In this issue of Blood, Dubois et al show a catalytic-independent role of the linear ubiquitin chain assembly complex (LUBAC) in lymphocyte activation and B-cell malignancy.1 These data add a new layer of versatility to the recently established role of LUBAC in nuclear factor-κB (NF-κB) signaling.
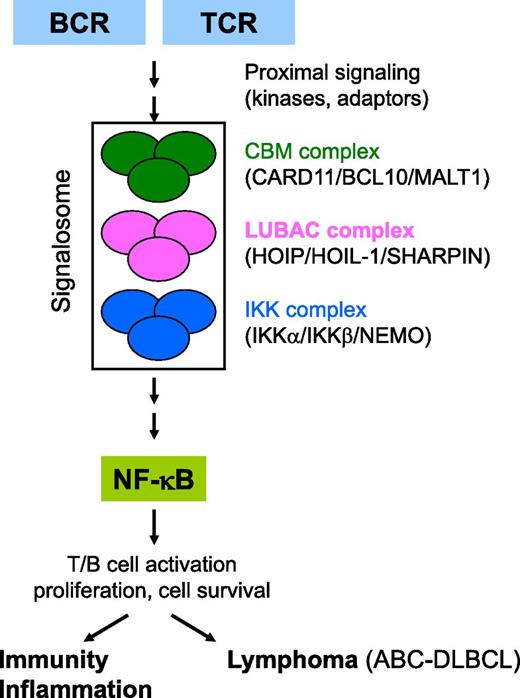
Hypothetical model for the role of LUBAC in antigen receptor–induced NF-κB signaling. TCR and BCR stimulation by antigens triggers the activation of proximal adaptors and kinases, followed by the rapid assembly of a signaling platform (known as the CBM complex) that contains the scaffold proteins CARD11/BCL10 and the paracaspase MALT1. In ABC-DLBCL, the CBM complex is preassembled, which often results from somatic mutations in positive or negative regulatory signaling proteins. Formation of the CBM complex results in the downstream activation of the IKK complex, consisting of 2 kinases (IKKα and IKKβ) and an adaptor protein NEMO. IKK-mediated phosphorylation results in the nuclear translocation of NF-κB where it induces the expression of several genes that mediate T- and B-cell activation and proliferation. In ABC-DLBCL, NF-κB is constitutively activated leading to the expression of anti-apoptotic genes and cell survival. How CBM connects to the IKK complex is still largely unclear. Dubois et al demonstrate that TCR- and BCR-induced NF-κB signaling also involves a third multiprotein complex known as LUBAC (consisting of HOIP, HOIL-1, and SHARPIN). LUBAC is best known for its role in tumor necrosis factor–induced NF-κB signaling, where it modifies specific signaling proteins with head-to-tail linked (linear) ubiquitin modules. However, the study of Dubois et al shows a catalytic-independent role of LUBAC in lymphocytes. Their results are compatible with a model in which antigen receptor stimulation induces the formation of a large signalosome in which LUBAC functions as an adaptor between the CBM and IKK complexes.
Hypothetical model for the role of LUBAC in antigen receptor–induced NF-κB signaling. TCR and BCR stimulation by antigens triggers the activation of proximal adaptors and kinases, followed by the rapid assembly of a signaling platform (known as the CBM complex) that contains the scaffold proteins CARD11/BCL10 and the paracaspase MALT1. In ABC-DLBCL, the CBM complex is preassembled, which often results from somatic mutations in positive or negative regulatory signaling proteins. Formation of the CBM complex results in the downstream activation of the IKK complex, consisting of 2 kinases (IKKα and IKKβ) and an adaptor protein NEMO. IKK-mediated phosphorylation results in the nuclear translocation of NF-κB where it induces the expression of several genes that mediate T- and B-cell activation and proliferation. In ABC-DLBCL, NF-κB is constitutively activated leading to the expression of anti-apoptotic genes and cell survival. How CBM connects to the IKK complex is still largely unclear. Dubois et al demonstrate that TCR- and BCR-induced NF-κB signaling also involves a third multiprotein complex known as LUBAC (consisting of HOIP, HOIL-1, and SHARPIN). LUBAC is best known for its role in tumor necrosis factor–induced NF-κB signaling, where it modifies specific signaling proteins with head-to-tail linked (linear) ubiquitin modules. However, the study of Dubois et al shows a catalytic-independent role of LUBAC in lymphocytes. Their results are compatible with a model in which antigen receptor stimulation induces the formation of a large signalosome in which LUBAC functions as an adaptor between the CBM and IKK complexes.
T and B cells sense antigens via specific receptors that induce signaling cascades leading to the activation of multiple transcription factors such as those of the NF-κB family.2 NF-κB controls the expression of multiple genes essential for the immunogenic response and cell survival. Hyperactivation of NF-κB is involved in many autoimmune and inflammatory diseases and constitutive NF-κB activation is a characteristic of certain lymphoma types such as activated B-cell–like diffuse large B-cell lymphoma (ABC-DLBCL), where NF-κB signaling drives proliferation and cell survival.3,4 Understanding the early molecular events leading to NF-κB activation is an active area of research and much progress has been made during the last decade. Several molecules that specifically connect the T-cell receptor (TCR) and B-cell receptor (BCR) proximal adaptors and kinases to the central core of the NF-κB cascade (the IκB kinase [IKK] complex) have been identified. More specifically, the antigen receptor–induced assembly of a signaling platform containing the scaffold proteins CARD11 and BCL10 and the paracaspase MALT1 (known as the CBM complex) connects proximal antigen receptor signaling to the IKK complex. In ABC-DLBCL, the CBM complex is preassembled (which often results from somatic mutations in key regulatory proteins), leading to derailed signaling. Posttranslational modification of several components of the CBM and IKK complexes by phosphorylation and different types of polyubiquitination are believed to contribute to CBM-mediated NF-κB signaling, but the exact molecular mechanisms remain obscure.5
In this issue, Dubois et al provide new insights into the molecular basis of the link between CBM and NF-κB signaling.1 They performed a mass spectrometry proteomic screen in stimulated T cells after immunoprecipitation of casein kinase α, a kinase that binds to CARD11 and governs antigen receptor–induced NF-κB signaling. By this approach, they identified HOIP (also known as RNF31) as a new component of the CBM complex after antigen receptor stimulation. HOIP is 1 of the 3 proteins of the recently identified LUBAC that mediates NF-κB signaling in response to cytokines, bacteria, and genotoxic stress.6 Furthermore, coimmunoprecipitation experiments revealed that all 3 LUBAC components enter the CBM and IKK signalosome after antigen receptor stimulation. The authors further explored whether LUBAC is required for TCR-induced NF-κB signaling by assessing the outcome of small interfering RNA–mediated silencing of each of the 3 LUBAC components. Deficiency of HOIP and SHARPIN, but not HOIL-1, significantly reduced NF-κB activation and the binding of BCL10/MALT1 to the IKK adaptor protein NEMO. These data indicate that LUBAC contributes to optimal NF-κB activation in response to antigen receptor stimulation by enabling the interaction between CBM and IKK complexes.
HOIP is known as an E3 ubiquitin ligase whose catalytic activity is necessary for the LUBAC-mediated modification of specific NF-κB signaling proteins with multiple ubiquitin modules that are linked to each other via their N and C termini.6 This type of polyubiquitination is known as linear or M1-linked polyubiquitination and is the focus of intense research. Surprisingly, Dubois and colleagues found that expression of a catalytically inactive HOIP mutant is able to restore reduced NF-κB signaling in HOIP-deficient cells to normal levels, indicating that HOIP mediates TCR signaling independent of its catalytic activity. This is further supported by the observation that silencing of OTULIN, a negative regulator of linear polyubiquitination, did not change TCR-induced NF-κB signaling. It should be mentioned that some modest linear polyubiquitination is detected in TCR-stimulated cells, indicating a possible role for linear ubiquitination in other TCR signaling pathways than NF-κB signaling.
Dubois et al show that LUBAC is also part of the preassembled CBM complex in ABC-DLBCL cell lines and that combined silencing of all 3 LUBAC components inhibits constitutive NF-κB activation in these cells. Consistent with these findings and the known anti-apoptotic function of NF-κB, they show that LUBAC silencing also reduces cell survival. Together, these data indicate that LUBAC guarantees cell proliferation and survival of ABC-DLBCL by maintaining constitutive NF-κB activity.
The results of Dubois et al suggest a novel catalytic-independent role of LUBAC in lymphocytes and B-cell lymphoma. The underlying molecular mechanism is still unclear but the finding that HOIP is necessary for the association between CBM and IKK complexes is indicative for an adaptor function. The exact mechanism could, however, be more complex as many ill-defined components compose the CBM complex. The data of Dubois et al complement the recent demonstration that BCR-mediated NF-κB activation does not require LUBAC catalytic activity in splenocytes.7 In addition, another parallel study also reports that LUBAC associates with the CBM complex in ABC-DLBCL and is required for cell viability.8 However, the latter study shows that LUBAC mediates constitutive linear polyubiquitination of the IKK adaptor protein NEMO in ABC-DLBCL, and describes 2 rare HOIP germline mutations that promote LUBAC E3 ubiquitin ligase activity and activate NF-κB in ABC-DLBCL. At first look, these findings do not fit the catalytic-independent role of LUBAC that is proposed by Dubois et al in this issue. However, it should be mentioned that they only analyzed the dependency on HOIP catalytic activity in T cells and not in ABC-DLBCL cell lines. Nevertheless, the finding that LUBAC is part of the CBM complex and mediates NF-κB signaling and cell survival is of high importance for our understanding of the regulation of physiological and pathological signaling in adaptive immunity. The CBM complex is an attractive therapeutic target for diseases associated with aberrant lymphocyte activation and B-cell lymphomas, and recent developments using MALT1 protease inhibitors are very promising.9 A better knowledge of the function and regulation of LUBAC in the CBM complex may provide additional ways for therapeutic targeting.
Conflict-of-interest disclosure: The author declares no competing financial interests.