Key Points
LUBAC elements HOIP and SHARPIN participate in T-cell receptor-mediated NF-κB activation independently of HOIP catalytic activity.
LUBAC silencing compromises constitutive NF-κB activation and cell survival in ABC DLBCL lines.
Abstract
Antigen receptor-mediated nuclear factor κB (NF-κB) activation relies on the formation of a large multi-protein complex that contains CARMA1, BCL10, and MALT1 (CBM complex). This signalosome is pirated in the activated B-cell–like subgroup of diffuse large B-cell lymphoma (ABC DLBCL) to drive aberrant NF-κB activation, thereby promoting cell survival and propagation. Using an unbiased proteomic approach, we screened for additional components of the CBM in lymphocytes. We found that the linear ubiquitin chain assembly complex (LUBAC), which was previously linked to cytokine-mediated NF-κB activation, dynamically integrates the CBM and marshals NF-κB optimal activation following antigen receptor ligation independently of its catalytic activity. The LUBAC also participates in preassembled CBM complex in cells derived from ABC DLBCL. Silencing the LUBAC reduced NF-κB activation and was toxic in ABC DLBCL cell lines. Thus, our findings reveal a role for the LUBAC during lymphocyte activation and in B-cell malignancy.
Introduction
The activated B-cell like (ABC) subtype of diffuse large B-cell lymphoma (DLBCL) constitutes the most aggressive DLBCL entity.1 In contrast to germinal center B-cell–like (GCB) subtype of DLBCL, ABC DLBCL survival and proliferation require the constitutive activation of nuclear factor κB (NF-κB) transcription factors, which often results from somatic mutations in CD79B, CARD11 (also called CARMA1), MYD88, and TNFAIP3 genes.2 New perspectives for treatments restricted to the lymphoid compartment came from genomic-scale RNA interference screens, which unveiled that ABC DLBCL exploited a multi-protein complex that contains CARMA1, BCL10, and MALT1 (CBM complex) normally engaged in conveying NF-κB following antigen receptor engagement.3-6 Within the CBM, Lys-63 (K63)-linked ubiquitylation of BCL10 and MALT1 ensures the recruitment and activation of the inhibitor of NF-κB kinase (IKK, composed of IKKα, IKKβ, and NEMO) through IKKβ phosphorylation and NEMO poly-ubiquitylation.7-9 IKK subsequently authorizes NF-κB to shuttle in the nucleus and exert its transcriptional activity by phosphorylating its cognate inhibitors (IκBs), which further undergo proteasomal degradation.10
Here, we screened for additional CBM partners and identified the linear ubiquitin chain assembly complex (LUBAC), which comprises 2 E3 ligases, HOIL-1 and HOIP, and SHARPIN.11 Although this tripartite complex was previously linked to cytokine-, bacteria-, and genotoxic stress-mediated NF-κB signaling,12-17 its involvement in adaptive immunity remains unknown. We now show that the LUBAC binds to the CBM and governs NF-κB activation upon antigen receptor engagement independently of HOIP catalytic activity. In ABC DLBCL cells, the LUBAC is integral to preassembled CBM and its knockdown is lethal, as it hampers aberrant NF-κB activity.
Study design
Cells and reagents are described in the supplemental Methods (available on the Blood Web site). Knockdown was achieved by transfecting small interfering RNA (siRNA), or via retroviral infection of small hairpin RNA (shRNA) (supplemental Methods). Confocal microscopy, luciferase assays, enzyme-linked immunosorbent assay, immunoblots, and immunoprecipitations were performed as previously described.3 Fraction of GFP-positive cells over time following retroviral infection and DiOC6 staining were determined by flow cytometry. This study involved in vitro experiments with primary human T lymphocytes from healthy volunteer donors. Buffy coats from healthy volunteers were obtained from the “Etablissement Français du Sang” (EFS-Ile de France) according to an agreement between EFS and Institut National de la Santé et de la Recherche Médicale (INSERM) (convention 09EFS024, code 990029, SAP19DIV0336). The documents relative to patient free and inform consent, as well as the respect of confidentiality and privacy protection, are handled by EFS. This study was conducted in accordance with the Declaration of Helsinki.
Results and discussion
Promptly following antigen receptor engagement, the kinase CK1α binds the CBM and participates in NF-κB signaling.3 To uncover new NF-κB modulators, we performed a proteomic screen by mass spectrometry of CK1α partners in Jurkat T lymphoblastoid cells stimulated with phorbol 12 myristate 13-acetate plus ionomycin (P/I) to mimic T-cell receptor (TCR) ligation. In addition to known interactants, 2 peptides covering HOIP were isolated (supplemental Table 1; supplemental Figure 1). As expected,13,15,16 HOIP was constitutively tethered to SHARPIN and HOIL-1, regardless of stimulation (supplemental Figure 2). Coimmunoprecipitation experiments showed that LUBAC and CBM components bound to CK1α following TCR engagement (Figure 1A; supplemental Figures 3 and 4). CARMA1 and HOIP also precipitated with BCL10/MALT1 heterodimers in stimulated cells. Likewise, SHARPIN dynamically recruited BCL10, MALT1, CK1α, and NEMO. Corroborating these findings, LUBAC and CBM components coprecipitated with NEMO upon stimulation (Figure 1A). Similar results were obtained in P/I-stimulated BJAB B cells (supplemental Figure 5). Hence, LUBAC components integrate a signalosome containing both CBM and IKK complexes following antigen receptor engagement.
The LUBAC binds to CBM and IKK complexes and participates in antigen receptor-mediated NF-κB activation. (A) Jurkat T lymphocytes were stimulated with 20 ng.mL−1 PMA plus 300 ng.mL−1 ionomycin (P/I) or with 1 μg.mL−1 anti-CD3 and anti-CD28 (CD3/28) for 0, 10, and 20 minutes. Cell extracts were prepared and immunoprecipitated (IP) with antibodies to CK1α, BCL10, SHARPIN, or NEMO, and immunoblots (IBs) were performed as indicated. Closed circle and asterisk indicate the protein of interest and nonspecific bands, respectively. Ub, ubiquitin. Molecular weight markers (kDa) are indicated. (B) Jurkat cells were transfected with a nonspecific control siRNA (NS) or with 3 individual siRNAs against HOIP (HOIP.1-3). Three days later, cells were transfected with pNF-κB-Luc reporter and pTKRL control plasmids for an additional 24 hours, prior stimulation with 0.5 μg.mL−1 anti-CD3 and anti-CD28 (3/28), or with P/I as in (A), or with 10 ng.mL−1 TNFα for 6 hours. Histograms represent the mean ± standard error of the mean (SEM) of 3 independent experiments. **P < .001; ****P < .0001 compared with cells transfected with NS siRNA (analysis of variance [ANOVA]). GAPDH, glyceraldehyde-3-phosphate dehydrogenase; ns, nonsignificant; RLU, relative light unit; Unst, unstimulated cells. Inset IB shows the level of protein knockdown. (C) NS- and HOIP-silenced Jurkat T cells were stimulated with 1 µg.mL−1 anti-CD3 and anti-CD28 (CD3/28) for 0, 30, and 60 minutes. Nuclear and cytosolic fractions were purified, and IBs were performed as indicated. Tubulin and poly (ADP-ribose) polymerase (PARP) served as loading controls. (D-E) Confocal microscopy micrographs of NF-κB p65 (green) in Jurkat T cells transfected with an NS or HOIP siRNA and stimulated with 1 µg.mL−1 anti-CD3 and anti-CD28 (CD3/28) for 1 hour. Nuclei were also stained with 4′,6-diamidino-2-phenylindole (in blue). In (E), the percentage of cells with nuclear p65 was calculated by scoring >150 cells for each sample. Shown is the mean ± SEM from 3 independent experiments (****P < .0001 by ANOVA). (F) NS- or HOIP-silenced human peripheral blood mononuclear cells were stimulated with 1 and 100 ng.mL−1 anti-CD3 and anti-CD28 for 16 hours. Interleukin-2 secretion in the culture supernatants was determined by enzyme-linked immunosorbent assay. Histograms represent the mean ± SEM of 3 independent experiments (****P < .0001 by ANOVA). Inset immunoblots show the knockdown efficiency. (G) Cell extracts from NS- and HOIP-silenced Jurkat cells stimulated as in (C) for 0, 10, 20, and 30 minutes were analyzed by IB. (H) NS- and HOIP-silenced BJAB B cells were stimulated with 10 ng.mL−1 PMA plus 300 ng.mL−1 ionomycin (P/I) for 0, 10, 20, and 30 minutes. Cell lysates were prepared and analyzed by IB as indicated. (I) NEMO binding to HOIP, BCL10, and MALT1 was assessed by IP/IB in NS- and HOIP-silenced Jurkat cells stimulated with P/I as in (A). Lys., lysate. (J) Cell extracts from Jurkat cells stimulated with (P/I) as in (A) or with 10 ng.mL−1 TNFα for 0, 10, and 20 min were IP with an antibody against M1-linked Ub. IBs were performed as indicated. The asterisk shows the anti-M1-Ub. (K) Jurkat cells were retrovirally infected to express GFP, GFP plus an shRNA against human HOIP (HOIPsh), RNAi-resistant HOIP wild type plus HOIPsh, and catalytically inactive HOIP (HOIP-CS) plus HOIPsh. Cells were stimulated as in (J) for 30 minutes and analyzed as in (E). Shown is the mean ± SEM from 3 independent experiments (ns, ***P < .001; ****P < .0001 by ANOVA). Cell lysates were prepared and IB performed as indicated. Molecular weight markers (kDa) are shown. Data are representative of 2 (I) or at least 3 independent experiments (A-H, J-K).
The LUBAC binds to CBM and IKK complexes and participates in antigen receptor-mediated NF-κB activation. (A) Jurkat T lymphocytes were stimulated with 20 ng.mL−1 PMA plus 300 ng.mL−1 ionomycin (P/I) or with 1 μg.mL−1 anti-CD3 and anti-CD28 (CD3/28) for 0, 10, and 20 minutes. Cell extracts were prepared and immunoprecipitated (IP) with antibodies to CK1α, BCL10, SHARPIN, or NEMO, and immunoblots (IBs) were performed as indicated. Closed circle and asterisk indicate the protein of interest and nonspecific bands, respectively. Ub, ubiquitin. Molecular weight markers (kDa) are indicated. (B) Jurkat cells were transfected with a nonspecific control siRNA (NS) or with 3 individual siRNAs against HOIP (HOIP.1-3). Three days later, cells were transfected with pNF-κB-Luc reporter and pTKRL control plasmids for an additional 24 hours, prior stimulation with 0.5 μg.mL−1 anti-CD3 and anti-CD28 (3/28), or with P/I as in (A), or with 10 ng.mL−1 TNFα for 6 hours. Histograms represent the mean ± standard error of the mean (SEM) of 3 independent experiments. **P < .001; ****P < .0001 compared with cells transfected with NS siRNA (analysis of variance [ANOVA]). GAPDH, glyceraldehyde-3-phosphate dehydrogenase; ns, nonsignificant; RLU, relative light unit; Unst, unstimulated cells. Inset IB shows the level of protein knockdown. (C) NS- and HOIP-silenced Jurkat T cells were stimulated with 1 µg.mL−1 anti-CD3 and anti-CD28 (CD3/28) for 0, 30, and 60 minutes. Nuclear and cytosolic fractions were purified, and IBs were performed as indicated. Tubulin and poly (ADP-ribose) polymerase (PARP) served as loading controls. (D-E) Confocal microscopy micrographs of NF-κB p65 (green) in Jurkat T cells transfected with an NS or HOIP siRNA and stimulated with 1 µg.mL−1 anti-CD3 and anti-CD28 (CD3/28) for 1 hour. Nuclei were also stained with 4′,6-diamidino-2-phenylindole (in blue). In (E), the percentage of cells with nuclear p65 was calculated by scoring >150 cells for each sample. Shown is the mean ± SEM from 3 independent experiments (****P < .0001 by ANOVA). (F) NS- or HOIP-silenced human peripheral blood mononuclear cells were stimulated with 1 and 100 ng.mL−1 anti-CD3 and anti-CD28 for 16 hours. Interleukin-2 secretion in the culture supernatants was determined by enzyme-linked immunosorbent assay. Histograms represent the mean ± SEM of 3 independent experiments (****P < .0001 by ANOVA). Inset immunoblots show the knockdown efficiency. (G) Cell extracts from NS- and HOIP-silenced Jurkat cells stimulated as in (C) for 0, 10, 20, and 30 minutes were analyzed by IB. (H) NS- and HOIP-silenced BJAB B cells were stimulated with 10 ng.mL−1 PMA plus 300 ng.mL−1 ionomycin (P/I) for 0, 10, 20, and 30 minutes. Cell lysates were prepared and analyzed by IB as indicated. (I) NEMO binding to HOIP, BCL10, and MALT1 was assessed by IP/IB in NS- and HOIP-silenced Jurkat cells stimulated with P/I as in (A). Lys., lysate. (J) Cell extracts from Jurkat cells stimulated with (P/I) as in (A) or with 10 ng.mL−1 TNFα for 0, 10, and 20 min were IP with an antibody against M1-linked Ub. IBs were performed as indicated. The asterisk shows the anti-M1-Ub. (K) Jurkat cells were retrovirally infected to express GFP, GFP plus an shRNA against human HOIP (HOIPsh), RNAi-resistant HOIP wild type plus HOIPsh, and catalytically inactive HOIP (HOIP-CS) plus HOIPsh. Cells were stimulated as in (J) for 30 minutes and analyzed as in (E). Shown is the mean ± SEM from 3 independent experiments (ns, ***P < .001; ****P < .0001 by ANOVA). Cell lysates were prepared and IB performed as indicated. Molecular weight markers (kDa) are shown. Data are representative of 2 (I) or at least 3 independent experiments (A-H, J-K).
We next investigated whether the LUBAC participates in antigen receptor-mediated NF-κB activation. First, HOIP knockdown with 3 individual siRNA sequences curtailed both TCR- and tumor necrosis factor (TNF)α-mediated NF-κB activation in a luciferase gene reporter assay (Figure 1B). TCR-driven redistribution of NF-κB p65 into the nucleus and downstream interleukin-2 production were blunted in HOIP-silenced human primary peripheral blood mononuclear cells and in Jurkat lymphocytes (Figure 1C-F; supplemental Figures 6 and 7). In addition, IKK and IκBα phosphorylation, which reflects NF-κB activation,10 was diminished (Figure 1G). Similarly, IκBα phosphorylation and degradation were reduced in HOIP-silenced BJAB cells stimulated with P/I (Figure 1H). Even though CK1α normally bound the CBM without HOIP (supplemental Figure 8), NEMO interaction with BCL10 or MALT1, and NEMO poly-ubiquitylation were markedly decreased (Figure 1I; supplemental Figure 9). LUBAC stability is compromised in HOIL-1- or SHARPIN-deficient cells;13,15,16,18 however, this was partly the case when siRNAs were used (supplemental Figure 10). We found that silencing of SHARPIN or HOIP diminished TCR- and TNFα-mediated NF-κB activation (supplemental Figure 10). In contrast to fibroblasts in which it participates in TNFα-mediated NF-κB activation,14,18,19 HOIL-1 impact in Jurkat cells was only modest, suggesting a cell type-dependent modus operandi. Collectively, our data indicate that silencing HOIP perturbs the interaction between CBM and IKK complexes, thus diminishing IKK modifications and ensuing NF-κB activation.
LUBAC catalyzes linear Met-1 (M1)-ubiquitin chains that accumulate within cytokine receptors-driven signalosomes to favor IKK/NF-κB signaling.20-23 Although TNFα treatment efficiently assembled M1-ubiquitin chains that bound to TNFR and SHARPIN, little signal was detected following TCR stimulation (Figure 1J; supplemental Figure 11). Moreover, TNFα- but not TCR-mediated NF-κB activation was boosted when the negative regulator of LUBAC catalytic activity OTULIN20,21,24 was silenced (supplemental Figure 12). Last, plasmids that contain an shRNA against human HOIP followed by a RNAi-resistant HOIP wild type or a catalytically inactive mutant (C699S/C702S,25 HOIP-CS) allowed us to simultaneously silence and reconstitute HOIP in cells. Although important for TNFα signaling, HOIP catalytic activity was dispensable for NF-κB activation upon TCR engagement (Figure 1K; supplemental Figure 13). Hence, HOIP operates independently of its catalytic activity to convey NF-κB activation upon TCR engagement.
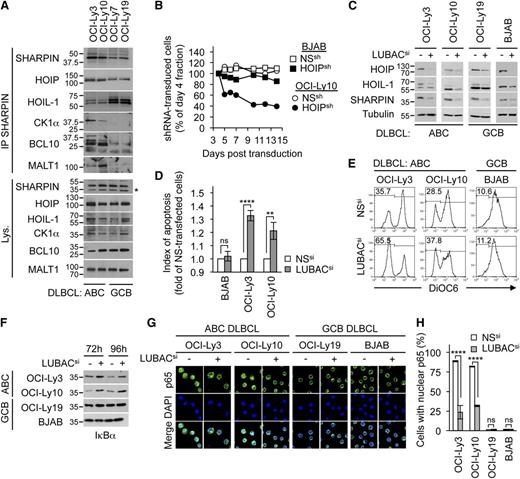
To evaluate the LUBAC contribution to ABC DLBCL piracy of antigen receptor-mediated NF-κB signaling pathway, we first examined its participation in the constitutively preassembled CBM complex.3,4 SHARPIN co-precipitated with the CBM in ABC DLBCL but not in GCB DLBCL cell lines (Figure 2A; supplemental Figure 14). In addition, CBM and LUBAC elements were also found in CK1α pull-downs in ABC DLBCL lines (supplemental Figure 14). Next, DLBCL lines were retrovirally infected to express shRNA against HOIP together with GFP. As expected, BJAB cells stably expressing HOIP shRNA displayed reduced IκBα phosphorylation upon P/I treatment (supplemental Figure 15). The expression of HOIP shRNA decreased the fraction of GFP-positive cells in OCI-Ly10 cells, but not in the BJAB cells over time (Figure 2B), suggesting that HOIP knockdown is selectively toxic in ABC DLBCL. Similar results were obtained with the ABC DLBCL U2932 line (supplemental Figure 15). Knocking down with siRNA the LUBAC components, individually or collectively, significantly, albeit modestly, increased apoptosis in OCI-Ly3 and OCI-Ly10 as measured by mitochondrial transmembrane potential dissipation, phosphatidylserines exposure, and propidium iodide incorporation (Figure 2C-E; supplemental Figure 16). The same was true when CARMA1 was silenced (supplemental Figure 16). Consonant with an aberrant NF-κB activity in ABC DLBCL cells, IκBα was constitutively degraded, and this was reversed when LUBAC components were silenced (Figure 2F). By contrast, no overt changes in IκBα levels were observed in GCB DLBCL cells. Furthermore, p65 no longer accumulated in the nucleus of LUBAC-silenced ABC DLBCL lines (Figure 2G-H; supplemental Figure 17). Altogether, our data suggest that the LUBAC is part of constitutively preassembled NF-κB activating signalosome in ABC DLBCL lines and contributes to NF-κB–dependent cell survival.
The LUBAC contributes to aberrant activation of NF-κB and survival of ABC DLBCL lines. (A) Cell lysates from ABC DLBCL lines (OCI-Ly3 and OCI-Ly10) and from GCB DLBCL lines (OCI-Ly7 and OCI-Ly19) were IP with antibodies to SHARPIN, and IBs were performed as indicated. The asterisk indicates nonspecific bands. (B) DLBCL lines were infected with a retrovirus that expressed a control nonspecific (NSsh) or a HOIPsh shRNA together with GFP. Shown is the fraction of GFP-positive cells over time relative to day 4 postinfection. (C) Lysates from OCI-Ly3, OCI-Ly10, OCI-Ly19, and BJAB cells transfected with control NS siRNA or with siRNA against the LUBAC (HOIP plus HOIL-1 plus SHARPIN) for 48 hours were analyzed by IB as indicated. (D-E) DLBCL lines were transfected as in (C) for 72 hours and stained with DiOC6 and analyzed by flow cytometry. Histograms in (D) represent the fold of NS-treated sample. Shown is the mean ± SEM from 3 independent experiments (ns, **P < .001; ****P < .0001 by ANOVA). (F) IκBα levels were assessed by IB 72 and 96 hours posttransfection in lysates as in (C). (G-H) ABC DLBCL lines (OCI-Ly3 and OCI-Ly10) and GCB DLBCL lines (OCI-Ly19 and BJAB) were transfected as in (C) for 72 hours. NF-κB p65 subcellular location was examined by confocal microscopy. Nuclei were illuminated with 4′,6-diamidino-2-phenylindole (DAPI). Histograms in (H) show the mean ± SEM from 3 independent experiments (ns, ****P < .0001 by ANOVA). Data are representative of 3 independent experiments.
The LUBAC contributes to aberrant activation of NF-κB and survival of ABC DLBCL lines. (A) Cell lysates from ABC DLBCL lines (OCI-Ly3 and OCI-Ly10) and from GCB DLBCL lines (OCI-Ly7 and OCI-Ly19) were IP with antibodies to SHARPIN, and IBs were performed as indicated. The asterisk indicates nonspecific bands. (B) DLBCL lines were infected with a retrovirus that expressed a control nonspecific (NSsh) or a HOIPsh shRNA together with GFP. Shown is the fraction of GFP-positive cells over time relative to day 4 postinfection. (C) Lysates from OCI-Ly3, OCI-Ly10, OCI-Ly19, and BJAB cells transfected with control NS siRNA or with siRNA against the LUBAC (HOIP plus HOIL-1 plus SHARPIN) for 48 hours were analyzed by IB as indicated. (D-E) DLBCL lines were transfected as in (C) for 72 hours and stained with DiOC6 and analyzed by flow cytometry. Histograms in (D) represent the fold of NS-treated sample. Shown is the mean ± SEM from 3 independent experiments (ns, **P < .001; ****P < .0001 by ANOVA). (F) IκBα levels were assessed by IB 72 and 96 hours posttransfection in lysates as in (C). (G-H) ABC DLBCL lines (OCI-Ly3 and OCI-Ly10) and GCB DLBCL lines (OCI-Ly19 and BJAB) were transfected as in (C) for 72 hours. NF-κB p65 subcellular location was examined by confocal microscopy. Nuclei were illuminated with 4′,6-diamidino-2-phenylindole (DAPI). Histograms in (H) show the mean ± SEM from 3 independent experiments (ns, ****P < .0001 by ANOVA). Data are representative of 3 independent experiments.
In summary, we provide evidence that the LUBAC favors the association of CBM and IKK complexes and participates in NF-κB activation following TCR stimulation. This function we ascribe to the LUBAC expands its pivotal role in fine-tuning NF-κB to adaptive immunity. Although it deserves further investigation, our data suggest that HOIP rather functions as an adaptor upon TCR engagement, because its catalytic activity is dispensable for NF-κB signaling. In line with this, B-cell receptor-mediated NF-κB activation occurs in splenocytes that lack HOIP catalytic activity.22 We also show that the LUBAC is integral to preassembled CBM complex in ABC DLBCL lines and guarantees cell survival by maintaining constitutive NF-κB activity. Hence, strategies aimed at targeting the LUBAC might be relevant in the context of ABC DLBCL.
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank H. Walczak and S. Kupka (University College London Cancer Institute, London) for providing reagents and N. Cordeiro and A. Le Moignic for helpful assistance.
This work was supported by grants from the French National Research Agency (ANR-10-JCJC-1306), Fondation ARC, Ligue Nationale contre le Cancer, Fondation pour la Recherche Médicale, and Institut National du Cancer (INCA_6508). S.M.D. is supported by a fellowship from Université Paris Sud.
Authorship
Contribution: S.M.D. designed the research, conducted experiments, analyzed the data, and wrote the manuscript; C.A., H.M.L., C.L., and E.S. designed and performed experiments and analyzed the data; Y.W., T.F., K.T., and Z.J.C. provided essential tools; J.G. analyzed the data; and N.B. conceived the project, analyzed the data, and wrote the manuscript. All authors read and approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Nicolas Bidère, INSERM U1014, Bâtiment INSERM Lavoisier, Hôpital Paul Brousse, 14 Avenue Paul Vaillant-Couturier, 94800 Villejuif, France; e-mail: nicolas.bidere@inserm.fr.

![Figure 1. The LUBAC binds to CBM and IKK complexes and participates in antigen receptor-mediated NF-κB activation. (A) Jurkat T lymphocytes were stimulated with 20 ng.mL−1 PMA plus 300 ng.mL−1 ionomycin (P/I) or with 1 μg.mL−1 anti-CD3 and anti-CD28 (CD3/28) for 0, 10, and 20 minutes. Cell extracts were prepared and immunoprecipitated (IP) with antibodies to CK1α, BCL10, SHARPIN, or NEMO, and immunoblots (IBs) were performed as indicated. Closed circle and asterisk indicate the protein of interest and nonspecific bands, respectively. Ub, ubiquitin. Molecular weight markers (kDa) are indicated. (B) Jurkat cells were transfected with a nonspecific control siRNA (NS) or with 3 individual siRNAs against HOIP (HOIP.1-3). Three days later, cells were transfected with pNF-κB-Luc reporter and pTKRL control plasmids for an additional 24 hours, prior stimulation with 0.5 μg.mL−1 anti-CD3 and anti-CD28 (3/28), or with P/I as in (A), or with 10 ng.mL−1 TNFα for 6 hours. Histograms represent the mean ± standard error of the mean (SEM) of 3 independent experiments. **P < .001; ****P < .0001 compared with cells transfected with NS siRNA (analysis of variance [ANOVA]). GAPDH, glyceraldehyde-3-phosphate dehydrogenase; ns, nonsignificant; RLU, relative light unit; Unst, unstimulated cells. Inset IB shows the level of protein knockdown. (C) NS- and HOIP-silenced Jurkat T cells were stimulated with 1 µg.mL−1 anti-CD3 and anti-CD28 (CD3/28) for 0, 30, and 60 minutes. Nuclear and cytosolic fractions were purified, and IBs were performed as indicated. Tubulin and poly (ADP-ribose) polymerase (PARP) served as loading controls. (D-E) Confocal microscopy micrographs of NF-κB p65 (green) in Jurkat T cells transfected with an NS or HOIP siRNA and stimulated with 1 µg.mL−1 anti-CD3 and anti-CD28 (CD3/28) for 1 hour. Nuclei were also stained with 4′,6-diamidino-2-phenylindole (in blue). In (E), the percentage of cells with nuclear p65 was calculated by scoring >150 cells for each sample. Shown is the mean ± SEM from 3 independent experiments (****P < .0001 by ANOVA). (F) NS- or HOIP-silenced human peripheral blood mononuclear cells were stimulated with 1 and 100 ng.mL−1 anti-CD3 and anti-CD28 for 16 hours. Interleukin-2 secretion in the culture supernatants was determined by enzyme-linked immunosorbent assay. Histograms represent the mean ± SEM of 3 independent experiments (****P < .0001 by ANOVA). Inset immunoblots show the knockdown efficiency. (G) Cell extracts from NS- and HOIP-silenced Jurkat cells stimulated as in (C) for 0, 10, 20, and 30 minutes were analyzed by IB. (H) NS- and HOIP-silenced BJAB B cells were stimulated with 10 ng.mL−1 PMA plus 300 ng.mL−1 ionomycin (P/I) for 0, 10, 20, and 30 minutes. Cell lysates were prepared and analyzed by IB as indicated. (I) NEMO binding to HOIP, BCL10, and MALT1 was assessed by IP/IB in NS- and HOIP-silenced Jurkat cells stimulated with P/I as in (A). Lys., lysate. (J) Cell extracts from Jurkat cells stimulated with (P/I) as in (A) or with 10 ng.mL−1 TNFα for 0, 10, and 20 min were IP with an antibody against M1-linked Ub. IBs were performed as indicated. The asterisk shows the anti-M1-Ub. (K) Jurkat cells were retrovirally infected to express GFP, GFP plus an shRNA against human HOIP (HOIPsh), RNAi-resistant HOIP wild type plus HOIPsh, and catalytically inactive HOIP (HOIP-CS) plus HOIPsh. Cells were stimulated as in (J) for 30 minutes and analyzed as in (E). Shown is the mean ± SEM from 3 independent experiments (ns, ***P < .001; ****P < .0001 by ANOVA). Cell lysates were prepared and IB performed as indicated. Molecular weight markers (kDa) are shown. Data are representative of 2 (I) or at least 3 independent experiments (A-H, J-K).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/123/14/10.1182_blood-2013-05-504019/4/m_2199f1.jpeg?Expires=1763495091&Signature=xU7w0UWt7HGX-P~vZ-1Wp7cr0P0dyRt3fCWXxF2U74TXUT2OQUvJuWWEKiOG4DVuNjnceDlz40W43V1lD0MRXO2h~6wBUx4g8exB4s-Z2wde0nM2ETFUd2fufQ-8OaYNzM6uJI2xGuyP3T1Va96R6c-pu9Prxe~FgUlO7ttNGqs8yrRp7iVwT6HQT-wSCc24yb69RutqMkbLBA-WbtvezOM~958nhmHJul4mgvJELmDa~0nb1n2E03qCVtrRI-JYkeqg-UF9vo0Ywi7YrFUl5jTBMT82mVv~w1G5oYo9mKafTakhz8Pjzd2UnsJPvvTC7dmSoS5v8CJK57x2j9vMjQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal