Key Points
CXCR7 is a key actor of the cell cycling and survival promoting effect of CXCL12 on primary human CD34+ HSPCs through Akt signaling.
β-arrestins are signaling hubs downstream of CXCL12-activated receptors in primary human CD34+ HSPCs.
Abstract
In addition to its well-known effect on migration and homing of hematopoietic stem/progenitor cells (HSPCs), CXCL12 chemokine also exhibits a cell cycle and survival-promoting factor for human CD34+ HSPCs. CXCR4 was suggested to be responsible for CXCL12-induced biological effects until the recent discovery of its second receptor, CXCR7. Until now, the participation of CXCR7 in CXCL12-induced HSPC cycling and survival is unknown. We show here that CXCL12 was capable of binding CXCR7 despite its scarce expression at CD34+ cell surface. Blocking CXCR7 inhibited CXCL12-induced Akt activation as well as the percentage of CD34+ cells in cycle, colony formation, and survival, demonstrating its participation in CXCL12-induced functional effects in HSPCs. At steady state, CXCR7 and β-arrestin2 co-localized near the plasma membrane of CD34+ cells. After CXCL12 treatment, β-arrestin2 translocated to the nucleus, and this required both CXCR7 and CXCR4. Silencing β-arrestin expression decreased CXCL12-induced Akt activation in CD34+ cells. Our results demonstrate for the first time the role of CXCR7, complementary to that played by CXCR4, in the control of HSPC cycling, survival, and colony formation induced by CXCL12. We also provide evidence for the involvement of β-arrestins as signaling hubs downstream of both CXCL12 receptors in primary human HSPCs.
Introduction
Hematopoiesis is tightly orchestrated by a precise quiescence/cycling equilibrium that is required for the continuous controlled production of differentiated blood cells. Chemokines and their receptors play an essential role in maintaining the hematopoietic stem/progenitor cells (HSPCs) pool within stem cell niches.
In addition to its well-known effect on HSPC trafficking, the CXCL12/SDF-1 chemokine is a key regulator of hematopoiesis homeostasis, acting at low concentrations as a survival and cell cycle–promoting factor for CD34+ HSPCs.1,2 We recently demonstrated the pivotal role of 2 downstream effectors of the PI3K/Akt pathway, FoxO3a and mTOR, as key signaling nodes in CXCL12/transforming growth factor-β–induced control of the cycling/quiescence switch.3
CXCR4 was the first identified receptor for CXCL124 and was long considered as the only CXCL12 receptor until the discovery of a second receptor, CXCR7, also known as RDC1.5,6 Studies using CXCR7-deficient mice permitted the demonstration of a crucial role for this receptor in endothelial, cardiac, and B-cell development. However, no obvious hematopoietic phenotype could be shown in these mice.7 The absence of hematopoietic phenotype contrasts with the defects observed in CXCL12- and CXCR4-deficient mice8-10 and supports the hypothesis of distinct developmental roles for CXCR7 and CXCR4.
It has been proposed that, like CXCR4, CXCR7 is involved in the migration and survival of various cell types and could provide tumor cells with a proliferative advantage.5,6,11 In the hematopoietic system, the expression of CXCR7 is controversial. Some studies reported the expression of this receptor at the cell surface of lymphocytes and monocytes,5,12,13 whereas other investigators could not find any detectable plasma membrane expression.14 In HSPCs, the low or undetectable plasma membrane expression of CXCR7 observed on CD34+ cells isolated from bone marrow, mobilized peripheral blood (PB), or umbilical cordon blood14,15 contrasts with its abundant intracellular level.16
It has been proposed that CXCR7 might be a nonsignaling “decoy” receptor, unable to generate intracellular signals via G-protein activation.17 However, ligand binding to CXCR7 can activate downstream signaling pathways through β-arrestins, 2 scaffolding proteins that coordinate multiple signaling pathways in addition to their roles in promoting receptor desensitization and trafficking.18-22 In the hematopoietic system, it was proposed that CXCR7 can heterodimerize with CXCR4 in differentiated cells such as T lymphocytes and contribute to CXCL12-mediated chemotaxis through CXCR4-mediated G-protein signaling.7,17 Whereas CXCR7 has been proposed to be involved in CXCL12-triggered integrin activation in cooperation with CXCR4,16 its participation in CXCL12-induced HSPC cycling and survival is unknown. In this context, the present study was aimed to study the role of CXCR7 in the CXCL12-induced effects on human HSPC cycling and survival and to identify its downstream signalization. We made use of CD34+ cells purified from the PB of healthy unmobilized donors that are mainly in G0 to perform a synchronized study of the CXCL12-induced G0-G1 transition and proliferation.
Our results show that CXCR7 is nearly undetectable at the plasma membrane of CD34+ cells from PB. In contrast, its intracellular expression is high and partially co-localized with that of CXCR4. Despite the scarce expression of CXCR7 at the plasma membrane of CD34+ cells, specific CXCL12 binding can be documented. We demonstrate that CXCR7 participates, together with CXCR4, in the promoting effects of CXCL12 on CD34+ cell cycling, survival, and proliferation through Akt activation and that β-arrestins are signaling hubs downstream of the 2 receptors.
Materials and methods
Immunomagnetic Lin– and CD34+ cell separation
Lin– and CD34+ cells were purified from the unmobilized PB of healthy donors with their informed consent by the MACS technology (Miltenyi-Biotech) according to the local institutional review board (Centre de Transfusion Sanguine des Armées, Clamart, France). Studies were conducted in accordance with the Declaration of Helsinki. CD34+ cells were selected directly after density gradient separation (Inc–) or after (Inc+) an overnight incubation as previously described.2 CD34+ cells were verified to be 95% to 98% pure by flow cytometry analysis.
Surface and intracellular CXCR4 and CXCR7 labeling
CD34+ cells were labeled with anti–CXCR4-PE, –CXCR7-PE, or –CD34-Percp antibodies or with their corresponding isotype controls (supplemental Table 1; available on the Blood Web site). Intracellular labeling was performed as previously described.3
CXCL12AF647 binding assay
CD34+ cells were incubated either with CXCL12 (1.6 µg/mL corresponding to 200 nM), CXCL11 (3.32 µg/mL corresponding to 400 nM), anti-CXCR4 and/or -CXCR7 blocking antibodies (10 µg/mL), CXCR7 inhibitors (1 µM), or respective controls, and then incubated with fluorescent CXCL12AF647 (10 ng/mL; Almac Sciences, Scotland, United Kingdom) for 3 hours at 37°C (supplemental Table 1).
Quantitative reverse transcription polymerase chain reaction
Reverse transcription (RT) of total RNA from CD34+ cells was performed as previously described.3 Specific primer sequences are detailed in supplemental Table 2. Data were normalized on the TATA box binding protein (TBP) housekeeping gene using the 2ΔΔCT method.
Confocal microscopy
CD34+ cells were stained with anti–CXCR4-FITC, anti–CXCR7-PE, anti–β-arrestin2 antibodies, or isotype controls (supplemental Table 1) in PBS1X containing 0.5%BSA/0.1%Triton/5%goat serum. For β-arrestin2 translocation experiments, cells were incubated with CXCR4 and/or CXCR7 blocking antibodies or respective controls (10 µg/mL) and then incubated at 37°C for 45 minutes with CXCL12 (0.5 ng/mL). Staining was examined with a TC5 SP5 confocal microscope (Leica, Wetzlar, Germany). Images were processed using Photoshop and ImageJ software.
CXCR4 and CXCR7 coimmunoprecipitation
A coimmunoprecipitation experiment was performed as previously described23 using anti-CXCR7 or anti-CXCR4 antibodies (supplemental Table 1).
Western blot
An equivalent number of CD34+ cells per condition were lysed as previously described.23 Proteins were loaded in an SDS-polyacrylamide gel and transferred to a polyvinylidene fluoride membrane. Primary antibodies included an anti–phospho-Akt, anti–total Akt, anti-actin or anti–β-arrestin2 (supplemental Table 1). Alexa Fluor-680 anti-rabbit (Invitrogen) or -800 anti-mouse (Thermo-Scientific) antibodies were added to detect specific proteins using an Odyssey scanner.
β-arrestin silencing
Freshly purified CD34+ cells were transfected with 2 µM ON-TARGET plus SMART pool siRNA targeting human β-arrestin2 (Thermo-Scientific) or ON-TARGET plus nontargeting pool siRNA (Thermo-Scientific) using an Amaxa Human CD34+ Cell Nucleofector Kit (Lonza) according to the manufacturer’s instructions and were transferred in SynH medium supplemented with TPO, Flt3-L, and SCF at 37°C for 3 days (Abcell-Bio).
CD34+ cell cultures
Freshly purified CD34+ cells were incubated in a serum- and cytokine-free StemαA medium (Stem-α, Beaucaire, France) in the presence or absence of CXCL12 (0.5 ng/mL), blocking anti-CXCR4, -CXCR7 or isotype control (10 µg/mL). In some experiments, cells were incubated with CXCR7 inhibitory compounds: CCX704 (control), or CCX771 and CCX733 (1 µM or 100 nM).
For colony formation, PB CD34+ cells were plated at a density of 2000 cells/mL in methylcellulose media supplemented with cytokines according to the manufacturer’s instructions (Methocult GF+ H4435;Stem Cell Technologies).
Intracellular detection of cyclin expression, cell cycle fractions, and phospho-Akt level
Cyclin expression and cell cycle analysis were performed using anti–Ki67-FITC or anti–cyclin A, B1, D1, or D3-FITC antibodies (supplemental Table 1) as previously described.3
For phospho-Akt detection, cells were stimulated 15 minutes in a serum- and cytokine-free StemαA medium in the presence or absence of CXCL12 (0.5 ng/mL) and stained with an anti–phospho-Akt antibody.
Statistical analysis
Data were expressed as means ± SD. The significance between the different conditions and their control was determined by Student t test for paired samples. A P value ≤ .05 was considered significant.
Results
CXCR7 is mainly expressed in intracellular compartments and is partly co-localized with CXCR4 in PB CD34+ cells
We first verified the specificity of anti-CXCR7 antibodies (9C4 and 11G8 clones) in SkBr3 cells expressing no CXCR7 mRNA (supplemental Methods and supplemental Figure 1A). When CXCR7– SkBr3 cells were transfected with a CXCR7-YFP plasmid, we showed that only CXCR7-YFP plasmid–transfected cells were stained by both anti-CXCR7 antibodies (supplemental Figure 1B-C).
We further showed that CXCR7 was expressed at the cell surface of Lin– cells from unmobilized PB and that a very low percentage of Lin– cells co-expressed CXCR7 and CD34 (0.126% ± 0.06%, n = 3, Figure 1A). However, CXCR7 was mostly undetectable at the membrane of freshly purified (Inc–) PB CD34+ cells (Figure 1B). Whereas a low percentage of CD34+ cells expressed CXCR4 at the plasma membrane (6.8% ± 1.6%, n = 3, Figure 1B), >85% of PB CD34+ cells expressed both intracellular CXCR7 and CXCR4 (87.8 ± 10.9% and 95.3% ± 5.8%, respectively; n = 3-5, Figure 1C).
CXCR7 is mainly expressed in intracellular compartments and is partly co-localized with CXCR4 in PB CD34+cells. (A) The CXCR7 protein expression level in Lin– immunomagnetically selected cells was analyzed on a FACScalibur flow cytometer after labeling with specific antibodies. Results are representative of 1 experiment of the 3 we performed. (B-C) PB CD34+ cells were purified immediately after density gradient separation (Inc–) or after incubation on a plastic support (Inc+). The CXCR4 and CXCR7 extracellular and intracellular expression were analyzed on a FACScalibur flow cytometer after labeling with specific antibodies. Results are representative of 1 experiment of the 3 to 6 performed. Histograms show the percentage of CXCR4- and CXCR7-expressing CD34+ cells and are expressed as mean ± SD. ***Significance between experimental conditions (P ≤ .001). (D) The CXCR4 and CXCR7 mRNA expression levels in freshly purified PB CD34+ cells (Inc–) were analyzed by quantitative RT polymerase chain reaction. The expression levels of each receptor are normalized on the TBP housekeeping gene by a relative quantification. ns, no significance between both receptors. Results are representative of 1 experiment of the 7 performed. (E) PB CD34+ cells were incubated for 45 minutes in a serum- and cytokine-free medium in the presence or absence of CXCL12 (0.5 ng/mL). The CXCR4 and CXCR7 protein expression of cells were analyzed by a TC5 SP5 confocal microscope (Leica, Wetzlar, Germany) after being labeled with specific antibodies. The scale bar represents 10 µm. (F) KG1 cells (4.107 ) were incubated with or without CXCL12 (0.5 ng/mL) for 5 minutes, lysed, immunoprecipated with anti-CXCR4 or anti-CXCR7 antibodies, and immunoblotted with anti-CXCR7 antibody.
CXCR7 is mainly expressed in intracellular compartments and is partly co-localized with CXCR4 in PB CD34+cells. (A) The CXCR7 protein expression level in Lin– immunomagnetically selected cells was analyzed on a FACScalibur flow cytometer after labeling with specific antibodies. Results are representative of 1 experiment of the 3 we performed. (B-C) PB CD34+ cells were purified immediately after density gradient separation (Inc–) or after incubation on a plastic support (Inc+). The CXCR4 and CXCR7 extracellular and intracellular expression were analyzed on a FACScalibur flow cytometer after labeling with specific antibodies. Results are representative of 1 experiment of the 3 to 6 performed. Histograms show the percentage of CXCR4- and CXCR7-expressing CD34+ cells and are expressed as mean ± SD. ***Significance between experimental conditions (P ≤ .001). (D) The CXCR4 and CXCR7 mRNA expression levels in freshly purified PB CD34+ cells (Inc–) were analyzed by quantitative RT polymerase chain reaction. The expression levels of each receptor are normalized on the TBP housekeeping gene by a relative quantification. ns, no significance between both receptors. Results are representative of 1 experiment of the 7 performed. (E) PB CD34+ cells were incubated for 45 minutes in a serum- and cytokine-free medium in the presence or absence of CXCL12 (0.5 ng/mL). The CXCR4 and CXCR7 protein expression of cells were analyzed by a TC5 SP5 confocal microscope (Leica, Wetzlar, Germany) after being labeled with specific antibodies. The scale bar represents 10 µm. (F) KG1 cells (4.107 ) were incubated with or without CXCL12 (0.5 ng/mL) for 5 minutes, lysed, immunoprecipated with anti-CXCR4 or anti-CXCR7 antibodies, and immunoblotted with anti-CXCR7 antibody.
As was previously reported,2 overnight incubation on plastic (Inc+) increased CXCR4 cell surface expression (6.77% ± 1.61% vs 65.27% ± 6.38%, P ≤ .001, n = 3, Figure 1B). In contrast, CXCR7 remained mostly undetectable at the plasma membrane after overnight incubation on plastic (Figure 1B). However, the percentage of cells expressing intracellular CXCR4 or CXCR7 was unchanged between Inc– and Inc+ cells (95.3% ± 5.8% vs 85% ± 21.9% and 87.8 ± 10.9% vs 84.8 ± 7.7%, respectively; Figure 1C). We also demonstrated the presence of CXCR7 mRNA in freshly purified PB CD34+ cells and showed that its relative expression was similar to that of CXCR4 (0.7 ± 0.4 vs 1.0 ± 0.4 AU, respectively; Figure 1D).
Because of the presence of intracellular CXCR4 and CXCR7 in CD34+ cells, we investigated whether both receptors were co-localized on PB CD34+ cells. Confocal microscopy analysis showed that a small proportion of CXCR4 and CXCR7 were co-localized in the CD34+ cell cytoplasm and that a 1-hour incubation with CXCL12 (0.5 ng/mL) did not modify their cell distribution (Figure 1E). Receptor co-localization prompted us to investigate whether CXCR4/CXCR7 heterodimers exist in HSPCs. Given the small number of CD34+ cells purified from unmobilized PB, co-immunoprecipitation experiments were performed in KG1 cells as a model of CD34+ HSPCs that expressed a similar scarce plasma membrane CXCR7. Figure 1F shows the presence of CXCR4/CXCR7 heterodimers in KG1 CD34+ cells regardless of whether they were treated with CXCL12 (0.5 ng/mL).
Altogether, our results show that CXCR7 is expressed in PB CD34+ cells, where it is partly co-localized with CXCR4, and that its expression is mainly restricted to the intracellular compartment.
CXCL12 binds to CXCR7 in PB CD34+ cells
CXCR7 was reported to internalize constitutively and to rapidly recycle back to the cell surface.16,21 By using Alexafluor-647–coupled CXCL12 (CXCL12AF647), a fully functional and specific CXCL12 chemokine derivative,24,25 we showed that CXCL12AF647 could bind to CD34+ HSPCs (n = 4, Figure 2A). CXCL12AF647 binding was specifically inhibited by the addition of CXCL12 (9.5 ± 2.6 AU vs 100 AU, P ≤ .001, n = 4, Figure 2B). Pretreatment with either CXCR4- and/or CXCR7-blocking antibodies (9C4 and 11G8 clones) inhibited CXCL12AF647 binding compared with control IgG (41.6 ± 5.0 AU vs 100 AU, P ≤ .001, n = 4; 70.6 ± 9.5 AU vs 100 AU, P ≤ .01, n = 4; 84.5 ± 11.1 AU vs 100 AU, P ≤ .05, n = 4, respectively; Figure 2A-B). A similar inhibitory effect was observed after treatment with CCX733 or CCX771, 2 CXCR7 antagonists (64.5 ± 5.9 AU vs 100 AU, P ≤ .001, n = 4; 72.2 ± 13.2 AU vs 100 AU, P ≤ .05, n = 4, respectively; Figure 2A-B). Moreover, the inhibitory effect of CXCR7 and CXCR4 antibodies was additive (69.2 ± 5.4 vs 61.2 ± 5.2%, P ≤ .05, n = 4, Figure 2C). More importantly, CXCL12AF647 binding was also inhibited by the addition of CXCL11, the other natural ligand of CXCR7 (65.8 ± 17.4 AU vs 100 AU, P ≤ .05, n = 4, Figure 2B).
CXCL12 binds to CXCR7 in PB CD34+cells. Fresh PB CD34+ cells were incubated in serum- and cytokine-free medium supplemented with CXCL12AF647 (10 ng/mL) for 3 hours and CXCL12-, CXCL11-, CXCR4-, and/or CXCR7- (9C4 and 11G8) blocking antibodies, CXCR7 inhibitors, isotype controls, or CCX704 control. Analysis was performed on a BD Fortessa flow cytometer. (A) Histograms show CXCL12AF647 binding CD34+ cells in each condition. Results are representative of 1 experiment of the 3 performed. (B) Histograms show MFI of cells determined by a BD Fortessa flow cytometer in each experimental condition and expressed as mean ± SD (n = 4). Control was normalized at 100 arbitrary units in each experimental condition. (C) Histograms illustrate CXCL12AF647 binding inhibition in CD34+ cells for each condition. The percentage of CXCL12AF647 binding inhibition was determined using the formula previously described24 : (1−[MFI−MFINC]/[MFIPC−MFINC])*100. MFI corresponds to mean fluorescence intensity of cells incubated with CXCL12AF647 and CXCL12, CXCL11, CXCR4, and/or CXCR7 (9C4 and 11G8) blocking antibodies or CXCR7 inhibitors; MFIPC to the cells incubated with CXCL12AF647 and respective controls; and MFINC to cell autofluorescence. Asterisks indicate significance vs control conditions (*P ≤ .05, **P ≤ .01, ***P ≤ .001). Deltas indicate significance between experimental conditions (ΔP ≤ .05, ΔΔP ≤ .01, ΔΔΔP ≤ .001).
CXCL12 binds to CXCR7 in PB CD34+cells. Fresh PB CD34+ cells were incubated in serum- and cytokine-free medium supplemented with CXCL12AF647 (10 ng/mL) for 3 hours and CXCL12-, CXCL11-, CXCR4-, and/or CXCR7- (9C4 and 11G8) blocking antibodies, CXCR7 inhibitors, isotype controls, or CCX704 control. Analysis was performed on a BD Fortessa flow cytometer. (A) Histograms show CXCL12AF647 binding CD34+ cells in each condition. Results are representative of 1 experiment of the 3 performed. (B) Histograms show MFI of cells determined by a BD Fortessa flow cytometer in each experimental condition and expressed as mean ± SD (n = 4). Control was normalized at 100 arbitrary units in each experimental condition. (C) Histograms illustrate CXCL12AF647 binding inhibition in CD34+ cells for each condition. The percentage of CXCL12AF647 binding inhibition was determined using the formula previously described24 : (1−[MFI−MFINC]/[MFIPC−MFINC])*100. MFI corresponds to mean fluorescence intensity of cells incubated with CXCL12AF647 and CXCL12, CXCL11, CXCR4, and/or CXCR7 (9C4 and 11G8) blocking antibodies or CXCR7 inhibitors; MFIPC to the cells incubated with CXCL12AF647 and respective controls; and MFINC to cell autofluorescence. Asterisks indicate significance vs control conditions (*P ≤ .05, **P ≤ .01, ***P ≤ .001). Deltas indicate significance between experimental conditions (ΔP ≤ .05, ΔΔP ≤ .01, ΔΔΔP ≤ .001).
We also performed a 125I-CXCL12 radiobinding assay on KG1 cells as a model of CD34+ cells and showed that the addition of either CXCL12, CXCL11, anti-CXCR7 antibody (11G8 clone), or CCX771 inhibitor inhibited 125I-CXCL12 binding to CXCR7 (supplemental Figure 2).
These data demonstrate that CXCR7 can bind its natural ligands CXCL12 and CXCL11 despite its low membrane expression in CD34+ cells.
The cell cycling–promoting effect of CXCL12 on CD34+ cells is mediated by both CXCR7 and CXCR4
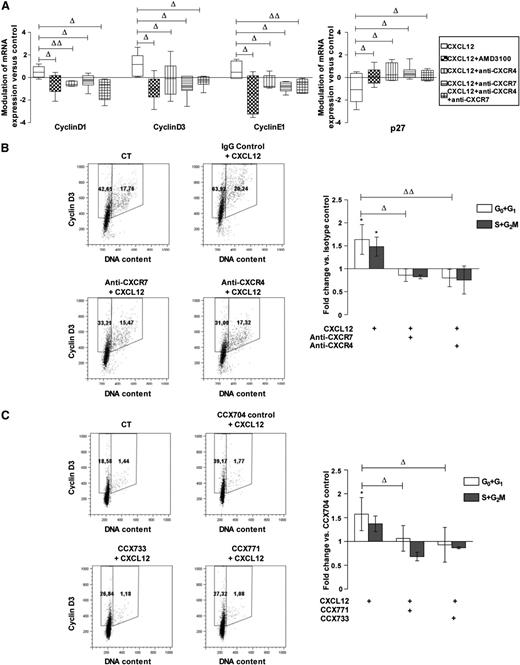
We analyzed the respective contributions of CXCR7 and CXCR4 in the modulation of cyclin and inhibitor of cyclin-dependent kinase levels by CXCL12.3 Figure 3A shows that the addition of either AMD3100 (a CXCR4 antagonist) or CXCR4 or CXCR7-blocking antibodies, alone or in combination, inhibited the effect of a 24-hour incubation with CXCL12 (0.5 ng/mL) on transcripts of D1, D3, and E1 cyclins and of p27, an inhibitor of cyclin-dependent kinases. We next studied the effect of CXCR4- and CXCR7-inhibiting molecules on cyclin expression in CD34+ cells. Figure 3B-C shows that, consistent with its effect on transcripts, CXCL12 (0.5 ng/mL) increased the percentage of cells expressing cyclin D3, an early G1 cyclin, compared with controls. Similar results were obtained for cyclins D1, A, and B (supplemental Figure 3). Treatment with either anti-CXCR4 or anti–CXCR7-blocking antibodies or with CCX771 or CCX733 inhibitors, significantly inhibited the percentage of cells in G0+G1 expressing cyclin D3 upon CXCL12 addition (fold change vs isotypic-treated control: 0.80 ± 0.19, 0.86 ± 0.14 for anti-CXCR4 and anti-CXCR7, respectively, n = 4; 1.06 ± 0.27, 0.93 ± 0.36 for CCX771 and CCX733, respectively, n = 3). In contrast, treatment with control IgGs or with CCX704, a compound that does not bind to CXCR7, did not significantly affect cyclin expression (data not shown). The simultaneous neutralization of CXCR4 and CXCR7 did not cause any additive inhibition of CXCL12-induced cyclin D3 augmentation compared with the individual neutralization of CXCR4 or CXCR7 (Figure 3B-C), suggesting their cooperativity in the regulation of cyclin D3 levels.
CXCR7 participates with CXCR4 in CXCL12-induced cyclin overexpression in PB CD34+cells. (A) CD34+ cells corresponding to a pool of 9 to 19 normal PB samples were stimulated 24 hours in a serum- and cytokine-free medium in the presence or absence of CXCL12 (0.5 ng/mL) and supplemented with AMD3100, blocking anti-CXCR4 and/or -CXCR7 (11G8) antibodies or isotype control for mRNA expression. The cyclinD1, D3, and E1, and cyclin-dependent kinase inhibitor p27 mRNA levels were quantified by quantitative RT polymerase chain reaction and compared with control conditions. Modulations are normalized on the TBP housekeeping gene by a relative quantification and expressed as a box and whisker plot. Deltas indicate significance between experimental conditions (ΔP ≤ .05, ΔΔP ≤ .01). (B) PB CD34+ cells were stimulated for 48 hours in a serum- and cytokine-free StemαA medium in the presence or absence (CT) of CXCL12 (0.5 ng/mL), blocking anti-CXCR4 and/or -CXCR7 (11G8) antibodies or isotype control, or (C) CCX704, CCX771, or CCX733 compounds. After cyclin D3/PI labeling, the percentages of cyclin D3–expressing cells in G0/G1 or S+G2/M phases were determined by flow cytometry. Dot plots show the results from 1 representative experiment (n = 3-4). Histograms show the modulations of the percentages of cyclin D3–expressing cells in G0/G1 or S+G2/M phases vs control cells as the mean of fold changes ± SD. *Significance vs control cells (P ≤ .05). Deltas indicate significance between conditions (ΔP ≤ .05, ΔΔP ≤ .01).
CXCR7 participates with CXCR4 in CXCL12-induced cyclin overexpression in PB CD34+cells. (A) CD34+ cells corresponding to a pool of 9 to 19 normal PB samples were stimulated 24 hours in a serum- and cytokine-free medium in the presence or absence of CXCL12 (0.5 ng/mL) and supplemented with AMD3100, blocking anti-CXCR4 and/or -CXCR7 (11G8) antibodies or isotype control for mRNA expression. The cyclinD1, D3, and E1, and cyclin-dependent kinase inhibitor p27 mRNA levels were quantified by quantitative RT polymerase chain reaction and compared with control conditions. Modulations are normalized on the TBP housekeeping gene by a relative quantification and expressed as a box and whisker plot. Deltas indicate significance between experimental conditions (ΔP ≤ .05, ΔΔP ≤ .01). (B) PB CD34+ cells were stimulated for 48 hours in a serum- and cytokine-free StemαA medium in the presence or absence (CT) of CXCL12 (0.5 ng/mL), blocking anti-CXCR4 and/or -CXCR7 (11G8) antibodies or isotype control, or (C) CCX704, CCX771, or CCX733 compounds. After cyclin D3/PI labeling, the percentages of cyclin D3–expressing cells in G0/G1 or S+G2/M phases were determined by flow cytometry. Dot plots show the results from 1 representative experiment (n = 3-4). Histograms show the modulations of the percentages of cyclin D3–expressing cells in G0/G1 or S+G2/M phases vs control cells as the mean of fold changes ± SD. *Significance vs control cells (P ≤ .05). Deltas indicate significance between conditions (ΔP ≤ .05, ΔΔP ≤ .01).
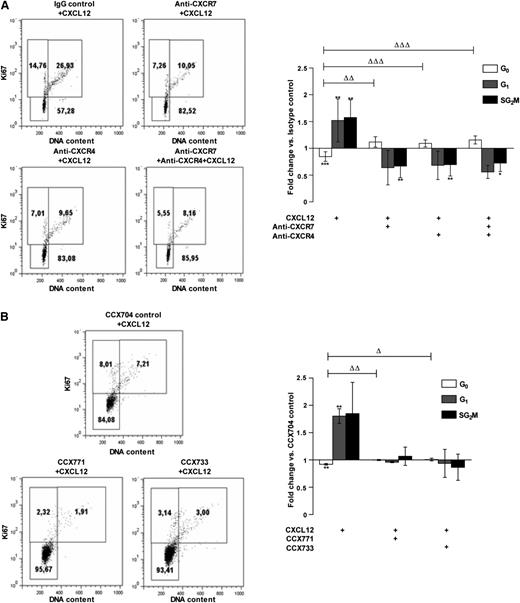
We further determined the respective roles of CXCR4 and CXCR7 on CD34+ cell cycling in response to CXCL12. PB CD34+ cells were stimulated or not with CXCL12 (0.5 ng/mL) for 48 hours in StemαA medium in the presence or absence of either CXCR4- or CXCR7-blocking antibodies (Figure 4A) or CCX771/CCX733 CXCR7 antagonists (Figure 4B). IgGs and CCX704 were used as controls. Incubation with CXCL12 (0.5 ng/mL) decreased the percentage of CD34+ cells in G0 and increased their percentage in G1 and S+G2/M phases, compared with nonstimulated cells (fold change vs untreated controls; Figure 4A: 0.84 ± 0.09, 1.52 ± 0.40, 1.57 ± 0.32 with P ≤ .001, ≤ .01, and ≤ .01 for cells in G0, G1, and S+G2/M, respectively; n = 3-5; Figure 4B: 0.92 ± 0.01, 1.80 ± 0.13, 1.85 ± 0.57 for cells in G0, G1, and S+G2/M with P ≤ .01 and ≤ .01 for cells in G0 and G1, respectively; n = 3-5).
The cell cycling–promoting effect of CXCL12 on PB CD34+cells is mediated by both CXCR7 and CXCR4. (A) PB CD34+ cells were stimulated for 48 hours in a serum- and cytokine-free StemαA medium in the presence or absence of CXCL12 (0.5 ng/mL), blocking anti-CXCR4 and/or -CXCR7 (11G8) antibodies, or isotype control; or (B) CCX704, CCX771, or CCX733 compounds. After Ki67/PI labeling, the percentages of cells in the G0, G1 or S+G2/M phases were determined by flow cytometry. Dot plots show the results from 1 representative experiment (n > 3). The histograms show the modulations of the percentages of cells in G0, G1, or S+G2/M phases vs control cells as a mean of fold changes ± SD (n > 3). Asterisks indicate significance vs control cells (*P ≤ .05, **P ≤ .01). Deltas indicate significance between conditions (ΔP ≤ .05, ΔΔP ≤ .01, ΔΔΔP ≤ .001).
The cell cycling–promoting effect of CXCL12 on PB CD34+cells is mediated by both CXCR7 and CXCR4. (A) PB CD34+ cells were stimulated for 48 hours in a serum- and cytokine-free StemαA medium in the presence or absence of CXCL12 (0.5 ng/mL), blocking anti-CXCR4 and/or -CXCR7 (11G8) antibodies, or isotype control; or (B) CCX704, CCX771, or CCX733 compounds. After Ki67/PI labeling, the percentages of cells in the G0, G1 or S+G2/M phases were determined by flow cytometry. Dot plots show the results from 1 representative experiment (n > 3). The histograms show the modulations of the percentages of cells in G0, G1, or S+G2/M phases vs control cells as a mean of fold changes ± SD (n > 3). Asterisks indicate significance vs control cells (*P ≤ .05, **P ≤ .01). Deltas indicate significance between conditions (ΔP ≤ .05, ΔΔP ≤ .01, ΔΔΔP ≤ .001).
The addition of blocking anti-CXCR4 or anti-CXCR7 antibodies alone or in combination significantly inhibited the cycling of CD34+ cells induced by CXCL12, as shown by a reduction of cells in the S+G2/M phase (0.69 ± 0.21, 0.67 ± 0.21, 0.72 ± 0.16; P ≤ .01, ≤ .01, ≤ .05, n = 3-5 for anti-CXCR4, anti-CXCR7, and anti-CXCR4+anti-CXCR7 vs CXCL12 untreated controls, respectively; Figure 4A). The simultaneous neutralization of CXCR4 and CXCR7 did not display any additive effect, compared with the separate neutralization of CXCR4 or CXCR7. Treatment with either the CCX771 or CCX733 CXCR7 antagonist inhibited the G0/G1 transition in CXCL12-treated cells, as shown by a significant reduction of cells in G1 and in S+G2/M phases (0.96 ± 0.01, 1.07 ± 0.17 and 0.94 ± 0.26, 0.86 ± 0.24) and a parallel increase of cells in G0 (1.01 ± 0.007 and 1.02 ± 0.01) for CCX733 and CCX771, respectively, compared with CCX704-treated controls (P ≤ .05 and ≤ .01, n = 3-5, respectively; Figure 4B).
Altogether, these data indicate that CXCR7 and CXCR4 cooperate to mediate the cell-cycling effect of CXCL12 in PB CD34+ cells.
CXCR7 participates in the CXCL12 priming effect on PB CD34+ cell survival and colony formation
We analyzed the effect of blocking CXCR4/CXCR7 monoclonal antibodies or of CXCR7 antagonists on PB CD34+ cell survival in response to CXCL12 (0.5 ng/mL) in a serum- and cytokine-free StemαA medium. A 48-hour treatment with either control IgG or CCX704 did not significantly modify the number of viable CD34+ cells compared with untreated cells (data not shown). As expected, a treatment with CXCL12 significantly increased the CD34+ cell number, compared with treatment with IgG or CCX704 (1.20 ± 0.05 and 1.26 ± 0.06, P ≤ .05 and ≤ .05, n = 3, respectively; Figure 5A).1,2 In contrast, the addition of anti-CXCR7 or anti–CXCR4-blocking antibodies, separately or in combination, significantly inhibited the CXCL12-promoted survival of CD34+ cells in StemαA medium (0.83 ± 0.08, 0.89 ± 0.04, P ≤ .05 and ≤ .05, respectively, n = 3; Figure 5A). The concurrent inhibition of CXCR4 and CXCR7 did not display any additive inhibition on the CXCL12-promoted survival effect (Figure 5A). Similarly, the CXCR7 antagonists CCX733 (0.85 ± 0.05; P ≤ .05, n = 3) or CCX771 (0.76 ± 0.10; P ≤ .05, n = 3) significantly inhibited the effect of CXCL12 on CD34+ cell survival, whereas the CCX704 control compound was ineffective (Figure 5A). The addition of CXCR4- and CXCR7-blocking antibodies or inhibiting compounds also reduced the CXCL12 priming effect on colony formation by PB CD34+ cells (Figure 5B). This reduction similarly affected multipotent progenitors (CFU-Mix) as well as progenitors of erythroid (BFU-E), granulomacrophagic (CFU-GM), and granulocytic (CFU-G) lineages (Figure 5B).
CXCR7 participates in the CXCL12 priming effect on PB CD34+cell survival and colony formation. (A) PB CD34+ cells were stimulated for 48 hours in a serum- and cytokine-free StemαA medium in the presence or absence of CXCL12 (0.5 ng/mL), blocking anti-CXCR4 and/or -CXCR7 (11G8) antibodies or isotype control, or CCX704 or CCX733 compounds. After trypan blue labeling, the number of live cells were determined by counting on a Malassez cell. Histograms show live cell number modulations vs control as a mean of fold changes ± SD (n = 3). Asterisk indicates significance vs control (*P ≤ .05). Deltas indicate significance between conditions (ΔP ≤ .05, ΔΔP ≤ .01). (B) PB CD34+ cells were stimulated (2.105 cells/mL) for 24 hours in serum- and cytokine-free StemαA medium in the presence or absence of CXCL12 (0.5 ng/mL), blocking anti-CXCR4 and/or -CXCR7 (11G8) antibodies or isotype control, or CCX704 or CCX733 compound. Cells were then placed at a density of 2000 cells/mL in methylcellulose media supplemented with cytokines in 35-mm Petri dishes. Colonies were scored using an inverted microscope after 14 days of culture on the basis of morphological criteria. Histograms show modulations of the total number of colonies including BFU-E, CFU-G, CFU-GM, and CFU-MIX vs untreated cells. Results are expressed as the mean of fold changes ± SD (n = 3). Asterisks indicate significance vs untreated cells (*P ≤ .05, **P ≤ .01). Deltas indicate significance between conditions (ΔP ≤ .05, ΔΔP ≤ .01).
CXCR7 participates in the CXCL12 priming effect on PB CD34+cell survival and colony formation. (A) PB CD34+ cells were stimulated for 48 hours in a serum- and cytokine-free StemαA medium in the presence or absence of CXCL12 (0.5 ng/mL), blocking anti-CXCR4 and/or -CXCR7 (11G8) antibodies or isotype control, or CCX704 or CCX733 compounds. After trypan blue labeling, the number of live cells were determined by counting on a Malassez cell. Histograms show live cell number modulations vs control as a mean of fold changes ± SD (n = 3). Asterisk indicates significance vs control (*P ≤ .05). Deltas indicate significance between conditions (ΔP ≤ .05, ΔΔP ≤ .01). (B) PB CD34+ cells were stimulated (2.105 cells/mL) for 24 hours in serum- and cytokine-free StemαA medium in the presence or absence of CXCL12 (0.5 ng/mL), blocking anti-CXCR4 and/or -CXCR7 (11G8) antibodies or isotype control, or CCX704 or CCX733 compound. Cells were then placed at a density of 2000 cells/mL in methylcellulose media supplemented with cytokines in 35-mm Petri dishes. Colonies were scored using an inverted microscope after 14 days of culture on the basis of morphological criteria. Histograms show modulations of the total number of colonies including BFU-E, CFU-G, CFU-GM, and CFU-MIX vs untreated cells. Results are expressed as the mean of fold changes ± SD (n = 3). Asterisks indicate significance vs untreated cells (*P ≤ .05, **P ≤ .01). Deltas indicate significance between conditions (ΔP ≤ .05, ΔΔP ≤ .01).
These results indicate that CXCR7, in cooperation with CXCR4, mediates the survival and colony formation–promoting effects of CXCL12 on PB CD34+ cells.
CXCR7 and CXCR4 mediate the CXCL12-induced Akt phosphorylation in PB CD34+ cells through β-arrestins
Confocal analysis showed that β-arrestin2 is expressed in the cytoplasm of freshly purified CD34+ cells and was partially co-localized with CXCR7 (Figure 6A). A 45-minute incubation with CXCL12 (0.5 ng/mL) induced translocation of β-arrestin2 to the nucleus (Figure 6A). This nuclear translocation was inhibited after neutralization of either CXCR4 or CXCR7 with specific antibody or antagonist (0.63 ± 0.06 and 0.53 ± 0.15 vs 0.88 ± 0.07 respectively, P ≤ .05; Figure 6B).
CXCR7 and CXCR4 mediate the CXCL12-induced Akt phosphorylation in PB CD34+cells through β-arrestins. (A) PB CD34+ cells were incubated or not in serum- and cytokine-free medium supplemented with CXCL12 (0.5 ng/mL) for 45 minutes. β-arrestin2 and CXCR7 localization were analyzed using a TC5 SP5 confocal microscope (Leica, Wetzlar, Germany) after labeling with specific antibodies. The scale bar represents 10 µm.. (B) PB CD34+ cells were incubated in serum- and cytokine-free medium supplemented with CXCL12 (0.5 ng/mL) and blocking anti-CXCR4 antibody or CXCR7 inhibitor (CCX733) for 45 minutes. The translocation of β-arrestin2 to the nucleus was analyzed with a TC5 SP5 confocal microscope (Leica) after labeling with specific antibody. Histograms show the ratio of β-arrestin2 nuclear translocated cells. Asterisks indicate significance between conditions (*P ≤ .05, **P ≤ .01). The scale bar represents 10 µm. (C) PB CD34+ cells were stimulated 15 minutes in a serum- and cytokine-free StemαA medium in the presence or absence of CXCL12 (0.5 ng/mL), CCX704, or CCX771 compounds, blocking anti-CXCR4 antibody, or isotype control IgG. After phospho-Akt-Ser473 labeling, the percentages of expressing cells were determined by flow cytometry by comparison with the isotype control profile. The histogram shows the percentage of phospho-Akt–expressing cells as mean ± SD (n = 3 to 4). Deltas indicate significance between conditions (ΔΔP ≤ .01, ΔΔΔP ≤ .001). (D) Scramble or β-arrestin2 siRNA–transfected CD34+ cells were incubated with CXCL12 (0.5 ng/mL) for 5, 10, 15, and 30 minutes. Western blot analysis was then done and the membrane was immunoblotted with specific antibodies against pAkt, total Akt, actin, and β-arrestin2. The histogram shows the percentage of β-arrestin2–expressing CD34+ cells after scramble or β-arrestin2 siRNA transfection. The asterisk indicates significance between conditions (*P ≤ .05).
CXCR7 and CXCR4 mediate the CXCL12-induced Akt phosphorylation in PB CD34+cells through β-arrestins. (A) PB CD34+ cells were incubated or not in serum- and cytokine-free medium supplemented with CXCL12 (0.5 ng/mL) for 45 minutes. β-arrestin2 and CXCR7 localization were analyzed using a TC5 SP5 confocal microscope (Leica, Wetzlar, Germany) after labeling with specific antibodies. The scale bar represents 10 µm.. (B) PB CD34+ cells were incubated in serum- and cytokine-free medium supplemented with CXCL12 (0.5 ng/mL) and blocking anti-CXCR4 antibody or CXCR7 inhibitor (CCX733) for 45 minutes. The translocation of β-arrestin2 to the nucleus was analyzed with a TC5 SP5 confocal microscope (Leica) after labeling with specific antibody. Histograms show the ratio of β-arrestin2 nuclear translocated cells. Asterisks indicate significance between conditions (*P ≤ .05, **P ≤ .01). The scale bar represents 10 µm. (C) PB CD34+ cells were stimulated 15 minutes in a serum- and cytokine-free StemαA medium in the presence or absence of CXCL12 (0.5 ng/mL), CCX704, or CCX771 compounds, blocking anti-CXCR4 antibody, or isotype control IgG. After phospho-Akt-Ser473 labeling, the percentages of expressing cells were determined by flow cytometry by comparison with the isotype control profile. The histogram shows the percentage of phospho-Akt–expressing cells as mean ± SD (n = 3 to 4). Deltas indicate significance between conditions (ΔΔP ≤ .01, ΔΔΔP ≤ .001). (D) Scramble or β-arrestin2 siRNA–transfected CD34+ cells were incubated with CXCL12 (0.5 ng/mL) for 5, 10, 15, and 30 minutes. Western blot analysis was then done and the membrane was immunoblotted with specific antibodies against pAkt, total Akt, actin, and β-arrestin2. The histogram shows the percentage of β-arrestin2–expressing CD34+ cells after scramble or β-arrestin2 siRNA transfection. The asterisk indicates significance between conditions (*P ≤ .05).
CXCL12-promoted signaling pathways include the PI3K/Akt axis.3 Therefore we investigated whether CXCR7 could affect Akt phosphorylation in response to CXCL12. As expected, CXCL12 (0.5 ng/mL) increased the percentage of phospho-Akt–expressing cells, compared with the basal level of phospho-Akt in cells treated with CCX704 or isotypic antibody controls (Figure 6C). Blocking either CXCR7 or CXCR4 prevented the CXCL12-induced Akt phosphorylation (Figure 6C), whereas simultaneous inhibition of both receptors did not show any additive effect (0.21 ± 0.21, 0.07 ± 0.04 and 0.13 ± 0.14, respectively, n = 3-4; Figure 6C).
We next investigated whether β-arrestin2 participated in CXCL12-induced Akt phosphorylation by silencing β-arrestin2 using siRNA. Such a treatment reduced β-arrestin2 protein expression by 61.7 ± 18.9% in CD34+ cells (P ≤ .05, n = 3; Figure 6D). CXCL12 (0.5 ng/mL) induced Akt phosphorylation in scramble siRNA-treated CD34+ cells, which progressively decreased after 5 minutes of stimulation. In contrast, β-arrestin2 siRNA markedly reduced the maximal level of CXCL12-induced Akt phosphorylation (Figure 6D). A similar inhibition was observed using another siRNA targeting both β-arrestin1 and β-arrestin2 (data not shown).
These results suggest that CXCL12 induces Akt phosphorylation in PB CD34+ cells via both CXCR7 and CXCR4 receptors and that it involves a β-arrestin2–dependent signaling.
Discussion
Hematopoiesis is a tightly regulated process resulting from a balance between HSPC quiescence for “stemness” maintenance, proliferation/differentiation for blood cell supply, and mobilization. The CXCL12/SDF-1 chemokine plays a crucial role in this equilibrium within stem cell niches.4,26,27 We have previously shown that CXCL12 induces HSPC survival and cycling at low concentrations (0.5 ng/mL)1,2 and that this process involves the activation of the Akt/mTOR/FoxO3a pathway.3 CXCL12 was initially thought to activate a single CXCR4 receptor, which participates in normal and malignant cell trafficking. Recently, a second receptor for CXCL12, named CXCR7, was discovered; however, its expression in HSPCs and its role in CXCL12-induced cell migration remained controversial.5,13-17,19 Furthermore, its involvement in the control of cell cycling and survival induced by CXCL12 had never been studied in human HSPCs.
The present study shows that CXCR7 is expressed in PB CD34+ HSPCs, where this receptor is found in the intracellular compartments, partly co-localized with CXCR4. Despite its scarce plasma membrane expression, CXCR7 binds CXCL12 and participates, together with CXCR4, to promote the survival and cycling effect of CXCL12 on HSPCs, via the β-arrestin–dependent activation of Akt.
In contrast to CXCR4, the expression profile of CXCR7 has not been extensively studied in CD34+ cells. In these cells, its expression has been reported to be mostly intracellular, whereas its membrane expression is controversial because it was reported to be either not expressed or expressed at very low levels in CD34+ cells purified from either bone marrow, cord blood, or mobilized PB.14-16 In the present study, we show that CXCR7 is expressed in CD34+ cells from unmobilized PB. However, whereas CXCR7 and CXCR4 mRNA levels are comparable, their subcellular distribution is different because, in contrast to CXCR4, CXCR7 is mainly restricted to the intracellular compartment. A low percentage of PB CD34+ Lin– cells express CXCR7 at the plasma membrane and become undetectable in purified PB CD34+ cells. Because CXCR7 expression is preferentially found on Lin– CD34low cells, it is possible that this subpopulation was lost during the purification procedure, which preferentially isolates CD34high cells.
Despite these quantitative limitations, CXCR7 can bind its 2 natural ligands CXCL12 and CXCL11 at the surface of PB CD34+ cells. The disconnection between the CXCR7 biological effect and the scarce plasma membrane expression detected on these cells is still not understood. Such discrepancy may reflect a posttranslational regulation of the protein as proposed in neurons.28 It may also attest for other mechanisms including GPCR oligomerization and regulation of GPCR export by escort proteins.29 A constant recycling of a small CXCR7 pool on the outer leaflet of the plasma membrane as reported in human T lymphocytes and umbilical cord blood CD34+ cells16 can also be suggested.
CXCR7 has been reported to play a role in the CXCL12-induced effects alone or together with CXCR4. Whereas CXCL12 regulated the adhesion, proliferation, and angiogenesis of endothelial progenitors through CXCR4 and CXCR7, it participated in their survival effect through CXCR7 alone.30 In lymphocytes, it is suggested that both receptors are involved in rapid CXCL12-triggered integrin activation16 and that CXCR4/CXCR7 heterodimers regulated CXCL12-promoted chemotaxis.16,17,30 Although CXCR7 has been shown to participate in the control of cell cycle gene expression in a murine adenoma cell line,31 its role in the survival and cell cycle–promoting effect of CXCL12 has not been reported in HSPCs. In the present study, the co-expression of CXCR7 and CXCR4 in PB CD34+ HSPCs led us to investigate their roles in CXCL12-induced cell cycle–promoting activity. Our data showing that the neutralization of either CXCR7 or CXCR4 alone or both together significantly inhibits (i) modulation of cyclins and p27 CDKI, (ii) cell cycle–promoting activity, (iii) survival, and (iv) colony formation of CD34+ cells in response to CXCL12, demonstrate the participation of CXCR7, together with CXCR4 in these biological effects. Our data are in agreement with recent studies reporting that although CXCR7 and CXCR4 can mediate distinct effects of CXCL12, they can also act in concert through heterodimerization16,17,32 for other biological effects.11,30 In our study, the detection of CXCR7-CXCR4 heterodimers in a CD34+ HSPC cell line by coIP combined with the data showing partial co-localization of the two receptors in freshly purified CD34+ cells by confocal microscopy are consistent with the hypothesis of heterodimeric complexes in CD34+ HSPCs. Interestingly, selective activation of CXCR7 does not induce CD34+ cell mobilization,15 strengthening the role of CXCR7-CXCR4 heterodimer activation in CXCL12-induced biological effects.
Signaling pathways activated in response to CXCL12 include MAPK and PI3K/Akt axes.16,32,33 It has been reported that CXCR7 participates in CXCL12-induced Akt activation in myelo-monocytic and prostate cancer cells15,33 but not in T lymphocytes,16,33 suggesting that the participation of CXCR7 in Akt activation could be cell dependent. We have previously shown that the PI3K/Akt pathway activation participates in CD34+ cell cycling in response to low concentrations of CXCL12.3 Our present results showing that the Akt phosphorylation is dependent on both receptors, demonstrates that CXCR7, together with CXCR4, activates the Akt signaling pathway in PB CD34+ cells.
Except in astrocytes,34 CXCR7 does not to activate G protein–dependent signaling pathways in response to CXCL12.17 However, it can transduce cell signaling through β-arrestins and was proposed to be a “β-arrestin–biased receptor.”19,22 β-arrestin is known to operate as a scaffold protein for modulating signal transduction18,22 and to participate in cell-cycle control and proliferation through direct activation of gene transcription.35 In the present study, we show that CXCR7 and β-arrestin2 co-localize close to the plasma membrane in freshly purified PB CD34+ cells. After CXCL12 treatment, most β-arrestin2 translocates into the nucleus. Although β-arrestin2 usually displays a cytoplasmic distribution caused by constitutive nuclear export,36 it also undergoes active nuclear import and has been shown to accumulate in the nucleus after stimulation of the odorant GPCR hOR17-4 in spermatozoa.37 In these cells, the nuclear accumulation of β-arrestin2 may be implicated in the control of gene expression during the early steps of fertilization. It is therefore possible that the translocation of β-arrestin2 to the CD34+ cell nucleus regulates some of the CXCL12-induced cell cycle–promoting effects through gene regulatory mechanisms.
β-arrestins have been shown to both inhibit and activate the PI3K/Akt pathway depending on the cell surface receptor that is stimulated. For example, downstream of the Ghrelin receptor38 or protease-activated-receptor-1 (PAR-1),39 β-arrestin promotes Akt phosphorylation, whereas after stimulation of the PAR-2,40 dopamine 2 receptor (D2R),41 or lysophosphatidic acid receptor 1 (LPA-1R),42 β-arrestins can inhibit the PI3K/Akt pathway through different mechanisms. Here, our results showing that the silencing of β-arrestin in PB CD34+ cells reduced the CXCL12-induced Akt phosphorylation demonstrate that β-arrestin plays a positive role in PI3K/Akt pathway activation. We showed that β-arrestin2 nuclear translocation and Akt phosphorylation are both dependent on CXCR7 and CXCR4 activation, suggesting that β-arrestins participate in the cross-talk between the 2 receptors in response to CXCL12 in CD34+ cells. Such a hypothesis is in agreement with data from Zabel et al in tumor cells.19
In conclusion, the present study reveals for the first time an essential role of CXCR7, together with CXCR4, in the control of CD34+ survival, cell cycling, and colony formation induced by CXCL12. It also provides evidence for the involvement of β-arrestins as signaling hubs downstream of CXCL12-activated receptors. Therefore, our results revisit the oversimplified model based on the role of the monogamous CXCR4/CXCL12 couple in HSPC regulation43 and integrate CXCR7 as an actor of the quiescence/cycling balance of HSPCs participating in hematopoiesis homeostasis and stem cell protection within bone marrow niches.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We are indebted to Général Marcel Joussemet (Jean Julliard Army Blood Transfusion Center, Clamart, France) for supplying human blood samples. We are very grateful to Mark E.T. Penfold from ChemoCentryx Inc. (Mountain View, CA) for providing the specific reagents for this study (one of the anti-CXCR7 antibodies [11G8 clone] and CCX704, CCX733, and CCX771 compounds), intellectual support, and final approval of the manuscript. We also thank Richard Proust, Jean-François Ottavi, and Emmanuel Dornier for their valuable technical advice.
This work was supported by grants from Ligue Nationale Contre le Cancer (EL2010 [M.-C.L.B.-K.]); Association pour la Recherche sur le Cancer (3332, 3878 [M.G.H.S.]); and Vaincre le Cancer-Nouvelles Recherches Biomédicales, Societé Française d’Hématologie, and Ligue Nationale Contre le Cancer associations (F.T.).
Authorship
Contribution: F.T., A.A., A.C., J.-J.L., and M.-C.L.B.-K. conceived and designed the study; F.T. collected and assembled data; A.A. collected the confocal data; D.C. collected and assembled the flow cytometry data; F.T., A.A., A.C., D.C., J.-J.L., and M.-C.L.B.-K. analyzed and interpreted data; A.A. performed the 125I-CXCL12 radio-labeling studies; B.G. provided the cell material and experimental design; L.B. and M.G.H.S. provided the study material; S.M. performed 125I-CXCL12 radio-labeling studies and interpretation; M.G.H.S. performed β-arrestin data analysis and interpretation; C.D. performed molecular data analysis and interpretation; F.T., M.G.H.S., J.-J.L., and M.-C.L.B.-K. wrote the manuscript; and F.T., A.A., S.M., M.G.H.S., J.-J.L., and M.-C.L.B.-K. gave final approval of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Marie-Caroline Le Bousse-Kerdilès, Inserm, U972, 14 Av Paul-Vaillant Couturier, Villejuif, F-94807, France; e-mail: caroline.le-bousse-kerdiles@inserm.fr.
References
Author notes
J.-J.L. and M.-C.L.B.-K. contributed equally to this study.


![Figure 2. CXCL12 binds to CXCR7 in PB CD34+ cells. Fresh PB CD34+ cells were incubated in serum- and cytokine-free medium supplemented with CXCL12AF647 (10 ng/mL) for 3 hours and CXCL12-, CXCL11-, CXCR4-, and/or CXCR7- (9C4 and 11G8) blocking antibodies, CXCR7 inhibitors, isotype controls, or CCX704 control. Analysis was performed on a BD Fortessa flow cytometer. (A) Histograms show CXCL12AF647 binding CD34+ cells in each condition. Results are representative of 1 experiment of the 3 performed. (B) Histograms show MFI of cells determined by a BD Fortessa flow cytometer in each experimental condition and expressed as mean ± SD (n = 4). Control was normalized at 100 arbitrary units in each experimental condition. (C) Histograms illustrate CXCL12AF647 binding inhibition in CD34+ cells for each condition. The percentage of CXCL12AF647 binding inhibition was determined using the formula previously described24: (1−[MFI−MFINC]/[MFIPC−MFINC])*100. MFI corresponds to mean fluorescence intensity of cells incubated with CXCL12AF647 and CXCL12, CXCL11, CXCR4, and/or CXCR7 (9C4 and 11G8) blocking antibodies or CXCR7 inhibitors; MFIPC to the cells incubated with CXCL12AF647 and respective controls; and MFINC to cell autofluorescence. Asterisks indicate significance vs control conditions (*P ≤ .05, **P ≤ .01, ***P ≤ .001). Deltas indicate significance between experimental conditions (ΔP ≤ .05, ΔΔP ≤ .01, ΔΔΔP ≤ .001).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/123/2/10.1182_blood-2013-05-500496/4/m_191f2.jpeg?Expires=1765030810&Signature=DTLXR7HPll4-IyKIFYrB6rfQHuBwZwAlllkK6YYC67d0TWo4akTHMqZa-cBIQ2FcLZY0QGjxhNxOY6MPmQ7PUrAc3V~KZ8ZcQO6NWSP2gXcT4oCkDRGZzRxD8cCBTU1uEU2Gd72k~kloou9~MHpK9H78udJ6A87~0T-9s3ceTD16sfIcVxsjdg-8frC5cdvD05DvRY3XDpjtriWuIwAi4hACL-29lWY1Lgcn2956LIzQh6QCFB5wYJxkJ78Aps8mq367RXAhIx~qdJy1zzIsPPtrk8EP1FMJKqSClD4vsGHOvgY0~DJZnNh70JgwTMz7eb0mGwedminfPxwkwV-nqQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal