Abstract
The inherited bone marrow failure (BMF) syndromes are a rare and diverse group of genetic disorders that ultimately result in the loss of blood production. The molecular defects underlying many of these conditions have been elucidated, and great progress has been made toward understanding the normal function of these gene products. This review will focus on perhaps the most well-known and genetically heterogeneous BMF syndrome: Fanconi anemia. More specifically, this account will review the current state of our knowledge on why the bone marrow fails in this illness and what this might tell us about the maintenance of bone marrow function and hematopoiesis.
Historical timeline
It is now 85 years since the Swiss pediatrician Guido Fanconi first described the case of 3 brothers with aplastic anemia associated with developmental defects.1 It was later found that the condition was inherited as a Mendelian recessive trait, thus setting the ground for the eventual determination of the molecular defect driving “Fanconi anemia” (FA). Over time, a single unifying feature of the disease emerged: cells from afflicted individuals were extremely sensitive to agents that cross-link the 2 strands of DNA together.2,3 Cross-linking chemicals, such as mitomycin C (MMC) and diepoxybutane, are a diverse class of molecules that are potently cytotoxic. Thus, the pivotal observation that cells derived from FA children accumulate broken chromosomes and die rapidly when exposed to cross-linking agents defined a cell autonomous molecular defect underpinning this condition. This linked FA to a failure to resolve a specific class of genomic damage. Armed with cross-linker–induced chromosome breakage as a simple cellular test for FA, clinicians rapidly identified many families throughout the world affected with this illness.4 In 1992, the first FA gene (FANCC) was identified by expression cloning and complementation of a patient-derived cell line.5 However, the coding sequence of the FANCC gene revealed nothing of how it may function to prevent FA; the FANCC polypeptide is completely devoid of any domain signatures. In the decades that followed, we came to realize that FA was genetically heterogeneous, and a worldwide race ensued to identify the many genes that constitute the 16 FA complementation groups.
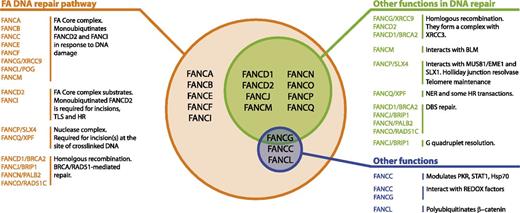
Multifunctionality of the FA proteins. This figure summarizes the known FA proteins and their functions. All FA proteins cooperate in the FA pathway to repair cross-linked DNA. Most of them have additional functions in other DNA repair transactions.12-14 Finally, some of them have been found to modulate cytokine responses or interact with REDOX sensing proteins, independently of their role in DNA repair.15,16,82-84
Multifunctionality of the FA proteins. This figure summarizes the known FA proteins and their functions. All FA proteins cooperate in the FA pathway to repair cross-linked DNA. Most of them have additional functions in other DNA repair transactions.12-14 Finally, some of them have been found to modulate cytokine responses or interact with REDOX sensing proteins, independently of their role in DNA repair.15,16,82-84
At the same time as the complex molecular genetics was unraveling, studies were underway to understand the molecular function of the FA gene products. The key questions to resolve were how the known components prevented the cellular chromosome breakage phenotype and, more challengingly, the clinical phenotype. Early studies indicated that the FANCC protein localized to the cytoplasm.6,7 This unexpected result immediately suggested that this FA protein might not exert a direct influence on DNA repair. It also brought into question whether the chromosomal breakage defect in FA cells was sufficient or indeed the primary reason for bone marrow failure (BMF). Credence for this view came from a body of work indicating that FA patient–derived cells were sensitive to certain cytokines.8,9 This unusual observation could not be easily reconciled with defective DNA repair as the primary reason why the bone marrow fails in FA. More recent studies indicated a more direct role for certain FA proteins in modulating signaling responses10,11 (Figure 112-16 ).
On the other hand, the majority of the FA gene products were shown to form a large nuclear complex, which also includes FANCC, because it was later found that FANCE clearly promotes its import into the nucleus.17 This multiprotein complex modified the key downstream FA gene product FANCD2, by conjugation of a single ubiquitin molecule (monoubiquitination). This critical modification could be stimulated by DNA damage, resulting in the localization of the FANCD2 protein to sites of nuclear DNA damage.18 Further strong support for the view that the primary function of the FA gene products was in the repair of DNA damage came from the eventual identification of mutations in bona fide DNA repair genes in rare FA patient complementation groups.19-21 Thus, 2 hypotheses (ie, defective DNA repair or abnormal cytokine response) emerged as rival and contrasting explanations of why the bone marrow fails in FA.
However, a key limitation was that they both lacked evidence that linked either of them directly to BMF. Subsequently, we set out the merits of these 2 hypotheses in the origins of marrow failure in FA. We review and assess the current state of knowledge and evidence to support each of them in this context. Finally, we discuss recent work, which has identified naturally derived aldehydes as drivers of BMF in FA.
FA gene products have a clear function in DNA repair
All 16 FA gene products clearly function in a common process to maintain genomic stability and to repair DNA interstrand cross-links (ICLs) (Figure 1). The majority of the FA proteins (FANCA, B, C, E, F, G, L, and M) assemble to form a large nuclear complex termed the “FA core complex.”22 The core complex functions as a multi-subunit E3 ubiquitin ligase with FANCL catalyzing the monoubiquitination of FANCD2 and FANCI after DNA damage18,23 (Figure 2). Loss of any one of the components of the core complex leads to its destabilization, abrogation of E3 ligase activity, and therefore, loss of robust FANCD2/FANCI ubiquitination in response to DNA damage. Ubiquitinated FANCD2 and FANCI form a heterodimer that is recruited to chromatin where it participates in the DNA repair process. However, an ICL is a complex lesion, requiring that multiple steps be removed. How then do the FA proteins participate in this removal?
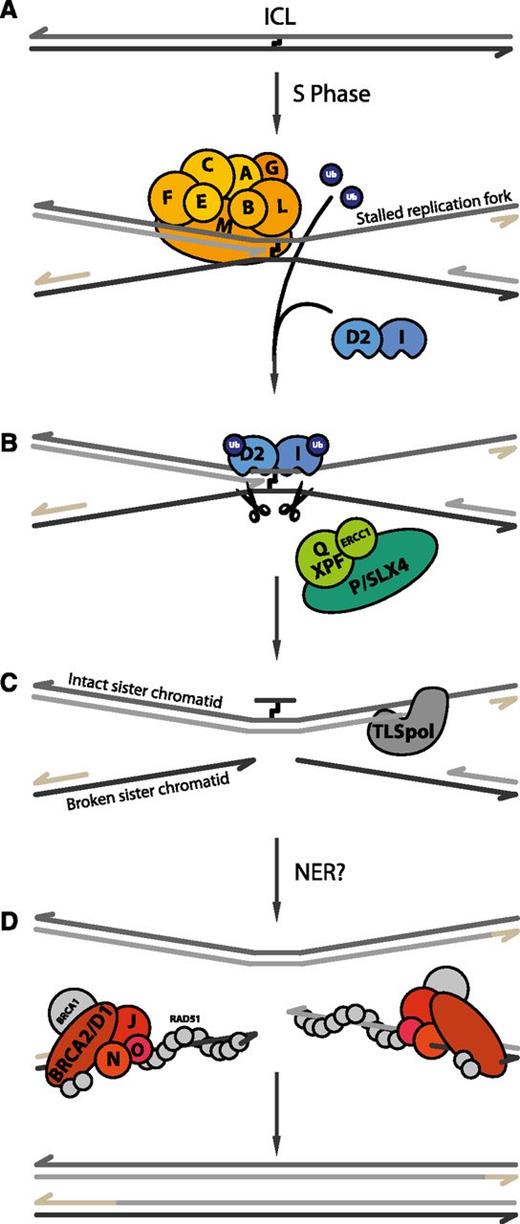
Current model of ICL repair by the FA pathway. (A) When DNA containing an ICL undergoes replication, the leading strands of 2 converging replication forks stop at the lesion. The FA core complex is recruited to chromatin, where it subsequently monoubiquitinates its 2 substrates, FANCD2 and FANCI. (B) Next, the 2 sister chromatids are uncoupled via dual incisions on either side of the ICL, possibly by SLX4-XPF-ERCC1. (C) Subsequently, a translesion DNA polymerase (TLS pol) extends the nascent strand beyond the ICL. (D) Finally, 2 fully repaired DNA duplexes are generated through the action of nucleotide excision repair (NER) on the top duplex and homologous recombination (HR) on the bottom duplex. The remaining FA proteins (FANCD1/BRCA2, FANCO/RAD51C, FANCN/PALB2, and FANCJ/BRIP1) are thought to be involved in these recombination transactions.
Current model of ICL repair by the FA pathway. (A) When DNA containing an ICL undergoes replication, the leading strands of 2 converging replication forks stop at the lesion. The FA core complex is recruited to chromatin, where it subsequently monoubiquitinates its 2 substrates, FANCD2 and FANCI. (B) Next, the 2 sister chromatids are uncoupled via dual incisions on either side of the ICL, possibly by SLX4-XPF-ERCC1. (C) Subsequently, a translesion DNA polymerase (TLS pol) extends the nascent strand beyond the ICL. (D) Finally, 2 fully repaired DNA duplexes are generated through the action of nucleotide excision repair (NER) on the top duplex and homologous recombination (HR) on the bottom duplex. The remaining FA proteins (FANCD1/BRCA2, FANCO/RAD51C, FANCN/PALB2, and FANCJ/BRIP1) are thought to be involved in these recombination transactions.
A comprehensive genetic analysis of DNA cross-link repair was carried out in a tractable genetic system offered by chicken DT40 cells. These studies revealed that the FA proteins must work in cooperation with 3 other key repair processes: excision repair, translesion synthesis, and double-strand break repair through homologous recombination (HR).24,25 The discovery that mutations in bona fide HR genes (like BRCA2) and in components of the excision nuclease machinery (SLX4 and, most recently, XPF) can all result in FA confirmed the observations made in genetic systems.19,20,26,27 However, the order and the regulation of these various processes could not be further defined using such genetic approaches, and the precise mechanism of how the FA proteins participated in the repair of a DNA cross-link remained elusive. The solution of the structure of FANCD2/FANCI provides some insight into this question. This showed that both proteins form a binding interface for both single- and double-stranded DNA; the DNA structures that form when replicating DNA are stalled at a cross-link.28
A key insight into how replication-coupled DNA cross-link repair is achieved came from work using Xenopus egg extracts. In this cell-free system, the replication of a plasmid bearing a single cross-link can be followed with single nucleotide resolution in vitro.29 First, replication stops before reaching the cross-linked DNA. One fork advances up to the cross-link, and at this point, the cross-link is excised. A translesion polymerase synthesizes across the adducted DNA creating an intact duplex strand of DNA.29,30 This duplex is then used to repair the double-strand break using HR.31 When FANCD2 is depleted from the nuclear extract or its monoubiquitination is abrogated, then cross-link repair is impeded because of a failure to initiate the nuclease incision step. This work unequivocally showed that the Fanconi pathway is critical for promoting endonucleolytic cleavage of cross-linked DNA, and that its loss leads to a specific molecular defect in DNA repair. However, our DNA is not normally exposed to nitrogen mustard, the chemical used to create this cross-link, so the question remained as to what the FA DNA repair pathway is repairing in a physiological context.
BMF in FA is attributable to a stem cell defect
Ultimately, most FA patients develop BMF culminating in pancytopenia. Blood counts at birth are typically normal, but at the median age of 7, hematologic complications arise. This initially manifests as low platelet counts, thrombocytopenia, followed by leukopenia before eventually developing into aplastic anemia.32,33 The fact that all blood lineages eventually become deficient strongly implies hematopoietic stem cell (HSC) dysfunction. Indeed, FA patients have very low numbers of CD34+ cells, a bone marrow fraction that is enriched for HSCs and is capable of producing all blood components upon transplantation.34 More recent work has shown significant depletion of the CD34+ fraction in very young FA patients, well before the onset of pancytopenia.35 The very low number of CD34+ cells in young FA patients points to a prenatal origin of the stem cell defect. In fact, knockdown of FA genes in human embryonic stem cells resulted in defective hematopoiesis.36 However, because of the limitations of studying the dynamics of hematopoiesis in humans, numerous labs set out to establish murine models of FA. Although BMF can be induced in these mice with the use of the cross-linking agent MMC,37 these mouse knockouts do not recapitulate the full severity of FA because they do not spontaneously develop the hematologic phenotype.38 In a few instances, mice deficient in the Fanconi pathway show hematologic dysfunction.39,40 However, importantly, FA knockout mice do show quantifiable lower numbers of HSCs coupled with a reduced ability to reconstitute blood production in irradiated recipients after transplantation.41,42 Furthermore, Fancc−/− embryos show reduced numbers of stem and progenitor pools with compromised repopulation activity.43 Collectively, these data suggest that FA patients have an impaired HSC pool. This defect begins in utero, worsens during childhood, and results in spontaneous BMF early in life (Figure 3).
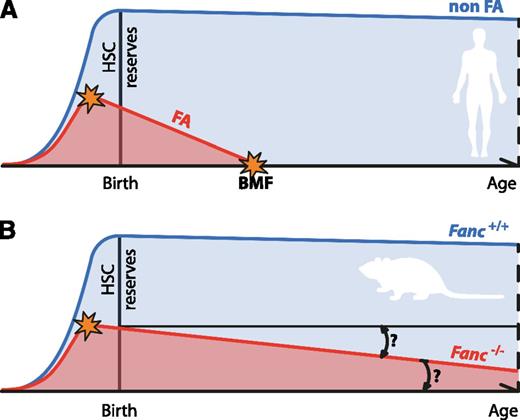
Progressive attrition of HSCs underlies BMF in FA. (A) The progressive decline of HSC numbers in FA patients leads to BMF early in life. The HSC defect is likely to start in utero. (B) Fanconi-deficient mice do not recapitulate the main feature of FA. For this reason, their usefulness as disease models has been questioned. However, FA-deficient mice do display a quantifiable defect in their HSC pool as well as reduced ability to reconstitute blood production in irradiated recipients, which is also present before birth. Even in the absence of BMF, it would be interesting to see if Fanconi-deficient mice display age-dependent attrition in the quality of their HSC pool, as has been observed for other DNA repair–deficient mice.
Progressive attrition of HSCs underlies BMF in FA. (A) The progressive decline of HSC numbers in FA patients leads to BMF early in life. The HSC defect is likely to start in utero. (B) Fanconi-deficient mice do not recapitulate the main feature of FA. For this reason, their usefulness as disease models has been questioned. However, FA-deficient mice do display a quantifiable defect in their HSC pool as well as reduced ability to reconstitute blood production in irradiated recipients, which is also present before birth. Even in the absence of BMF, it would be interesting to see if Fanconi-deficient mice display age-dependent attrition in the quality of their HSC pool, as has been observed for other DNA repair–deficient mice.
Is unresolved DNA damage the initiator of BMF?
Despite the overwhelming genetic and biochemical evidence for the role of FA gene products in repairing DNA cross-links, direct evidence to show that this function is essential to preserve bone marrow function has been lacking. Some circumstantial support comes from the fact that FA patients are cancer prone and blood malignancies from these patients often harbor gross chromosomal changes. Both myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML) are thought to originate at the stem or progenitor cell level, as the longevity and proliferative capacity of these cells renders them susceptible to the accumulation and transmission of mutations. Such cytogenetic changes can only occur as a consequence of misrepair of DNA damage, suggesting that FA patients are prone to genetic instability within their HSC and progenitor compartments.32,44-49
There are other aspects of the FA phenotype that might give us a further clue as to which function of the FA gene products results in the main feature of the human illness. FA is phenotypically heterogeneous; some patients present with a complex spectrum of congenital abnormalities, whereas others only have a mild phenotype. The hematologic features characteristic of the disease also vary; some patients succumb to marrow failure as early as age 3, whereas others might never develop it. With a few exceptions, most attempts at correlating genotype-phenotype have proved unsuccessful so far. However, what is clear is that children with severe developmental abnormalities also present with early onset of BMF.50 This observation, together with the fact that the HSC defect starts in utero, suggests that both developmental abnormalities and the contraction of the stem cell pool could be triggered by the same cause.
One of the main cellular responses to DNA damage is the induction of p53, which then initiates a response that results in cell cycle arrest and, in certain instances, apoptosis. Genetic ablation or deficiency of p53 enables cells to tolerate DNA damage, so it is no surprise that p53 deficiency can partially rescue DNA cross-linker sensitivity in FA-deficient cells. The lack of p53 also accelerates tumor formation in Fancd2−/− and Fancc−/− mice, suggesting that the FA DNA repair pathway and p53 cooperate to suppress tumorigenesis.51,52 Most recently, primary bone marrow cells from FA patients and Fancd2−/− and Fancg−/− mice were shown to have elevated levels of p53, and this was attributable to unresolved DNA damage. Finally, deletion or knockdown of p53 also rescued the defects of FA human and mouse hematopoietic progenitors.35 Although p53 inactivation allows HSCs to survive DNA damage, a note of caution must be sounded: this comes at the enormous cost of enhanced genomic instability and tumor formation. Collectively, these data strongly indicate that unresolved DNA damage in FA cells induces p53, which might then lead to HSC depletion and hence progressive BMF in FA. Knockdown experiments and gene expression analysis initially suggested that the p53-driven HSC elimination was mediated by p21 and cell cycle arrest.35 However, genetic ablation of p21 does not rescue the hematologic phenotype of Fancd2−/− mice, arguing against this approach.53 Although it is possible that the p53 response in HSCs differs between mice and humans, future genetic studies should address the contribution of p53-dependent apoptosis to BMF in FA patients. In support of this, a recent study has demonstrated an apoptotic response to cytokinesis failure in FA-deficient HSCs. It remains to be established whether Fanconi proteins have a distinct role during mitosis or the DNA bridges in FA-deficient cells are a consequence of unresolved DNA damage. However, this is one mechanism of HSC depletion that may contribute to BMF in FA patients.54
The bone marrow is one of the most radiosensitive tissues in the body, being the first organ system to fail following total body irradiation. It is DNA damage to the HSCs that ultimately limits the regeneration of hematopoiesis.55 Genetic evidence for the consequences of DNA damage on HSC function and bone marrow homeostasis is provided by the severe consequences of disrupting many different DNA repair pathways in mice.56-58 Mice bearing null or hypomorphic alleles for genes involved in DNA damage response (ATM−/−, conditional ATR−/−), nonhomologous end joining (Ku70−/−, Ku80−/−, DNA-PKc−/−, DNA-PKcs3A/3A, LigIVY288C/Y288C), HR (Rad50S/S), nucleotide excision repair (XPDTTD), and mismatch repair (Msh2−/−) show severe defects both in the quantity and functional quality of their HSC pools. In some cases, this resulted in spontaneous BMF (DNA-PKcs3A/3A, LigIVY288C/Y288C, Rad50S/S).
It is likely that the main driver of BMF in FA is DNA damage to the HSC pool. Genetic instability within FA-deficient HSCs results in the progressive attrition of this vital cell population or the genesis of neoplastic clones. This notion is supported by the observation that a strong p53 response mediates the FA hematologic phenotype and by the fact that different DNA repair deficiencies, unrelated to FA, also have profound consequences on HSC function.
What is the cause of DNA damage in HSCs?
Should unresolved DNA damage lead to HSC attrition in FA, then a key question is how such damage arises in the physiological setting. Without resolution of this key question, a DNA damage hypothesis for the cause of BMF would be based solely on the circumstantial evidence presented previously. Genomic DNA is intrinsically unstable, being subject to both spontaneous and enzymatic degradation. Moreover, metabolism can generate a battery of reactive molecules that are capable of attacking DNA, the most ubiquitous being reactive oxygen species (ROS).
The first clue that such molecules might contribute to the endogenous burden of DNA damage driving the FA phenotype was the hypersensitivity of patient-derived cell lines. ROS arise in cells as a consequence of normal cellular metabolism, namely, the electron transport chain and lipid peroxidation, but they also have important roles in cell signaling. ROS can damage DNA, RNA, and proteins, and they may also contribute to the aging process. It was demonstrated long ago that the frequency of chromosomal aberrations in FA lymphocyte cultures depends on oxygen tension.59 Since then, many groups have shown that FA-deficient cells grow better under hypoxic conditions than in ambient oxygen concentration.60 More recently, the use of low oxygen tension allowed the generation of FA-deficient iPS lines.61 Superoxide dismutases are a set of enzymes that metabolize superoxide radicals (·O2-) to molecular oxygen and hydrogen peroxide (H2O2), thus providing a defense against oxygen toxicity. Genetic evidence linking oxidative stress to bone marrow dysfunction in FA comes from Sod1−/−Fancc−/− double mutant mice.62 These double knockout mice showed bone marrow hypocellularity and decreased numbers of colony-forming units. However, these mice showed normal numbers of HSCs as seen by flow cytometry and did not display developmental defects or chromosomal aberrations typical of FA.
In search of a source of endogenous DNA damage, our laboratory has focused on simple aldehydes. Small aldehydes such as formaldehyde and acetaldehyde are ubiquitously found in the environment and are also by-products of cellular metabolism. They are also highly reactive, being able to form DNA adducts in vitro and in vivo.63,64 Moreover, FA-deficient cells have been shown to be hypersensitive to both of these compounds and to accumulate double-strand breaks and chromosomal aberrations.65-68 Finally, acetaldehyde treatment induces the activation of the FA pathway in wild-type cells.69
To genetically test if endogenous aldehydes are a source of DNA damage, our laboratory generated mice harboring disruptions of the key FA gene Fancd2 in combination with aldehyde dehydrogenase 2 (Aldh2). Aldh2 oxidizes acetaldehyde to acetate, thereby preventing the accumulation of this genotoxic agent. Therefore, if metabolically produced aldehydes are indeed DNA-damaging agents normally counteracted by the FA pathway, then the simultaneous disruption of both Aldh2 and Fancd2 should have a synergistic effect on the phenotype of these mice.65 Most strikingly, the presence of aldehyde catabolism in the mother was found to be essential for the development of Aldh2−/−Fancd2−/− embryos. Surprisingly, if the mother expressed Aldh2 (Aldh2+/−), then double mutant mice could be born, suggesting that the mother was capable of breaking down acetaldehyde and compensating for the Aldh2 deficiency in the fetus. When born, the Aldh2−/−Fancd2−/− mice had increased prevalence of developmental abnormalities such as kinked tails or eye defects. Most Aldh2−/−Fancd2−/− mice succumbed to acute lymphoblastic leukemia within the first 6 months of life. In an attempt to link acetaldehyde to the phenotype of double knockout mice, these animals were challenged with ethanol, a precursor of acetaldehyde. The treatment not only potentiated the developmental abnormalities but also precipitated myelotoxicity in adult double mutant mice.
Analysis of the hematopoietic compartment of double knockout mice revealed a profound defect in the stem and progenitor cell pool, with a more than 600-fold reduction in their HSC pool.70 It has been known for a long time that HSCs possess high levels of aldehyde dehydrogenase activity, and this was shown, in the case of mouse HSCs, to be attributable to Aldh2. When this protection was taken away, the HSCs were particularly compromised, as Aldh2−/−Fancd2−/− HSCs seemed to be far more sensitive to acetaldehyde than more mature progenitors. This increased sensitivity also correlated with the accumulation of DNA damage, as measured by the induction of γ-H2AX. Most strikingly, a small group of Aldh2−/−Fancd2−/− mice that did not get leukemia developed spontaneous BMF. All of this data put together led us to the conclusion that BMF in FA patients could result from endogenous aldehyde-induced toxicity, which then leads to the depletion of HSCs (Figure 4). Despite this recent discovery, the question remains as to why single FA knockout mice so poorly replicate the human hematologic phenotype. It is only upon exposure to the exogenous cross-linking agent MMC or by taking away aldehyde catabolism in Fancd2−/− mice that the key features of this human illness can be recapitulated. We provide some speculations on this paradox.
A 2-tier protection mechanism preserves HSC function. (A) HSCs display high Aldh2 activity that protects their genome against the toxic effects of reactive aldehydes. If aldehydes should evade this protection mechanism, then DNA damage is dealt with by the FA DNA repair pathway. (B) In Aldh2−/−Fancd2−/− mice, the HSC pool is exposed to a greater burden of reactive aldehydes, and in the absence of DNA repair, DNA damage persists and leads to neoplastic transformation or HSC loss and spontaneous BMF.
A 2-tier protection mechanism preserves HSC function. (A) HSCs display high Aldh2 activity that protects their genome against the toxic effects of reactive aldehydes. If aldehydes should evade this protection mechanism, then DNA damage is dealt with by the FA DNA repair pathway. (B) In Aldh2−/−Fancd2−/− mice, the HSC pool is exposed to a greater burden of reactive aldehydes, and in the absence of DNA repair, DNA damage persists and leads to neoplastic transformation or HSC loss and spontaneous BMF.
First, FA patients show an age-dependent decrease in the frequency of CD34+ cells, which correlates with the worsening of clinical signs and results in the onset of hematologic abnormalities at the median age of 7.32 It is possible that the 2-year life span of mice does not allow for the complete exhaustion of the stem cell pool. It would be interesting to analyze Fanc-deficient mice to ask if there are age-related changes in the quantity/quality of the HSC pool (Figure 3). In support of this, it was shown that mice deficient in different genomic maintenance pathways (NER, nonhomologous end joining [NHEJ], and telomere maintenance) show a severe decrease in the functional capacity of their HSCs with age.71 Additionally, Fancc−/− HSCs were found to perform poorly at serial transplantation.43
Second, laboratory mice spend their lives in a highly controlled environment with the same food source throughout their lives. It is possible that this limits their exposure to exogenous DNA-damaging agents. Finally, the murine metabolism could produce less endogenous genotoxins, or, alternatively, the protection against them could be higher in mice than in humans. The metabolic rate of mice is much greater than that of larger mammals.72 This could result in the more rapid and efficient detoxification of toxic metabolites. It is possible that it is necessary to take this protection away in order to reveal the FA phenotype in mice.
An important prediction is that mutations in ALDH2 will lead to more severe clinical features in FA patients. In humans, ALDH2 is mutated in ∼1 billion people. This dominant-negative polymorphism (E487K) causes a dramatic decrease in ALDH2 activity, and, because it is most common in Southeast Asia, it is known as the Asian flushing syndrome.73 Hira and colleagues have recently determined the ALDH2 genotype of a group of Japanese FA patients. They found that ALDH2 deficiency dramatically accelerates BMF and increases the frequency of malformation in some tissues. Most strikingly, those patients entirely deficient for ALDH2 developed BMF within the first 7 months of life. These results unequivocally confirm that reactive aldehydes play an important role in the pathogenesis of FA.74
Although Aldh2 commonly oxidizes acetaldehyde, it has broad substrate specificity, being able to oxidize other aldehydes including 4-hydroxynonenal, acrolein, propionaldehyde, and butyraldehyde.75 It remains to be clarified if endogenous acetaldehyde is solely responsible for the phenotype observed in Aldh2−/−Fancd2−/− mice. Conversely, Aldh2 is 1 member of a family of 18 enzymes, any of which could also be offering protection against various aldehydes.76 Finally, there is a synthetic lethal interaction between FANCD2 and alcohol dehydrogenase 5 (ADH5) in the chicken B-cell line DT40.68 Adh5 is responsible for the detoxification of formaldehyde, the simplest and most toxic aldehyde, which can be produced close to DNA by means of DNA and histone demethylation. It will be interesting to see what the consequences of Adh5 disruption in Fanconi-deficient mice are.
Do the FA gene products modulate cytokine responses?
So far, we have discussed the very strong biochemical and genetic evidence for a function of the FA genes in DNA repair and how this function relates to BMF. However, we have already alluded to the unusual sensitivity that FA cells display to certain inflammatory cytokines.8,9 Three crucial observations underpin this discovery: First, FA cells are prone to apoptosis when exposed in vitro to tumor necrosis factor α (TNF-α) and interferon γ (IFN-γ).9,77,78 Second, exposing FA-deficient mice to these cytokines results in bone marrow dysfunction.79,80 Third, elevated levels of these and other cytokines have been reported in FA patients and are overproduced by FA-deficient cells in vitro.13,81 These observations underpin a general hypothesis that the hematopoietic phenotype of FA is attributable to the overproduction of precisely the cytokines to which FA cells are hypersensitive.
Most work on understanding the hypersensitivity of FA cells to cytokine overproduction has been conducted with the FANCC gene product, as well as cells derived from this complementation group and Fancc−/− knockout mice. This is probably because of the fact that FANCC was the first FA gene to be cloned and its product was initially shown to localize to the cytoplasm. FANCC has been proposed to have a completely separate role in hematopoiesis and to modulate cytokine responses by suppressing protein kinase R activation and its interactions with the chaperone HSP70 and STAT1, a key effector of IFN-γ signaling82-84 (Figure 5B85,86 ). It has been suggested that understanding all potential functions of FA proteins outside of DNA repair is a prerequisite for defining the pathogenesis of marrow failure in FA. On the other hand, BMF is a universal feature of FA, and this will be explained not by the independent functions of some FA proteins, but by understanding the function common to all 16 FA proteins (Figure 1).
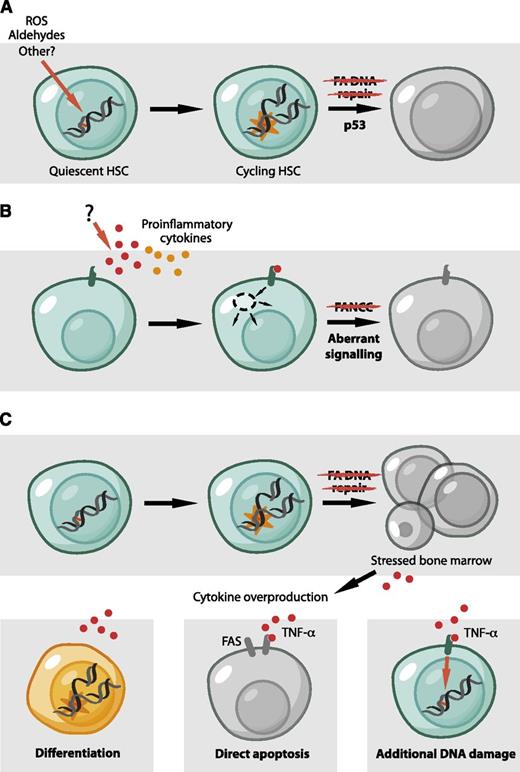
Models for BMF in FA. (A) The accumulation of DNA damage in the HSC pool leads to p53-dependent HSC depletion by diminishing the ability of HSCs to proliferate and self-renew. Endogenously generated aldehydes are an important source of such damage. (B) The molecular basis for cytokine overproduction and hypersensitivity by FA cells remains unclear. However, some FA proteins were proposed to have a direct role in hematopoiesis and to regulate the response to inflammatory signals, independent of DNA repair. (C) Reconciliation of the roles of FA proteins in hematopoiesis. A defect in DNA repair could be the initiating event that would lead to cell death, tissue injury, and the production of inflammatory cytokines. These could then contribute to bone marrow dysfunction in a number of ways. TNF-α and IFN-γ can cause apoptosis in stem cells and progenitors, mediated, for example, by the Fas receptor.78 Alternatively, TNF-α could produce additional DNA damage by the induction of reactive molecules, like ROS.85 Finally, inflammatory cytokines like TNF-α and IFN-γ have classically been considered to inhibit HSC and limit stem cell function. However, recent studies are challenging this view and propose a role for inflammatory signals in the direct regulation of HSCs.86 It is conceivable that these signals could lead to the differentiation of HSCs, either directly or indirectly, causing them to encounter new DNA damage in the context of replication.
Models for BMF in FA. (A) The accumulation of DNA damage in the HSC pool leads to p53-dependent HSC depletion by diminishing the ability of HSCs to proliferate and self-renew. Endogenously generated aldehydes are an important source of such damage. (B) The molecular basis for cytokine overproduction and hypersensitivity by FA cells remains unclear. However, some FA proteins were proposed to have a direct role in hematopoiesis and to regulate the response to inflammatory signals, independent of DNA repair. (C) Reconciliation of the roles of FA proteins in hematopoiesis. A defect in DNA repair could be the initiating event that would lead to cell death, tissue injury, and the production of inflammatory cytokines. These could then contribute to bone marrow dysfunction in a number of ways. TNF-α and IFN-γ can cause apoptosis in stem cells and progenitors, mediated, for example, by the Fas receptor.78 Alternatively, TNF-α could produce additional DNA damage by the induction of reactive molecules, like ROS.85 Finally, inflammatory cytokines like TNF-α and IFN-γ have classically been considered to inhibit HSC and limit stem cell function. However, recent studies are challenging this view and propose a role for inflammatory signals in the direct regulation of HSCs.86 It is conceivable that these signals could lead to the differentiation of HSCs, either directly or indirectly, causing them to encounter new DNA damage in the context of replication.
Alanine scanning mutation of FANCC identified a putative conserved motif that mediates these interactions. Mutation of this motif renders cells resistant to cross-linkers but sensitive to inflammatory cytokines, suggesting that the cytokine sensitivity and DNA repair function of FANCC can be decoupled. Cells bearing a naturally occurring mutation of FANCC (c.67delG, previously c.322delG) carry a hypomorphic 50-kDa FANCC polypeptide reinitiated at methionine 55 (FP-50 or M55).87 The M55 polypeptide preserves the conserved motif required for normal STAT1 activation and HSP70 interaction but does not rescue the hypersensitivity to MMC. However, patients with the c.67delG (c.322delG) mutation have milder congenital malformations but share the hematologic phenotype of other FA patients.88 This critical genetic observation does not support a role for the conserved STAT1/HSP70 binding motif in BMF in FA. Additionally, it remains to be explained how the aberrant signaling by FANCC might contribute to cytokine hypersensitivity and BMF in patients from other complementation groups.8,80 Although it is clear that FA cells are sensitive to inflammatory cytokines, it has not been emphatically established that this is independent of defective DNA repair or, indeed, that this points to a distinct role for the FA proteins in limiting the production and/or in resistance to these molecules. Hopefully, in the future, genetic dissection of the various components (cytokine, cytokine receptors, and downstream effector molecules), in combination with FA knockout mice, should clarify these potentially important questions.
The molecular basis for the overproduction of inflammatory cytokines in FA is unclear. A key question in this respect is whether this is a primary defect attributable to the function of the FA proteins or if this is a secondary phenomenon. In fact, high levels of cytokines can be induced by cytotoxic agents.89 Recent work from Matsui and colleagues has, for the first time, looked at cytokine production by bone marrow cells from patients with different inherited BMF syndromes. This work contradicts previous studies because no evidence for the overproduction of TNF-α or IFN-γ by unstimulated Fanconi-deficient T cells could be found. Cells from FA patients tended toward higher cytokine levels following lipopolysaccharide treatment, but this was not specific to FA cells and was seen in bone marrow samples from all syndromes.90 It is therefore plausible that raised cytokines levels are a generic response to bone marrow dysfunction, chronic inflammation, and tissue injury (Figure 5C).
Future directions
Over the past decade, much evidence for the role of FA gene products in DNA repair has been established. However, until recently, we have had little insight into what damages the DNA in the first place. The identification of reactive aldehydes as potent genotoxins in FA-deficient HSCs strengthens the link between DNA repair and the onset of BMF in FA. It will be crucial to define the sources of these reactive molecules and how they can be neutralized. However, it is not yet clear if these chemicals are the only or indeed main drivers of endogenous DNA damage in HSCs. Similar genetic approaches may reveal a role for other reactive molecules as endogenous genotoxins.
At present, we do not completely understand the precise nature of the DNA damage caused by aldehydes that is repaired by the FA pathway, and whether it is direct or indirect. For instance, is the lesion a DNA-DNA cross-link, DNA-protein cross-link, or simply an adducted base? At a more mechanistic and fundamental level, much work needs to be done to determine the biochemical and structural basis of how endogenous DNA damage is repaired by the FA proteins. All these potentially fertile avenues of research look set to keep this fascinating rare bone failure syndrome at the center of basic and translational biomedical research.
Acknowledgments
The authors would like to thank the members of the Patel laboratory for critically reading the manuscript.
Authorship
Contribution: J.I.G. and K.J.P. wrote the paper and had final approval of the submitted manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Juan I. Garaycoechea, MRC Laboratory of Molecular Biology, Francis Crick Ave, Cambridge Biomedical Campus, Cambridge CB2 0QH, United Kingdom; e-mail: juang@mrc-lmb.cam.ac.uk; and K. J. Patel, MRC Laboratory of Molecular Biology, Francis Crick Ave, Cambridge Biomedical Campus, Cambridge CB2 0QH, United Kingdom; e-mail: kjp@mrc-lmb.cam.ac.uk.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal