Key Points
PAD4-mediated chromatin decondensation and release by neutrophils exacerbate injury after MI/R.
Combining reduction of neutrophil recruitment with extracellular DNA cleavage could be a new approach to reduce cardiac damage after MI.
Abstract
Innate immune cells play a major role in the early response to myocardial ischemia/reperfusion (MI/R) injury. Recombinant human ADAMTS13 (rhADAMTS13), cleaving von Willebrand factor (VWF), reduces leukocyte recruitment in mice. Death of cardiomyocytes and the possible formation of neutrophil extracellular traps (NETs) may result in chromatin release that is prothrombotic and cytotoxic. We investigated the pathophysiological role of extracellular chromatin during MI/R to evaluate the therapeutic potential of targeting extracellular DNA and VWF by using DNase I with/without rhADAMTS13. Finally, we examined the impact of histone citrullination and NETosis by peptidylarginine deiminase 4 (PAD4) on MI/R. We used a 24-hour MI/R mouse surgical model. MI/R injury caused an increase in plasma nucleosomes, abundant neutrophil infiltration, and the presence of citrullinated histone H3 at the site of injury. Both monotherapies and coadministration of DNase I and rhADAMTS13 revealed a cardioprotective effect, resulting in subsequent improvement of cardiac contractile function. PAD4−/− mice, which do not produce NETs, were also significantly protected from MI/R and DNase I treatment had no further beneficial effect. We demonstrate that extracellular chromatin released through NETosis exacerbates MI/R injury. Targeting both VWF-mediated leukocyte recruitment and chromatin removal may be a new therapeutic strategy to reduce ischemia-related cardiac damage.
Introduction
Cardiovascular disease is a growing health care problem, is the leading cause of morbidity and mortality worldwide, and is projected to account for 25 million deaths annually by 2030.1 Acute myocardial infarction (AMI) is one of the major clinical manifestations of cardiovascular disease, caused by intraluminal coronary thrombosis due to a disrupted atherosclerotic plaque.2 Current clinical guidelines advocate early reperfusion as the cornerstone of AMI management.3 Both thrombolytic therapy and/or primary percutaneous coronary intervention aim to limit the extent of myocardial damage.4 Nevertheless, a large body of experimental and clinical evidence suggests that reperfusion per se can further aggravate myocardial injury upon restoration of blood flow through the obstructed coronary arteries.5,6 Therefore, novel cardioprotective therapeutic strategies are needed to minimize the ischemia-reperfusion (I/R)–induced myocardial damage and improve clinical outcomes.
Infarct size is directly associated with long-term prognosis in patients with AMI.7 There are various mechanisms that contribute to the final myocardial infarct size including oxidative stress, inflammation, thrombosis, cardiomyocyte calcium overload, rapid restoration of pH, and others.8,9 Neutrophils and proinflammatory monocytes are actively recruited to the site of infarction shortly after ischemia occurs, thus mediating the early responses to tissue injury. Besides their phagocytic properties, both neutrophils and monocytes promote the release of reactive oxygen species, numerous inflammatory mediators, and proteolytic enzymes, all of which may mediate deleterious effects in the postischemic myocardium.10,11 As a result of the ongoing cell death during AMI, a substantial amount of cell-free DNA and histones are released into the circulation.12 During hyperactivation, neutrophils can expel nuclear chromatin via a distinct cell death pathway, known as neutrophil extracellular trap (NET) formation. NETs are composed of chromatin covered by neutrophil granule proteins (elastase, cathepsin G, and myeloperoxidase [MPO]) and cytoplasmic proteins.13,14 Although the process of NET formation (NETosis) is likely designed to operate as a host defense mechanism against bacterial infections, experimental studies indicate that extracellular DNA traps are involved in the pathogenesis of several clinical conditions such as thrombosis, sepsis, and lung injury.15-20 In fact, NETs are observed in coronary thrombi removed from patients with AMI.21 Extracellular chromatin and histones exacerbate tissue injury in experimental models of cerebral and hepatic I/R,22,23 and impair the clearance of apoptotic neutrophils,24 a process pivotal to the resolution of inflammation.25,26 Histone citrullination by peptidylarginine deiminase 4 (PAD4) is a posttranslational histone modification process, necessary for chromatin decondensation during NET formation.27-30 PAD4−/− mice were generated and shown to lack NETosis in vitro and in vivo.29 Our recent work also indicates that PAD4-mediated release of NETs is a crucial mechanism in the pathogenesis of venous thrombosis.31
We hypothesized that extracellular DNA, chromatin, and NETs, released during myocardial ischemia, could exacerbate I/R-induced myocardial injury. Combined pharmacologic approaches targeting extracellular chromatin formation and limiting neutrophil-mediated tissue injury are of great clinical interest. Recent findings reveal potent antithrombotic and cytoprotective effects of deoxyribonuclease I (DNase I), whose primary role is to degrade DNA,19,22,32 and of ADAMTS13 (a disintegrin and metalloproteinase with a thrombospondin type-1 motif, member 13),33-35 the enzyme responsible for the proteolytic degradation of von Willebrand factor (VWF). ADAMTS13-mediated cleavage of VWF has a protective anti-inflammatory effect during myocardial ischemia/reperfusion (MI/R) in mice, and ADAMTS13 deficiency exacerbates MI injury in a VWF-dependent manner by increasing neutrophil infiltration.33,36,37 We therefore sought to investigate the effects of DNase I monotherapy vs combination therapy with ADAMTS13 on MI/R injury and their impact on postischemic cardiac function in mice. Given the essential role of PAD4 in NETs formation and thrombosis, we further used PAD4−/− mice to examine PAD4 involvement during myocardial infarction.
Materials and methods
Mice
Wild-type (WT) C57Bl/6J mice were purchased from The Jackson Laboratory. PAD4−/− mice were on a C57Bl/6J background and were backcrossed for at least 8 generations (supplemental Materials and methods, available on the Blood Web site). All animals used in this work were males, 8 to 10 weeks old. All experimental procedures were reviewed and approved by the Institutional Animal Care and Use Committee of Boston Children’s Hospital (protocol no. 11-03-1919).
Induction of myocardial ischemia
An “open-chest” mouse surgical model of 24 hours MI/R injury (1 hour occlusion of the left anterior descending artery [LAD] followed by 23 hours reperfusion) was performed under electrocardiogram (ECG) control using a 3-lead ECG system (Powerlab 4/30 with Labchat 7 software; AD Instruments) as previously described33 (supplemental Materials and methods).
DNase I and rhADAMTS13 treatment
Treatment with recombinant human DNase I (Pulmozyme; Genentech) was performed after 1 hour of LAD occlusion and repeated 11 hours after reperfusion. DNase I was injected by an intraperitoneal administration of 50 μg and an intravenous administration of 10 μg. For vehicle treatment, Pulmozyme buffer (8.77 mg/mL sodium chloride and 0.15 mg/mL calcium chloride) was diluted in sterile saline and administered in the same way. Preparation of recombinant human ADAMTS13 (rhADAMTS13; kindly provided by F. Scheiflinger and H. Rottensteiner, Baxter Bioscience) has been previously described.33 Treatment with rhADAMTS13 was carried out 1 hour after LAD occlusion via retro-orbital intravenous administration at a dose of 3460 U/kg.34 Combination therapy with DNase I and rhADAMTS13 was performed immediately after 1 hour ischemia in the same doses and routes of administration as described above for monotherapy with DNase I or rhADAMTS13.
Assessment of infarct size
After 23 hours of reperfusion, mice were sacrificed and the hearts were harvested. The hearts were then sectioned transversely into four 2-mm slices, starting from the site of LAD ligation. Each ventricular section was incubated with 1.0% 2,3,5-triphenyltetrazolium chloride (TTC; Sigma-Aldrich) for 15 minutes at 37°C and the amount of infarcted area (percentage of left ventricular)) was calculated as previously described33 (supplemental Materials and methods).
Echocardiography
Cardiac function and heart dimensions were measured in healthy 8- to 10-week-old male WT and PAD4−/− mice (control) and in mice subjected to 24 hours of MI/R injury followed by treatment. Images were obtained using Vevo 2100 (Visual Sonics) ultrasound at the Small Animal Imaging Laboratory at Boston Children’s Hospital. The mice were anesthetized using 1.5% isoflurane and 4 limbs were attached to the corresponding ECG electrodes. M-mode of the parasternal short-axis view was used to evaluate left ventricular (LV) internal dimension, LV interventricular septum, and LV posterior wall at end diastole and end systole. Echocardiograms were stored digitally and ejection fraction (EF; percentage) was calculated using Vevostrain software. An investigator blinded to the study groups performed echocardiography and data analysis.
Plasma collection
For blood collection, mice were anesthetized using 3.5% isoflurane. Blood was collected from the retro-orbital sinus using 0.5M EDTA (1 volume to 100 volumes of blood). Platelet-poor plasma was prepared immediately after blood collection by centrifuging anticoagulated whole blood for 5 minutes at 2300g. Plasma supernatant was carefully removed and centrifuged again for 10 minutes at 16 100g to remove any remaining blood cells. Plasma samples were immediately stored at −80°C until analysis.
Determination of plasma nucleosome level
Nucleosome levels were measured using the Cell Death Detection ELISAPLUS kit (Roche) in plasma samples from 24-hour sham-operated animals and all experimental groups after 24 hours of MI/R. The assay allows relative quantification of histone-complexed DNA fragments (mono- and oligonucleosomes). Results are presented as fold increase for each mouse over the baseline levels measured before MI/R (or sham) procedure.
Immunologic assays
Mouse hearts were harvested after 24 hours of MI/R, infarct size measured (percentage of LV), and then they were incubated with a zinc fixative overnight. Four heart samples from each mouse were processed through graded alcohols and xylene, embedded in paraffin, and sectioned into 8-μm sections. Tissue sections were stained with hematoxylin and eosin for morphologic evaluation, and immunostained. Immunohistochemistry for anti-mouse monoclonal Gr-1 (Ly-6G and Ly-6C) antibody (dilution 1:500, clone RB6-8C5; BD Pharmingen) was used to determine the origin of cells infiltrating infarcted myocardium.33 For immunofluorescence analysis, rabbit polyclonal antibody to citrullinated histone H3 (H3cit) (dilution 1:1000, citrulline 2+8+17; Abcam) was used as first antibody. The number of Gr-1+ and H3cit+ cells were quantified and expressed per 1 mm2 of infarcted area. Double immunofluorescence staining was performed using antibodies for Gr-1 and H3cit (supplemental Materials and methods).
Blood clotting and platelet aggregation
We measured the effect of DNase I on ex vivo blood clotting (time to and rate of fibrin generation) and platelet aggregation (maximal aggregation amplitude and aggregation rate) (supplemental Materials and methods; supplemental Figure 1).
Neutrophil isolation and treatment with rhADAMTS13
Peripheral neutrophils were isolated from male WT mice16 and treated with the equivalent of 3460 U/kg rhADAMTS13 (0.0346 U/μL) or equal volume of vehicle for 15 minutes. Cells were then stimulated using 4-μM ionomycin (Invitrogen) and quantification analysis of NETs was performed (supplemental Materials and methods).
Statistical analysis
Data are expressed as mean ± SEM. Data sets were assessed for normality using the Kolmogorov-Smirnov test or the Bartlett test for homogeneity of variance. Data were compared using the unpaired 2-tailed t test or 1-way analysis of variance, followed by the Newman-Keuls post hoc test for multiple comparisons. In cases of nonnormal distribution, nonparametric tests such as the Mann-Whitney or Kruskall-Wallis test with Dunn post hoc analysis were used as appropriate. Statistical analysis was carried out using Prism (Version 5.00; GraphPad Software Inc.). P values < .05 were considered statistically significant.
Results
Coadministration of DNase I with rhADAMTS13 reduces the size of myocardial infarction
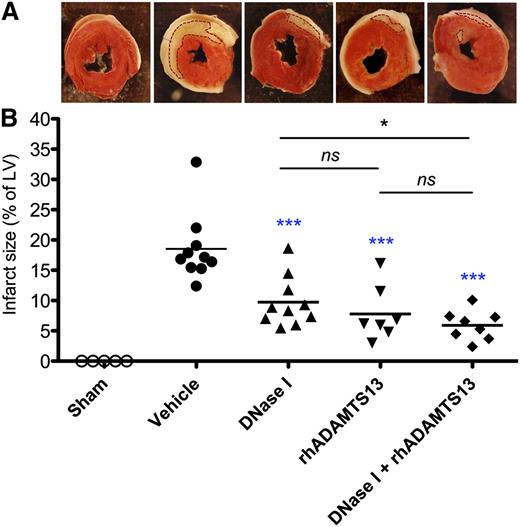
We evaluated the effects of DNase I, rhADAMTS13, or a combination of DNase I and rhADAMTS13 on MI/R-induced injury after 1 hour of LAD occlusion and 23 hours of reperfusion. DNase I did not affect blood clotting and platelet aggregation ex vivo (supplemental Figure 1). We found that either monotherapy with DNase I or rhADAMTS13 significantly reduced the infarct size compared with mice receiving vehicle (9.73% ± 1.31% or 7.77% ± 1.70% vs 18.53% ± 1.78%; P < .001; Figure 1). Of interest, coadministration of DNase I with rhADAMTS13 resulted in a significantly smaller infarcted area (5.90% ± 0.86%) than mice treated with DNase I alone (P < .05) or with vehicle (P < .001) (Figure 1). All sham-operated mice, which underwent the same surgery without LAD occlusion, showed negative results for TTC staining. Taken together, these data indicate that DNase I, rhADAMTS13, and their coadministration all have a protective role in MI/R injury.
Infarct volumes after LAD occlusion following reperfusion in sham-operated mice, mice treated with vehicle, DNase I, rhADAMTS13, or a combination of DNase I with rhADAMTS13. (A) Representative TTC stains of transverse sections of the LV of the 5 experimental groups. Infarctions appear as white and are outlined with dotted lines. The surrounding white layers represent the pericardium and were not included in the analysis. (B) Myocardial infarct size (expressed as % of LV) measured at 24 hours after LAD occlusion. *P < .05, ***P < .001 (blue asterisks represent comparison with vehicle; black, between treated groups).
Infarct volumes after LAD occlusion following reperfusion in sham-operated mice, mice treated with vehicle, DNase I, rhADAMTS13, or a combination of DNase I with rhADAMTS13. (A) Representative TTC stains of transverse sections of the LV of the 5 experimental groups. Infarctions appear as white and are outlined with dotted lines. The surrounding white layers represent the pericardium and were not included in the analysis. (B) Myocardial infarct size (expressed as % of LV) measured at 24 hours after LAD occlusion. *P < .05, ***P < .001 (blue asterisks represent comparison with vehicle; black, between treated groups).
Combination therapy with DNase I and rhADAMTS13 improves cardiac function
We then studied the effects of DNase I and rhADAMTS13 on cardiac function, which had not been previously evaluated. Echocardiography revealed a significant decrease in EF after 24-hour MI/R in all studied groups (Figure 2A). Vehicle-treated mice demonstrated significantly impaired LV EF as compared with the control (no MI) group (57.18% ± 1.83% vs 72.73% ± 0.60%; P < .001; Figure 2A). Both monotherapy with DNase I (65.51% ± 1.64%) or rhADAMTS13 (62.40% ± 1.25%) significantly improved EF as compared with vehicle-treated animals (P < .01 for DNase I treatment and P < .05 for rhADAMTS13; Figure 2A). The combination of DNase I with rhADAMTS13 (67.93% ± 1.68%) showed a significantly better effect on improvement of cardiac function, as compared with rhADAMTS13 administration alone (P < .05) or vehicle treatment (P < .001) (Figure 2A). Using echocardiography, we did not observe any structural differences or changes in heart dimensions among all experimental groups (supplemental Figure 2).
Monotherapy and combination treatment of DNase I and rhADAMTS13 improve cardiac function after 24 hours of MI/R. (A) Echocardiography analysis displayed a significant decrease in EF (%) in all treated groups of mice after 24 hours MI/R in contrast to control group (##P < .01; ###P < .001; # represents comparison with control). EF decreased significantly less in all groups of treated mice as compared with vehicle-treated animals, respectively (*P < .05; **P < .01; ***P < .001). (B) Correlation analysis including all experimental groups revealed a significant inverse relationship between the infarct size (% of LV) and EF (%) (Spearman r coefficient = −0.441, P = .027). In contrast to all mice assigned to the vehicle arm of the study (●), mice treated with either DNase I monotherapy (▲), rhADAMTS13 (▼), or combination therapy (♦) showed a significantly smaller myocardial infarction after MI/R injury and markedly improved LV systolic function.
Monotherapy and combination treatment of DNase I and rhADAMTS13 improve cardiac function after 24 hours of MI/R. (A) Echocardiography analysis displayed a significant decrease in EF (%) in all treated groups of mice after 24 hours MI/R in contrast to control group (##P < .01; ###P < .001; # represents comparison with control). EF decreased significantly less in all groups of treated mice as compared with vehicle-treated animals, respectively (*P < .05; **P < .01; ***P < .001). (B) Correlation analysis including all experimental groups revealed a significant inverse relationship between the infarct size (% of LV) and EF (%) (Spearman r coefficient = −0.441, P = .027). In contrast to all mice assigned to the vehicle arm of the study (●), mice treated with either DNase I monotherapy (▲), rhADAMTS13 (▼), or combination therapy (♦) showed a significantly smaller myocardial infarction after MI/R injury and markedly improved LV systolic function.
Because changes in cardiac pump function are known to reflect myocardial viability after MI/R injury, we performed correlation analysis between the infarct size (percent of LV) and EF (percent), the latter evaluated 23 hours after reperfusion. There was a significant inverse correlation between those 2 parameters (Spearman r coefficient = −0.441, P = .027; Figure 2B), indicating that smaller infarct volumes after MI/R injury were significantly associated with improvement of LV systolic function.
Combination therapy with DNase I and rhADAMTS13 substantially reduces circulating nucleosome levels generated during MI/R
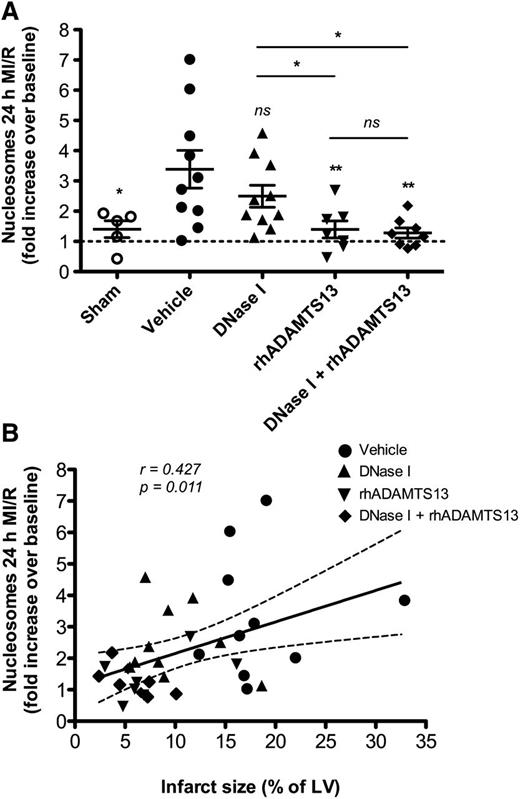
As cell death is directly associated with extracellular DNA/chromatin generation, we measured the nucleosome (histone-DNA fragments) levels in plasma. MI/R-induced injury caused a threefold increase in plasma nucleosome levels over baseline and the levels were significantly higher in vehicle-treated than in sham-operated mice (3.39 ± 0.62 vs 1.40 ± 0.28, respectively; P < .05; Figure 3A). The levels of circulating nucleosomes were significantly lower after MI/R in mice treated with rhADAMTS13 alone or DNase I in combination with rhADAMTS13 (1.40 ± 0.28 and 1.28 ± 0.17, respectively) compared with the vehicle-treated group (P < .01; Figure 3A). Mice on DNase I monotherapy did not show a significant change in plasma nucleosome levels 24 hours after ischemia/reperfusion (2.49 ± 0.36; P = .34; Figure 3A). In correlation analysis, including mice from all experimental groups, we observed a significant positive correlation between the increase in plasma nucleosome levels after MI/R and infarct size (percent of LV) (Spearman r coefficient = 0.427, P = .011; Figure 3B).
Effect of applied treatments on the level of nucleosomes in plasma. (A) Treatment with rhADAMTS13 and its coadministration with DNase I significantly decreased the levels of circulating nucleosomes in plasma 24 hours after MI/R. Nucleosome levels are expressed as a fold increase over the baseline (*P < .05, **P < .01, as compared with vehicle-treated mice). (B) This graph depicts a significant positive association between plasma nucleosome levels and the degree of cardiac damage (infarct size, % of LV) among mice from all experimental groups.
Effect of applied treatments on the level of nucleosomes in plasma. (A) Treatment with rhADAMTS13 and its coadministration with DNase I significantly decreased the levels of circulating nucleosomes in plasma 24 hours after MI/R. Nucleosome levels are expressed as a fold increase over the baseline (*P < .05, **P < .01, as compared with vehicle-treated mice). (B) This graph depicts a significant positive association between plasma nucleosome levels and the degree of cardiac damage (infarct size, % of LV) among mice from all experimental groups.
DNase I alone and more so in combination with rhADAMTS13 reduces neutrophil infiltration and presence of H3cit in the ischemic myocardium
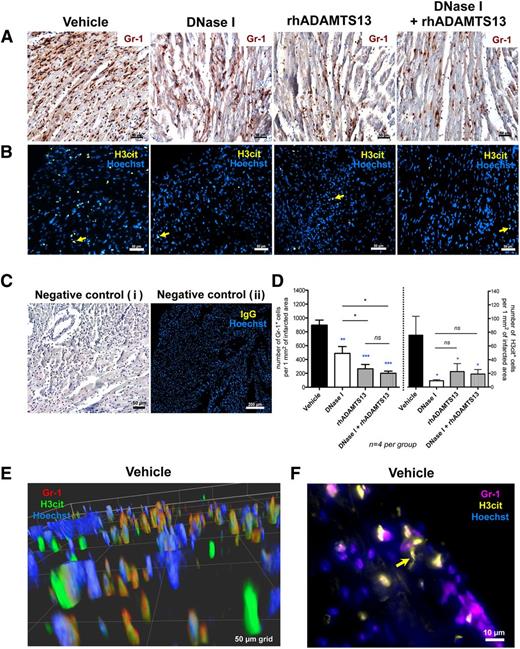
To provide a better understanding of the cardioprotective mechanisms involved, we looked for a possible anti-inflammatory effect of the agents used in this study. Because MI/R injury caused an increase in the levels of circulating nucleosomes, we investigated the presence of citrullinated histones in the infarcted myocardium that could be indicative of NETosis. Heart sections of MI/R mice treated with vehicle, DNase I, rhADAMTS13, or combination therapy were stained for Gr-1 and H3cit, respectively. In heart sections of mice receiving vehicle treatment, we found numerous Gr-1–positive cells, as observed previously,33 recognized as neutrophils or a subset of monocytes infiltrating the infarcted myocardium (Figure 4A). Monotherapy with DNase I significantly decreased the number of infiltrating neutrophils as compared with vehicle-treated mice (488.9 ± 97.8 vs 894.1 ± 74.3 cells per 1 mm2 of infarcted area; P < .01; Figure 4D, left graph). Monotherapy with rhADAMTS13 (265.3 ± 61.8 cells per 1 mm2 of infarcted area) and combination treatment of DNase I with rhADAMTS13 (201.4 ± 30.1 cells per 1 mm2 of infarcted area) displayed a more robust anti-inflammatory effect in comparison with DNase I- (P < .05) or vehicle-treated (P < .001) mice (Figure 4D, left graph).
Treatment with DNase I and rhADAMTS13 significantly reduces leukocyte infiltration and H3cit accumulation in the infarcted area. (A) Immunohistochemical staining revealed abundant presence of Gr-1–positive cells (brown) infiltrating the ischemic myocardium in vehicle-treated mice and significantly less in heart sections of mice treated with DNase I monotherapy, rhADAMTS13 monotherapy, or DNase I in combination with rhADAMTS13. (B) Immunofluorescence analysis showed H3cit-positive cells in the infarcted area (yellow arrows). Numerous H3cit-positive cells were found in the vehicle-treated group, whereas only a few H3cit-expressing cells were found after treatment with DNase I, rhADAMTS13, or a combination of DNase I and rhADAMTS13. (C) Incubation with only secondary antibody served as a negative control (i). Negative control was performed using specific IgG instead of primary antibody (ii). (D) Numbers of cells positive for Gr-1 (left) and H3cit (right) were counted (4 mice per group) and presented as positive cells per 1 mm2 of infarcted area, respectively (*P < .05, **P < .01, ***P < .001; blue asterisks represent comparison with vehicle, and black between treated groups). (E) Representative deconvolved 3D image of double staining for Gr-1 (red) and H3cit (green) of the infarcted myocardium in a vehicle-treated mouse. Most H3cit-positive cells are associated with Gr-1 antigen. (F) Double immunofluorescence analysis for Gr-1 (purple) and H3cit (yellow). Yellow arrow indicates NET-like H3cit-positive structure. Scale bars: 50 μm (A-B, Ci), 200 μm (Cii), 50-μm grid (E), 10 μm (F).
Treatment with DNase I and rhADAMTS13 significantly reduces leukocyte infiltration and H3cit accumulation in the infarcted area. (A) Immunohistochemical staining revealed abundant presence of Gr-1–positive cells (brown) infiltrating the ischemic myocardium in vehicle-treated mice and significantly less in heart sections of mice treated with DNase I monotherapy, rhADAMTS13 monotherapy, or DNase I in combination with rhADAMTS13. (B) Immunofluorescence analysis showed H3cit-positive cells in the infarcted area (yellow arrows). Numerous H3cit-positive cells were found in the vehicle-treated group, whereas only a few H3cit-expressing cells were found after treatment with DNase I, rhADAMTS13, or a combination of DNase I and rhADAMTS13. (C) Incubation with only secondary antibody served as a negative control (i). Negative control was performed using specific IgG instead of primary antibody (ii). (D) Numbers of cells positive for Gr-1 (left) and H3cit (right) were counted (4 mice per group) and presented as positive cells per 1 mm2 of infarcted area, respectively (*P < .05, **P < .01, ***P < .001; blue asterisks represent comparison with vehicle, and black between treated groups). (E) Representative deconvolved 3D image of double staining for Gr-1 (red) and H3cit (green) of the infarcted myocardium in a vehicle-treated mouse. Most H3cit-positive cells are associated with Gr-1 antigen. (F) Double immunofluorescence analysis for Gr-1 (purple) and H3cit (yellow). Yellow arrow indicates NET-like H3cit-positive structure. Scale bars: 50 μm (A-B, Ci), 200 μm (Cii), 50-μm grid (E), 10 μm (F).
Immunofluorescence analysis indicated a large amount of H3cit-positive cells in the vicinity of the reperfused LAD area (Figure 4B, yellow arrows) in vehicle-treated mice. Quantification analysis revealed that either monotherapy with DNase I or rhADAMTS13 (9.1 ± 1.7 and 22.4 ± 11.7 cells per 1 mm2 of infarcted area, respectively) and combined treatment (19.0 ± 6.6 cells per 1 mm2 of infarcted area) significantly reduced the number of H3cit-positive cells in ischemic myocardium as compared with vehicle-treated mice (75.7 ± 27.6 cells per 1 mm2 of infarcted area) (P < .05; Figure 4D, right graph). Of note, rhADAMTS13 treatment did not induce or inhibit NETosis in isolated peripheral neutrophils in vitro (supplemental Figure 3). Thus, the infusion of rhADAMTS13 reduced the number of H3cit-positive cells at the site of myocardial ischemia due to the known anti-inflammatory effect of this agent.33,35 Double immunofluorescence staining of infarcted myocardium from vehicle-treated mice revealed Gr-1–positive cells (red) with H3cit (green) (Figure 4E), and H3cit-positive patterns (yellow) with NET-like morphology in proximity of purple Gr-1–positive cells (Figure 4F, yellow arrow).
PAD4−/− mice are protected from MI/R injury
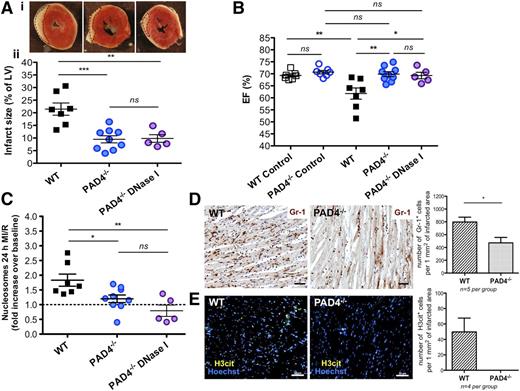
To understand the impact of histone citrullination, chromatin decondensation, and NETosis on MI/R injury, we performed experiments using PAD4−/− mice.29 It has been shown that PAD4 is required for histone citrullination, chromatin decondensation, and NETs formation.28 We found that after 24 hours of MI/R, PAD4−/− mice had significantly smaller infarction areas as compared with WT mice (9.47% ± 1.40% vs 21.46% ± 2.41%, respectively; P < .001; Figure 5A). Measurement of LV EF (%) in healthy WT and PAD4−/− mice showed no difference between these groups (69.31% ± 0.65% and 70.70% ± 0.73%, respectively; control in Figure 5B). Postischemic contractile cardiac function was significantly better in PAD4−/− mice as compared with WT (69.89% ± 1.03% vs 61.79% ± 2.34%, respectively; P < .01), and post-MI EF (percent) in PAD4−/− mice was comparable to the healthy controls (Figure 5B). The level of circulating nucleosomes generated during MI/R in PAD4−/− mice was comparable to baseline and was significantly lower after MI/R than in the WT group (1.20 ± 0.13-fold vs 1.83 ± 0.21-fold increase over baseline, respectively; P < .05; Figure 5C). Of interest, in contrast to WT, treatment with DNase I showed no beneficial effect in PAD4 mice on MI size (9.81% ± 1.51%; Figure 5A), EF (69.29% ± 1.34%; Figure 5B), and circulating nucleosome levels (0.79- ± 0.19-fold increase over baseline; Figure 5C). This indicates that in WT mice DNase I primarily clears chromatin generated by PAD4-mediated decondensation, ie, NETs.
PAD4−/−mice are protected from MI/R injury and their protection is not enhanced by DNase I treatment. (A) (i) Representative TTC stains of transverse sections of the LV 24 hours after MI/R of WT (left), PAD4−/− mice nontreated with DNase I (middle), and after DNase I infusion (right). Infarcted areas appear as white and are outlined with dotted lines. (ii) Myocardial infarction size in the studied groups (measured as % of LV), established at 24 hours after LAD occlusion (**P < .01, ***P < .001). A portion of PAD4−/− mice were treated with buffer as a control for DNase I infusion, but because we did not see a difference between buffer-treated and nontreated PAD4−/− mice, the PAD4−/− data were combined (blue solid circle). (B) No difference was observed in LV ejection function between healthy (control) WT and control PAD4−/− mice (blue open circle). Echocardiography analysis performed after 24-hour MI/R revealed a significantly higher EF (%) in PAD4−/− mice nontreated with DNase I (blue solid circle) as compared with WT group (**P < .01). DNase I infusion to PAD4−/− mice (purple circle) did not ameliorate cardiac function as compared with non-DNase I–treated PAD4−/− mice. (C) The level of circulating nucleosomes (fold increase over the baseline) remained unchanged both in PAD4−/− mice with or without DNase I treatment 24 hours after ischemia onset and was significantly lower as compared with WT mice (*P < .05, **P < .01). (D) Representative Gr-1 immunohistochemical staining of ischemic areas in WT and PAD4−/− mice. PAD4−/− mice showed significantly fewer Gr-1–positive cells infiltrating the infarcted myocardium than WT (*P < .05). (E) Immunofluorescence analysis revealed H3cit-positive cells in WT mice, whereas none were found in the PAD4−/− mice. Scale bars, 50 μm (D-E).
PAD4−/−mice are protected from MI/R injury and their protection is not enhanced by DNase I treatment. (A) (i) Representative TTC stains of transverse sections of the LV 24 hours after MI/R of WT (left), PAD4−/− mice nontreated with DNase I (middle), and after DNase I infusion (right). Infarcted areas appear as white and are outlined with dotted lines. (ii) Myocardial infarction size in the studied groups (measured as % of LV), established at 24 hours after LAD occlusion (**P < .01, ***P < .001). A portion of PAD4−/− mice were treated with buffer as a control for DNase I infusion, but because we did not see a difference between buffer-treated and nontreated PAD4−/− mice, the PAD4−/− data were combined (blue solid circle). (B) No difference was observed in LV ejection function between healthy (control) WT and control PAD4−/− mice (blue open circle). Echocardiography analysis performed after 24-hour MI/R revealed a significantly higher EF (%) in PAD4−/− mice nontreated with DNase I (blue solid circle) as compared with WT group (**P < .01). DNase I infusion to PAD4−/− mice (purple circle) did not ameliorate cardiac function as compared with non-DNase I–treated PAD4−/− mice. (C) The level of circulating nucleosomes (fold increase over the baseline) remained unchanged both in PAD4−/− mice with or without DNase I treatment 24 hours after ischemia onset and was significantly lower as compared with WT mice (*P < .05, **P < .01). (D) Representative Gr-1 immunohistochemical staining of ischemic areas in WT and PAD4−/− mice. PAD4−/− mice showed significantly fewer Gr-1–positive cells infiltrating the infarcted myocardium than WT (*P < .05). (E) Immunofluorescence analysis revealed H3cit-positive cells in WT mice, whereas none were found in the PAD4−/− mice. Scale bars, 50 μm (D-E).
Immunohistochemical analysis on heart sections obtained from PAD4−/− mice revealed significantly less Gr-1–positive cells than in WT mice (471.8 ± 84.7 vs 797.9 ± 75.2 cells per 1 mm2 of infarcted area, respectively; P < .05; Figure 5D). Immunofluorescence analysis for H3cit was negative in PAD4−/− mice, while H3cit-positive cells were found in ischemic myocardium of WT mice (49.7 ± 17.9 cells per 1 mm2 of infarcted area; Figure 5E). These data show that PAD4 deficiency reduced leukocyte recruitment to the infarcted myocardium and prevented nuclear histone citrullination occurring during MI/R injury. Importantly, lack of PAD4 and thus extracellular chromatin release due to NETosis had a cardioprotective effect during the 24 hours in the mouse model of AMI.
Discussion
Ischemia-related cardiomyocyte death (apoptosis and/or necrosis) rapidly triggers cytokine, chemokine, and adhesion molecule expression, resulting in leukocyte recruitment.10 Infiltrating neutrophils and mononuclear cells11 in turn mediate cardiomyocyte injury through the release of reactive oxygen species, proteases (neutrophil elastase and various metalloproteinases), and proinflammatory mediators.10,38 Pronounced delay of neutrophil apoptosis has been shown in patients with acute coronary syndrome, suggesting that neutrophils have enhanced proinflammatory activity during MI.39 Following myocardial ischemia, the membranes of dying cardiomyocytes may be disrupted with a subsequent increase of cell-free DNA in the circulation.12 We also found here that MI/R injury caused an increase in circulating nucleosomes, which correlated with MI size. This observation led us to postulate that extracellular chromatin generated during MI/R may worsen myocardial injury. Our group has previously shown that extracellular chromatin and histones contribute to ischemic stroke progression,22 participate in the process of venous thrombus formation32 and exert powerful prothrombotic effects.31,40,41 Histones are cytotoxic to endothelium in vitro and are lethal in mice.17 Extracellular histones significantly impair the clearance of apoptotic neutrophils by macrophages,24 suggesting that extracellular chromatin may delay the healing process after MI. It was important to specify the origin of released nucleosomal fragments during MI/R. Given the crucial role of neutrophils in the initial response to MI/R injury,10,11,38 we speculated that the extracellular chromatin could be generated through the formation of NETs. This hypothesis is supported by our observation of low plasma nucleosome generation in PAD4−/− mice that do not make NETs as compared with WT after MI (Figure 5C).
NETs are composed of DNA, histones, and specific granule proteins, such as neutrophil elastase and MPO.13,14 Of interest, plasma MPO-DNA complexes, considered a marker of NETs, were recently associated with the severity of coronary atherosclerosis in humans and occurrence of major adverse cardiac events.42 Moreover, neutrophil elastase present on NETs13 catalyzes the breakdown of collagen (type III and IV), fibronectin, and proteoglycans during MI,10 which could further hinder efficient myocardial repair. Histone citrullination is necessary for chromatin decondensation during NET formation.28 We show here that Gr-1–positive cells infiltrating the infarcted myocardium contained H3cit, suggesting an ongoing process of NETosis.28 It is known that the enzyme PAD4 mediates the citrullination and chromatin decondensation during NETosis.28,30 Recently, we reported that neutrophil histone modification by PAD4 is crucial for pathological thrombus formation, whereas PAD4 deficiency does not affect endothelial activation and platelet function.31 Here, we demonstrate that PAD4−/− mice were protected from MI/R injury, resulting in better postischemic cardiac function in comparison with WT mice. We observed a lack of H3cit staining at the site of myocardial injury in the PAD4−/− mice, indicating that other PAD enzymes cannot substitute for PAD4 in this process.
It is reasonable to suggest that chromatin/NET degradation and consequently extracellular histone removal would alleviate cardiac tissue damage after MI. DNase I facilitates rapid and effective chromatin breakdown,43,44 and DNase I treatment protects mice from deep vein thrombosis,32,45 cerebral I/R injury,22 and transfusion-related acute lung injury.19,20 Interestingly, several clinical studies have demonstrated an elevation of serum DNase I activity within 4 hours of the onset of symptoms in MI patients,46,47 suggesting a possible positive role for DNase I after AMI. Indeed, in our study, we show a significant cardioprotective effect of DNase I on MI/R injury. Of note, DNase I did not affect blood clotting and platelet aggregation ex vivo and we have not seen any signs of bleeding in the treated mice. DNase I infusion did not reduce the amount of circulating nucleosomes, but may rather affect DNA size and local concentration of extracellular DNA/histones, thus decreasing the number of H3cit-positive cells in the infarcted myocardium. DNase I treatment had an anti-inflammatory effect as it decreased neutrophil infiltration in the ischemic heart tissue to levels similar to those observed in the infarcted areas of PAD4-deficient mice. Of interest, DNase I infusion no longer had a cardioprotective effect when used for PAD4−/− mice treatment after MI (Figure 5), providing additional evidence that extracellular chromatin originated primarily from NETosis. Thus, lack of NETs during MI/R injury results in significant cardioprotective and anti-inflammatory effects.
NETs15 and histones48 are known to bind VWF, an adhesion molecule for platelets and leukocytes. In mice, ADAMTS13 was found to reduce thrombosis and inflammation35,49 and development of early atherosclerosis.50 We confirm here that mice treated with rhADAMTS13 have significantly smaller MI size33,36,37 and further show better postischemic LV function, as compared with the control group, likely due to the reduction of VWF-mediated leukocyte recruitment. VWF deficiency was also reported to reduce cardiomyocyte apoptosis after MI in mice.36 The number of accumulated neutrophils in the infarct zone has been shown to correlate with the extent of infarction.10 Importantly, we did not see an increased bleeding risk after administration of rhADAMTS13 in mouse models of both stroke34 and MI.33 The anti-inflammatory effect of rhADAMTS13 was significantly stronger than that of DNase I therapy, suggesting that VWF may affect leukocyte recruitment at all stages in the pathogenesis of MI, while DNase I may reduce only the secondary recruitment resulting from chromatin generation. Furthermore, we found that treatment with rhADAMTS13 significantly decreases the level of circulating nucleosomes and H3cit-positive nuclei at the site of MI/R injury, as compared with WT. This is likely due to reduced leukocyte infiltration and/or rhADAMTS13’s anti-inflammatory effects because rhADAMTS13 did not influence NET formation in vitro. Leukocyte activity post-MI has been considered as a therapeutic target for secondary prevention and leukocytosis is positively associated with disease progression.11 We hypothesized that coadministration of rhADAMTS13 together with DNase I could increase the cardioprotective effects of each treatment alone. In fact, combination therapy of rhADAMTS13 with DNase I leads to significantly smaller infarction than DNase I monotherapy and improved cardiac contractile function better than treatment with rhADAMTS13. In other instances, we did not see an additive effect. However, long-term studies are needed to better discriminate the effects of the different therapies on cardiac recovery after MI and prevention of heart failure. In addition, it will be important to develop and test inhibitors of the enzyme PAD4. By preventing NETosis, these inhibitors may much improve outcome after AMI as indicated by our results with the PAD4-deficient animals.
In conclusion, our study demonstrates that extracellular chromatin primarily originating from NETs exacerbates MI/R injury and might consequently affect postischemic cardiac function. We suggest that targeting VWF-mediated leukocyte recruitment by rhADAMTS13, as well as removal of extracellular chromatin generated at the site of infarction, could become a promising new approach in the management of MI.
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Hanspeter Rottensteiner and Friedrich Scheiflinger (Baxter Innovations GmbH, Vienna, Austria) for kindly providing rhADAMTS13, and Lesley Cowan for help with the preparation of the manuscript.
This work was supported by National Heart, Lung, and Blood Institute of the National Institutes of Health grants R01 HL041002 and R01 HL102101 (D.D.W.), and a basic research grant from Baxter Biosciences. Research on the PAD4−/− mouse model was supported by National Cancer Institute grant R01 CA136856 (Y.W.).
J.I.B. is a recipient of a Rubicon fellowship (825.11.019) from the Netherlands Organization for Scientific Research.
Authorship
Contribution: A.S.S. performed experiments, analyzed data, designed research, and wrote the paper; J.I.B., K.M., and A.B. performed experiments and analyzed data; M.G. and L.E. performed experiments; S.F.D.M. and Y.W. provided essential expertise on the mouse model and helpful discussion; and D.D.W. designed research, analyzed data, and cowrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for S.F.D.M. is Laboratory for Thrombosis Research, KU Leuven Kulak, Kortrijk, Belgium.
The current affiliation for A.B. is Centre for Cardiovascular Sciences, Institute of Biomedical Research, College of Medical and Dental Sciences, University of Birmingham, Birmingham, United Kingdom.
Correspondence: Denisa D. Wagner, Program in Cellular and Molecular Medicine, Boston Children's Hospital, 3 Blackfan Circle, 3rd Floor, Boston, MA 02115; e-mail: denisa.wagner@childrens.harvard.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal