we measured proliferation of CFSE-labeled CD8+ T cells from CMV+ donors with autologous DC subsets with CMV-derived proteins in the absence or presence of autologous CD4+ T cells. All DC induced efficient proliferation of CMV-specific CD8+ T cells when CD4+ T cells were present. In the absence of CD4+ T cells, only low CD8+ T-cell proliferation was induced in some donors by mDC2 (Figure 5C).
We conclude that both mDC1 and mDC2 can efficiently cross-present soluble protein antigens, but have different requirements for TLR stimulation. In contrast, pDC are less efficient but could nevertheless contribute to secondary expansions of CD8+ memory T cells.
DC require CD4 help and TLR stimulation for optimal CD8+ T-cell priming
A unique feature of DC is their ability to prime naive T cells, but the relative capacities of in vivo occurring human DC subsets to prime naive CD8+ T cells have not been analyzed yet. We therefore also assessed the capacities of DC subsets to prime naive CD4+ and CD8+ T cells in the absence and presence of TLR stimulation. Fluorescence-activated cell sorter–purified, naive CFSE-labeled CD4+ and CD8+ T cells were incubated alone or together with allogenic DC subsets in the absence or presence of TLR agonists, and proliferation of CD4+ or CD8+ T cells was assessed by CFSE dilution.
mDC1 and mDC2, and to a lesser extend pDC, induced naive CD4+ T-cell proliferation in the absence of TLR stimulation, whereas CD14+ monocytes or SLAN+ cells failed to do so (Figure 6A). However, DC induced only low or undetectable proliferation of naive CD8+ T cells under the same conditions (Figure 6B). Importantly, all DC subsets that received either appropriate TLR stimulation or CD4 help induced naive CD8+ T-cell proliferation, but optimal proliferation required both CD4 help and TLR stimulation (Figure 6B). Interestingly, whereas TLR-9 stimulation alone was sufficient for pDC to acquire priming capacities, mDC1 required combinational TLR stimulation. The higher capacity of TLR-stimulated DC to prime CD8+ T cells was associated with upregulation of MHC class I and CD86 expression as expected (Figure 6C). Thus, all DC subsets can prime CD4+ and CD8+ T cells, and they require TLR stimulation or CD4 help for CD8+ T-cell priming.
TLR stimulation and CD4 help license DC to prime CD8+ T cells. (A-B) Autologous naive CD4+ or CD8+ T cells from peripheral blood were CFSE-labeled and cultured alone or together with allogeneic DC or monocytes subsets in the absence (−) or presence of TLR agonists as indicated. Proliferation was assessed after 7 days by CFSE dilution and is shown as the percentage of divided CD4+ T cells (A) or CD8+ T cells (B). (C) MHC class I (upper panels) and CD86 (lower panels) expression on DC before (filled histograms) or after stimulation (open histograms) with LPS + R848 of mDC1 (left panels), with polyI:C + R848 of mDC2 (central panels) and with CpG of pDC (right panels). One representative donor of 3 is shown. *P < .05; **P < .005; ***P < .0005.
TLR stimulation and CD4 help license DC to prime CD8+ T cells. (A-B) Autologous naive CD4+ or CD8+ T cells from peripheral blood were CFSE-labeled and cultured alone or together with allogeneic DC or monocytes subsets in the absence (−) or presence of TLR agonists as indicated. Proliferation was assessed after 7 days by CFSE dilution and is shown as the percentage of divided CD4+ T cells (A) or CD8+ T cells (B). (C) MHC class I (upper panels) and CD86 (lower panels) expression on DC before (filled histograms) or after stimulation (open histograms) with LPS + R848 of mDC1 (left panels), with polyI:C + R848 of mDC2 (central panels) and with CpG of pDC (right panels). One representative donor of 3 is shown. *P < .05; **P < .005; ***P < .0005.
mDC1 efficiently induce cytotoxic molecules via IL-12
We next analyzed if proliferating naive CD8+ T cells primed by different DC subsets differentiated to cytotoxic T cells, which unlike naive cells secrete IFN-γ and express cytotoxic molecules.
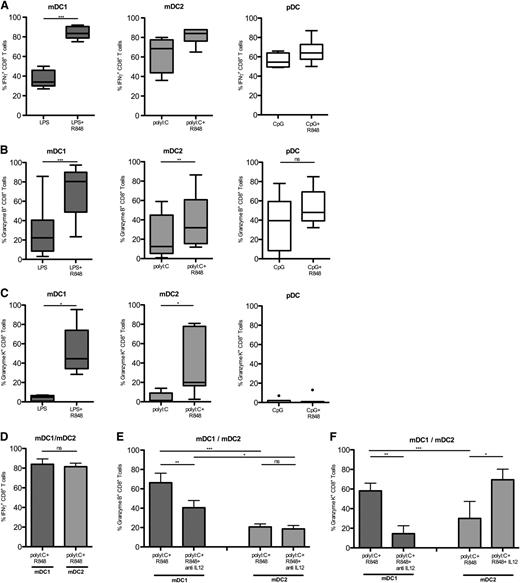
Naive CD8+ T cells that proliferated in response to TLR-stimulated DC subsets differentiated because they acquired IFN-γ–producing capacities (Figure 7A), and expressed cytotoxic molecules (Figure 7B-C). Following combinational TLR stimulation, mDC1 induced very high levels of IFN-γ production as well as Granzyme-B (Figure 7B) and Granzyme-K (Figure 7C). Also, pDC induced IFN-γ–producing capacities and Granzyme-B, but not Granzyme-K. Interestingly, upon optimal stimulation with polyI:C and R848, mDC2 induced IFN-γ production as efficiently as mDC1 (Figure 7D), but they induced significantly lower levels of Granzyme-B and Granzyme-K (Figure 7E-F). Importantly, neutralizing anti–IL-12 antibodies significantly reduced Granzyme-B induction by mDC1, whereas they had no effect on Granzyme-B induced by mDC2 (Figure 7E). Moreover, anti–IL-12 antibodies strongly reduced Granzyme-K expression in mDC1-primed CTL, whereas the addition of physiological amounts of IL-12 induced high levels of Granzyme-K in mDC2-primed CTL (Figure 7F).
Efficient and IL-12–dependent CTL generation by mDC1. Naive CFSE-labeled CD8+ T cells were cocultured for 7 days with allogeneic DC subsets in the presence of TLR agonists. (A) IFN-γ production of proliferating CD8+ T cells was assessed following brief restimulation with phorbol 12-myristate 13-acetate and ionomycin. Shown is the percentage of IFN-γ production among divided CD8+ T cells primed by mDC1 (left panel), mDC2 (central panel), or pDC (right panel), which had been stimulated with the indicated TLR agonists in 9 donors in different experiments. Expression of intracellular Granzyme B (B) or Granzyme K (C) in proliferating CD8+ T cells primed by the indicated DC subsets stimulated with the indicated TLR agonists. (D) Mean percentage of IFN-γ–producing cells among CD8+ T cells that had divided with mDC1 or mDC2 matured with polyI:C and R848. Granzyme-B (E) and Granzyme-K (F) expression in divided naive CD8+ T cells primed by mDC1 or mDC2 matured with PolyI:C and R848 in the absence or presence of neutralizing anti–IL-12 antibodies or 1 ng/mL IL-12 as indicated. Shown is the mean of at least 4 donors in at least 3 experiments. ns, not significant. *P < .05; **P < .005; ***P < .0005.
Efficient and IL-12–dependent CTL generation by mDC1. Naive CFSE-labeled CD8+ T cells were cocultured for 7 days with allogeneic DC subsets in the presence of TLR agonists. (A) IFN-γ production of proliferating CD8+ T cells was assessed following brief restimulation with phorbol 12-myristate 13-acetate and ionomycin. Shown is the percentage of IFN-γ production among divided CD8+ T cells primed by mDC1 (left panel), mDC2 (central panel), or pDC (right panel), which had been stimulated with the indicated TLR agonists in 9 donors in different experiments. Expression of intracellular Granzyme B (B) or Granzyme K (C) in proliferating CD8+ T cells primed by the indicated DC subsets stimulated with the indicated TLR agonists. (D) Mean percentage of IFN-γ–producing cells among CD8+ T cells that had divided with mDC1 or mDC2 matured with polyI:C and R848. Granzyme-B (E) and Granzyme-K (F) expression in divided naive CD8+ T cells primed by mDC1 or mDC2 matured with PolyI:C and R848 in the absence or presence of neutralizing anti–IL-12 antibodies or 1 ng/mL IL-12 as indicated. Shown is the mean of at least 4 donors in at least 3 experiments. ns, not significant. *P < .05; **P < .005; ***P < .0005.
In summary, although all DC can induce IFN-γ production upon CD8+ T-cell priming, mDC1 induce the highest levels of cytotoxic molecules because they produce high amounts of IL-12.
Discussion
BDCA-3+ DC (mDC2) are thought to be the human equivalent of murine CD8α+ DC, which are characterized by the production of high levels of IL-12 and their abilities to cross-prime CD8+ T-cell responses. We report here that human conventional mDC1, but not BDCA-3+mDC2, are potent producers of bioactive IL-12, can efficiently cross-present antigens, and that they promote cytotoxic CD8+ T-cell responses more efficiently than mDC2.
Human BDCA-3+mDC2 were shown to share several relevant characteristics with CD8α+DCs in the mouse, such as XCR1 and CLEC9A expression and the dependence on the transcription factor BATF3, but inconsistent results were published on the capacities of human DC subsets to cross-present soluble antigens and to secrete IL-12.23,26,31,33,34 Notably, however, there are also some relevant differences between the DC networks in men and mice. For example, murine mDC express TLR9 and secrete IFN-α,19,23 whereas in humans these are exclusive features of pDC.13
We identified here unequivocally conventional CD1c+mDC1 as the most potent human IL-12–producing APCs. Notably, mDC1 both from peripheral blood and tonsils produced IL-12. The high IL-12–producing capacity of mDC1 was missed in several previous studies, because it is tightly controlled and absolutely requires combinational stimulation of TLRs.33 The low amounts of IL-12 (<50 pg/mL) produced by mDC2 that we detected here are consistent with the low levels of mDC2-derived IL-12 reported previously.23,26,31 However, we detected IL-12 production by mDC2 only in response to polyI:C in combination with R848 and IFN-γ. Under this condition, mDC2 secreted very high amounts of IFN-λ, whereas mDC1 produced substantial amounts of IL-12. Thus, the characteristic cytokine of mDC2 in response to polyI:C is IFN-λ,29 and not IL-12. Because IFN-λ is induced upon TLR3 stimulation by double-stranded RNA and mediates antiviral protection, mDC2 might be particularly relevant to fight viruses that are not efficiently recognized by pDC, such as the hepatitis C virus.40,41 IL-12 production by mDC1 was boosted by IFN-γ and CD40L, showing that mDC1 have a similar regulation of IL-12 as human monocyte-derived DC and murine CD8α+DC.38 A notable exception to this rule is the negative effect of IL-4 on IL-12 produced by mDC1, because IL-4 paradoxically enhances IL-12 production in other DC.42 Moreover, in vitro–differentiated monocyte-derived DC can secrete even higher levels of IL-12.33 Nevertheless, because mDC1 efficiently prime naive T cells upon TLR stimulation, they are probably the relevant source of IL-12 in primary human T-cell responses in vivo.
CD8α+ DC in mice are considered to be the principal DC subset to cross-prime cytotoxic T-cell responses,17,18,43 although contributions from other DC subsets have also been reported under certain conditions.11,39,44 Notably, cross-priming and secondary expansions of CD8+ T cells in mice critically depends on CD4 help that is mediated largely by CD40/CD40L interactions.4,45-47 The role of CD4 help on human cytotoxic T-cell responses has been much less investigated, but given the strong evidence in mice it is considered to be relevant.48
We defined here for the first time the different stimulation requirements of in vivo–occurring human DC subsets to prime cytotoxic T-cell responses. We showed that DC maturation by TLR agonists and licensing by CD4+ T cells determined the capacity of all DC subsets to induce proliferation and differentiation of naive CD8+ T cells. As expected, this enhanced capacity of TLR-stimulated DC to stimulate CD8+ T cells was associated with an enhanced expression of MHC class I and of co-stimulatory molecules. Interestingly, priming of CD4+ T cells was quite efficiently induced by immature mDC subsets, consistent with the view that CD8+ T-cell priming has more stringent requirements.
Previous reports suggested that mDC2 have an higher intrinsic capacity to cross-present antigens in particular from necrotic cells,26 which might be particularly relevant for tolerance induction under steady-state conditions.49-51 Our findings that immature mDC2 could activate CMV-specific CD8+ memory T cells in the presence of soluble antigens in some donors is consistent with this notion. Nevertheless, efficient cross-presentation by both mDC1 and mDC2 required combinational TLR stimulation, possibly explaining inconsistent results of previous studies. Combinational TLR stimulation was shown to be important for IL-12 production,33 and we found here that it is also required for cross-presentation and CTL priming by mDC. This requirement for synergistic stimulation of surface and intracellular pathogen-sensing receptors might license DC that have taken up microbes, and consequently contain the highest levels of relevant antigens,38 to cross-prime cytotoxic T cells.
Consistent with our findings, 2 very recent studies showed that all 3 DC subsets can cross-present antigens if they are derived from tonsils or if antigen is delivered via appropriate surface receptors.52,53 Importantly, we identified here the different signals that induce cross-presentation in vitro and that probably mimic the stimuli that were received by tonsillar DC in vivo. Interestingly, mDC1 performed best upon TLR-4 plus TLR-8 stimulation, whereas mDC2 required agonists of TLR-3 and TLR-8. Given these subset-specific requirements, different adjuvants of protein vaccines could selectively license mDC1 or mDC2 to cross-prime cytotoxic T-cell responses.48 However, because mDC1 are much more abundant and efficiently induce CTL expressing high levels of cytotoxic molecules, they are more promising candidates than mDC2. Moreover, because we found that pDC express lower levels of MHC class I and CD86 are inefficient to cross-present antigens and fail to induce Granzyme-K, pDC are probably also less suited for antitumor vaccines than mDC1.12
In summary, although BDCA-3+mDC2 share many relevant characteristics, including IFN-λ secretion, with murine CD8α+DC, our findings also show some species-specific aspects of DC biology that have to be considered when results from mouse models are translated into the clinics. In particular, the tightly regulated capacities of conventional CD1c+DC to secrete high amounts of IL-12, cross-present antigens, and prime cytotoxic T cells have important implications for the design of human vaccines.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank P. Jähn (Miltenyi Biotech, Bergisch Gladbach, Germany) and A. Lanzavecchia (Institute for Research in Biomedicine, Bellinzona, Switzerland) for reagents and helpful discussion. The authors also thank M. Lösch and H. Kienapfel for kindly providing access to bone marrow samples.
This work was supported by the Deutsche Forschungsgemeinschaft, the Romeo and Enrica Invernizzi Foundation, and the Cariplo Foundation.
Authorship
Contribution: G.N., J.K., A.W., S.S., P.G., and A.B. performed research and analyzed data; B.S., M.M., and M.C. assisted with research; K.S., S.T., L.P., and C.S. provided clinical samples; F.F., C.R., P.N., R.D., and S.A. provided advice and assisted in the writing of the manuscript; and J.G. designed the study, analyzed the data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jens Geginat, INGM, Via Francesco Sforza 35, 20122 Milano, Italy; e-mail: geginat@ingm.org.
References
Author notes
J.K., A.W., and S.S. contributed equally to this study.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal