Key Points
First-line CPX-351 vs 7+3 control in newly diagnosed AML improves 60-day mortality, remission rate, and OS (HR = 0.46, P = .01) in sAML subset.
Abstract
CPX-351 is a liposomal formulation of cytarabine:daunorubicin designed to deliver synergistic drug ratios to leukemia cells. In this phase 2 study, newly diagnosed older acute myeloid leukemia (AML) patients were randomized 2:1 to first-line CPX-351 or 7+3 treatment. The goal was to determine efficacy and identify patient subgroups that may benefit from CPX-351 treatment. Response rate (complete remission + incomplete remission) was the primary end point, with event-free survival (EFS) and overall survival (OS) as secondary end points. The 126 patients entered were balanced for disease and patient-specific risk factors. Overall, CPX-351 produced higher response rates (66.7% vs 51.2%, P = .07), meeting predefined criteria for success (P < .1). Differences in EFS and OS were not statistically significant. A planned analysis of the secondary AML subgroup demonstrated an improved response rate (57.6% vs 31.6%, P = .06), and prolongation of EFS (hazard ratio [HR] = 0.59, P = .08) and OS (HR = 0.46, P = .01). Recovery from cytopenias was slower after CPX-351 (median days to absolute neutrophil count ≥1000: 36 vs 32; platelets >100 000: 37 vs 28) with more grade 3-4 infections but without increase in infection-related deaths (3.5% vs 7.3%) or 60-day mortality (4.7% vs 14.6%), indicating acceptable safety. These results suggest a clinical benefit with CPX-351, particularly among patients with secondary AML, and provide the rationale for a phase 3 trial currently underway in newly diagnosed secondary AML patients. This study is registered at Clinicaltrials.gov as #NCT00788892.
Introduction
Acute myeloid leukemia (AML) is a myeloid hematologic neoplasm associated with poor outcomes in older patients, with response rates to conventional induction chemotherapy regimens typically <50% and median survival usually <1 year.1,2 Multiple factors contribute to poor prognosis in older patients, including adverse disease biology and poor patient tolerance to chemotherapy. In fact, advanced age and comorbidities may preclude the administration of intensive induction therapy altogether.1,3-6
Current approaches to the delivery of cytotoxic chemotherapy assume that maximum therapeutic activity correlates with maximum dose intensity. However, this paradigm may ignore possible concentration-dependent drug interactions that ultimately influence whether drugs combine synergistically or antagonistically. In vitro studies have demonstrated that cytarabine and daunorubicin efficacy depend on the molar ratio of the 2 drugs, with the highest proportion of synergy and lowest level of antagonism occurring at a 5:1 molar ratio.7 This suggests that delivery of the maximally synergistic drug ratio to tumor cells may enhance treatment efficacy. CPX-351 is a liposomal formulation of cytarabine and daunorubicin packaged at a 5:1 molar ratio within 100-nm diameter liposomes, which in animal leukemia models markedly improved outcomes compared with the same drugs administered conventionally (ie, free drug) or delivered within liposomes at suboptimal or antagonistic ratios.8 In the absence of encapsulation, conventional drug administration will result in constantly changing drug ratios after dosing, thus preventing the maintenance of any particular drug ratio that could potentially optimize antitumor activity. The obstacle of fluctuating drug ratios may be overcome through the administration of fixed molar ratios within a nano-scale (ie, liposomal) drug delivery vehicle.
A randomized, controlled phase 2 trial design was chosen to evaluate the efficacy of CPX-351: to compare fixed molar ratio encapsulated cytarabine and daunorubicin (CPX-351) against the same 2 drugs administered conventionally (7+3 regimen) in the first-line setting. Consequently, this prospective comparison enables a test of the ratiometric dosing hypothesis: that delivery of a fixed molar ratio of cytarabine and daunorubicin, found to be consistently synergistic in vitro, will be more efficacious than conventional treatment. In addition, comparisons of efficacy in patient subsets, such as those with high-risk or secondary AML, were performed prospectively with the intent of identifying specific populations that may benefit from CPX-351 treatment and therefore justifying further clinical evaluation.
Patients and methods
Study design
This was a multicenter, randomized, open-label, parallel-arm study comparing CPX-351 against first-line conventional chemotherapy (7+3) (Figure 2). Patients from 18 sites in the United States and Canada were enrolled from November 2008 to October 2009. The study was fully compliant with the Declaration of Helsinki and the Code of Good Clinical Practices. Each study center participating in this clinical trial received institutional review board or research ethics board approval before initiation of the research.
Consenting eligible patients were stratified into “standard risk” and “high risk” groups. High risk was defined by any of the following: patients age 70 to 75 years, the presence of secondary AML, or complex karyotype (defined as ≥3 clonal abnormalities). Patients aged 60 to 69 with de novo AML with cytogenetics pending were assigned to the standard risk group. Patients were randomized 2:1 to receive either CPX-351 or 7+3. A 2:1 randomization scheme was used to maximize observations with CPX-351. Response was defined according to International Working Group Criteria and included morphologic complete remission (CR) and morphologic complete remission with incomplete blood count recovery (CRi).9 CRi was included as a form of response because more recent data demonstrate that CRi is clinically beneficial,10 and because achievement of CRi is sufficient grounds for referral for stem cell transplant. Cytogenetic risk categorization was assigned (ie, adverse, intermediate, or unknown) at the end of the study, according to National Comprehensive Cancer Network criteria11 and was used to extend the definition of the high risk group to cover all patients with poor prognosis on the basis of cytogenetic risk.
Control arm patients with persistent AML after 1 or 2 induction courses were permitted to cross over to receive CPX-351 as first salvage, based on the treating investigator’s determination of a very low probability of response to further 7+3 treatment (generally based on <50% reduction of bone marrow blasts compared with baseline). No crossover was permitted for nonresponding patients assigned to CPX-351.
Patient eligibility
Patients 60 to 75 years of age with newly diagnosed, pathologically confirmed AML were eligible. Patients with secondary AML (sAML), defined as having a history of antecedent hematologic disorder, usually with myelodysplastic syndrome (MDS), myeloproliferative neoplasm (MPN), or history of cytotoxic treatment of nonhematologic malignancy were also eligible. Patients were required to have Eastern Cooperative Oncology Group (ECOG) performance status ≤2, serum creatinine <2.0 mg/dL, total bilirubin <2.0 mg/dL, serum alanine aminotransferase and aspartate aminotransferase <3 × upper limit of normal, and left ventricular ejection fraction by echocardiogram or multiple-gated acquisition ≥50%. Patients with prior treatment of AML; evidence of active central nervous system leukemia, acute promyelocytic leukemia, or uncontrolled other malignancies; prior anthracycline exposure >368 mg/m2; cardiovascular disease resulting in heart failure (New York Heart Association Class III or IV); history of Wilson disease or other copper-handling disorders; and hypersensitivity to cytarabine, daunorubicin, or liposomal drugs were excluded. Patients with evidence of active fungal infection, known HIV disease, or active hepatitis C infection were also excluded. However, patients receiving treatment (ie, antibiotic, antifungal, or antiviral) for infections were eligible for enrollment if they were afebrile and hemodynamically stable for ≥72 hours. Patients were not permitted to receive any investigational or antineoplastic drug within 4 weeks of the first dose of study drug, except for hydroxyurea, which was permitted but had to be discontinued 24 hours before the start of study treatment. Patients unable to give full informed consent were ineligible.
Treatment
CPX-351 liposomes are composed of a 7:2:1 molar ratio of distearoyl phosphatidylcholine, distearoyl phosphatidylglycerol, and cholesterol. Each vial of CPX-351 contains 20 mL of CPX-351 suspension at a concentration of 5 U/mL. Each unit of CPX-351 contains 1.0 mg (±10%) cytarabine and 0.44 mg (±10%) daunorubicin. CPX-351 induction therapy was administered by 90-minute infusion at a dose of 100 U/m2 on days 1, 3, and 5 (delivering 100 mg/m2 cytarabine and 44 mg/m2 daunorubicin with each dose). Second induction and consolidation courses were administered at 100 U/m2 on days 1 and 3.12
Control arm treatment consisted of cytarabine 100 mg/m2 per day administered by 7-day continuous infusion and daunorubicin 60 mg/m2 per day administered on days 1, 2, and 3. Daunorubicin could be reduced to 45 mg/m2 per day at the investigator’s discretion for patients with advanced age, poor performance status, or reduced liver and/or kidney function. Choice of consolidation therapy was also at the discretion of the investigator, and recommended regimens included 100 to 200 mg/m2 cytarabine for 5 to 7 days with or without daunorubicin (5+2) or intermediate-dose cytarabine (1.0-1.5 g/m2/dose).
As many as 2 courses of induction and 2 courses of consolidation therapy were permitted in each arm. Postremission therapy with allogeneic hematopoietic stem cell transplant (HSCT) was also permitted.
Supportive care including premedication before chemotherapy, infection prophylaxis, and growth factor treatment was permitted according to local standards. Patients with mild or moderate hypersensitivity reactions could have treatment restarted with diphenhydramine or dexamethasone premedication.
Assessments
All patients receiving at least 1 dose of study medication were considered evaluable for efficacy and safety. Bone marrow examination at days 14 to 21 assessed achievement of marrow aplasia/hypoplasia. Hypoplasia was defined as <5% marrow blasts with <20% marrow cellularity.
Complete response was assessed according to the International Working Group Criteria9 and was defined as CR+CRi. CR required both the clearance of AML (<5% marrow blasts, absence of Auer rods, and disappearance of extramedullary disease and circulating blasts) and evidence of peripheral blood count recovery (neutrophil count ≥1000/μL and platelet count ≥100 000/μL). CRi required clearance of AML with incomplete recovery of blood counts (neutrophils <1000/μL or platelets <100 000/μL).
The primary end point for the study was complete response (CR+CRi) rate. Secondary end points included CR+CRi duration, EFS, and OS. An event for EFS was defined as documented persistent AML after induction, documented relapse after initial CR, initiation of nonprotocol therapy, or death. Patients receiving HSCT were censored for EFS at the start of the conditioning therapy. Patients alive and without progression were censored at the time of last contact for EFS and OS. Survival was calculated from the date of randomization to death. Patients in the control arm who later crossed over to receive CPX-351 were formally assessed for response and EFS based on their original therapy (7+3), even if subsequent response to CPX-351 was observed. These patients were not censored for OS at the time of crossover.
Patient follow-up was extended from 1 year to 2 years when it became evident that median survival would exceed 12 months in the CPX-351 arm.
Adverse event reporting was in compliance with Good Clinical Practice/International Conference on Harmonization guidelines. Adverse events were assessed using the Common Terminology Criteria for Adverse Events, V3.0. Deaths occurring at 30 and 60 days and hematologic and nonhematologic adverse events of grades 3 to 5 severity are presented.
Statistical methods
The primary efficacy end point was complete response (CR+CRi) rate. Enrollment of 120 patients (80 in the CPX-351 arm and 40 in the control arm) was sufficient to detect a 23% increase in response rate with at least 85% power and a one-sided significance level of 0.1. A P value of .1, rather than the traditional P < .05, was used because this study was not intended to replace a formal phase 3 study but rather to provide the basis for designing a subsequent phase 3 study. Chi-square, Fisher exact, and Kruskal-Wallis tests were used to assess differences in baseline patient characteristics. Time-to-event analyses for EFS and OS were performed using the Kaplan-Meier method (KM). All KM analyses were performed by Cancer Research and Biostatistics (Seattle, WA).
Results
Patient characteristics
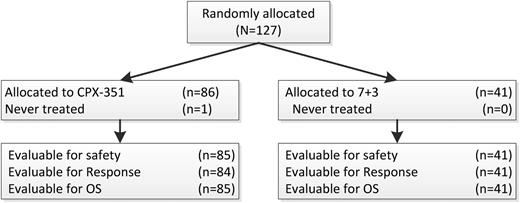
One-hundred twenty-seven patients were randomized, and 126 were treated from 18 sites within the United States and Canada, with 85 treated patients assigned to CPX-351 and 41 assigned to 7+3. Patient characteristics are shown in Table 1. The median age was 70 years, and stratification into the standard and high risk groups led to balanced allocation of patients in both treatment arms for cytogenetic risk, secondary AML status, and ECOG performance status. In addition, similar proportions of patients (39% vs 37%) had prior therapy with hypomethylating agents. One patient randomized and treated with CPX-351 was found to have Philadelphia chromosome–positive disease and was taken off the study after 1 week to begin treatment with imatinib. This patient was not counted for response but was included in the EFS and OS analyses. One patient in the CPX-351 arm required a 50% dose reduction for the last 2 doses of the first induction. All control arm patients received full-dose treatment with 60 mg/m2 daunorubicin. Similar proportions of patients had 2 inductions (17/85, 20% on CPX-351 vs 12/41, 29% on control). Of the 56 responding CPX-351 patients, 23 of 56 (41%) had 1 and 14 of 56 (25%) had 2 consolidations, whereas in the control arm, 6 of 21 (29%) had 1 and 9 of 21 (43%) had 2 consolidations. Among responding patients in the control arm who received consolidation (n = 15), approximately half (n = 7) received consolidation with “5+2”, whereas the remainder (n = 8) received an intermediate/high-dose cytarabine–based consolidation regimen. HSCT was used in place of consolidation for 3 (5%) CPX-351 and 3 (14%) control patients.
Patient characteristics and study throughput, induction, and consolidation treatments
| . | All patients . | High-risk* patients . | sAML patients . | |||
|---|---|---|---|---|---|---|
| CPX-351, n = 85 . | 7+3, n = 41 . | CPX-351, n = 61 . | 7+3, n = 29 . | CPX-351, n = 33 . | 7+3, n = 19 . | |
| Gender | ||||||
| % Male | 53 (62.4) | 25 (61.0) | 35 (57.4) | 16 (55.2) | 21 (63.6) | 9 (47.4) |
| Age (y) | ||||||
| 60-69 | 51 (60.0) | 26 (63.4) | 27 (44.3) | 14 (48.3) | 20 (60.6) | 11 (57.9) |
| 70-75 | 34 (40.0) | 15 (36.6) | 34 (55.7) | 15 (51.7) | 13 (39.4) | 8 (42.1) |
| Range | 60-77 | 61-77 | 60-77 | 61-77 | 60-75 | 61-75 |
| Median | 68 | 68 | 70 | 71 | 68 | 68 |
| Race | ||||||
| White | 79 (92.9) | 39 (95.1) | 58 (95.1) | 27 (93.1) | 31 (93.9) | 18 (94.7) |
| Performance status | ||||||
| 0 | 22 (25.9) | 12 (29.3) | 15 (24.6) | 10 (34.5) | 8 (24.2) | 6 (31.6) |
| 1 | 48 (56.5) | 24 (58.5) | 36 (59.0) | 15 (51.7) | 18 (54.5) | 9 (47.4) |
| 2 | 15 (17.6) | 5 (12.2) | 10 (16.4) | 4 (13.8) | 7 (21.2) | 4 (21.1) |
| AML type | ||||||
| de novo AML | 52 (61.2) | 22 (53.7) | 28 (45.9) | 10 (34.5) | — | — |
| Secondary AML | 33 (38.8) | 19 (46.3) | 33 (54.1) | 19 (65.5) | 33 (100) | 19 (100) |
| Cytogenetic risk | ||||||
| Intermediate/favorable | 55 (64.7) | 26 (63.4) | 33 (54.1) | 16 (55.2) | 20 (60.6) | 13 (68.4) |
| Adverse | 23 (27.1) | 13 (31.7) | 23 (37.7) | 13 (44.8) | 10 (30.3) | 6 (31.6) |
| Unknown | 7 (8.2) | 2 (4.9) | 5 (8.2) | 0 | 3 (9.1) | 0 |
| Stratification group | ||||||
| Standard risk | 33 (38.8) | 16 (39.0) | 10 (16.4) | 5 (17.2) | — | — |
| High risk | 52 (61.2) | 25 (61.0) | 51 (83.6) | 24 (82.8) | 30 (90.9) | 17 (89.5) |
| WBC count at entry | ||||||
| ≤20 × 109/L | 65 (76.5) | 32 (78.0) | 48 (78.7) | 22 (75.9) | 28 (84.8) | 15 (78.9) |
| >20-100 × 109/L | 19 (22.4) | 9 (22.0) | 12 (19.7) | 7 (24.1) | 5 (15.2) | 4 (21.1) |
| >100 × 109/L | 1 (1.2) | 0 | 1 (1.6) | 0 | 0 | 0 |
| Induction treatment | ||||||
| 1 course | 68 (80.0) | 29 (70.7) | 47 (77.0) | 21 (72.4) | 23 (69.7) | 15 (78.9) |
| 2 courses | 17 (20.0) | 12 (29.3) | 14 (23.0) | 8 (27.6) | 10 (30.3) | 4 (21.1) |
| Persistent AML/not assessed | ||||||
| After 1 or 2 inductions | 29 (34.1) | 20 (48.8) | 23 (37.7) | 15 (51.7) | 14 (42.4) | 13 (68.4) |
| Complete response CR + CRi† | ||||||
| After first course | 46 (54.8) | 13 (31.7) | 29 (48.3) | 9 (31.0) | 13 (39.4) | 4 (21.1) |
| After second course | 10 (11.9) | 8 (19.5) | 9 (15.0) | 5 (17.2) | 6 (18.2) | 2 (10.5) |
| Consolidation therapy (responders only) | ||||||
| None | 15 (26.8) | 3 (14.3) | 10 (26.3) | 2 (14.3) | 5 (26.3) | 0 |
| Chemotherapy only: 1 course | 15 (26.8) | 5 (23.8) | 12 (31.6) | 4 (28.6) | 6 (31.6) | 1 (16.7) |
| Chemotherapy only: 2 courses | 13 (23.2) | 8 (38.1) | 7 (18.4) | 5 (35.7) | 2 (10.5) | 3 (50.0) |
| Transplantation only | 4 (7.1) | 3 (14.3) | 4 (10.5) | 1 (7.1) | 2 (10.5) | 1 (16.7) |
| Chemotherapy followed by transplantation | 9 (16.1) | 2 (9.5) | 5 (13.2) | 2 (14.3) | 4 (21.1) | 1 (16.7) |
| Salvage therapy | ||||||
| Any chemotherapy | 39 (45.9) | 20 (48.8) | 23 (37.7) | 12 (41.4) | 10 (30.3) | 7 (36.8) |
| Patient crossover | — | 10 (24.4) | — | 6 (20.7) | — | 6 (31.5) |
| Transplant | 1 | 5‡ | 1 | 3§ | 1 | 2¶ |
| . | All patients . | High-risk* patients . | sAML patients . | |||
|---|---|---|---|---|---|---|
| CPX-351, n = 85 . | 7+3, n = 41 . | CPX-351, n = 61 . | 7+3, n = 29 . | CPX-351, n = 33 . | 7+3, n = 19 . | |
| Gender | ||||||
| % Male | 53 (62.4) | 25 (61.0) | 35 (57.4) | 16 (55.2) | 21 (63.6) | 9 (47.4) |
| Age (y) | ||||||
| 60-69 | 51 (60.0) | 26 (63.4) | 27 (44.3) | 14 (48.3) | 20 (60.6) | 11 (57.9) |
| 70-75 | 34 (40.0) | 15 (36.6) | 34 (55.7) | 15 (51.7) | 13 (39.4) | 8 (42.1) |
| Range | 60-77 | 61-77 | 60-77 | 61-77 | 60-75 | 61-75 |
| Median | 68 | 68 | 70 | 71 | 68 | 68 |
| Race | ||||||
| White | 79 (92.9) | 39 (95.1) | 58 (95.1) | 27 (93.1) | 31 (93.9) | 18 (94.7) |
| Performance status | ||||||
| 0 | 22 (25.9) | 12 (29.3) | 15 (24.6) | 10 (34.5) | 8 (24.2) | 6 (31.6) |
| 1 | 48 (56.5) | 24 (58.5) | 36 (59.0) | 15 (51.7) | 18 (54.5) | 9 (47.4) |
| 2 | 15 (17.6) | 5 (12.2) | 10 (16.4) | 4 (13.8) | 7 (21.2) | 4 (21.1) |
| AML type | ||||||
| de novo AML | 52 (61.2) | 22 (53.7) | 28 (45.9) | 10 (34.5) | — | — |
| Secondary AML | 33 (38.8) | 19 (46.3) | 33 (54.1) | 19 (65.5) | 33 (100) | 19 (100) |
| Cytogenetic risk | ||||||
| Intermediate/favorable | 55 (64.7) | 26 (63.4) | 33 (54.1) | 16 (55.2) | 20 (60.6) | 13 (68.4) |
| Adverse | 23 (27.1) | 13 (31.7) | 23 (37.7) | 13 (44.8) | 10 (30.3) | 6 (31.6) |
| Unknown | 7 (8.2) | 2 (4.9) | 5 (8.2) | 0 | 3 (9.1) | 0 |
| Stratification group | ||||||
| Standard risk | 33 (38.8) | 16 (39.0) | 10 (16.4) | 5 (17.2) | — | — |
| High risk | 52 (61.2) | 25 (61.0) | 51 (83.6) | 24 (82.8) | 30 (90.9) | 17 (89.5) |
| WBC count at entry | ||||||
| ≤20 × 109/L | 65 (76.5) | 32 (78.0) | 48 (78.7) | 22 (75.9) | 28 (84.8) | 15 (78.9) |
| >20-100 × 109/L | 19 (22.4) | 9 (22.0) | 12 (19.7) | 7 (24.1) | 5 (15.2) | 4 (21.1) |
| >100 × 109/L | 1 (1.2) | 0 | 1 (1.6) | 0 | 0 | 0 |
| Induction treatment | ||||||
| 1 course | 68 (80.0) | 29 (70.7) | 47 (77.0) | 21 (72.4) | 23 (69.7) | 15 (78.9) |
| 2 courses | 17 (20.0) | 12 (29.3) | 14 (23.0) | 8 (27.6) | 10 (30.3) | 4 (21.1) |
| Persistent AML/not assessed | ||||||
| After 1 or 2 inductions | 29 (34.1) | 20 (48.8) | 23 (37.7) | 15 (51.7) | 14 (42.4) | 13 (68.4) |
| Complete response CR + CRi† | ||||||
| After first course | 46 (54.8) | 13 (31.7) | 29 (48.3) | 9 (31.0) | 13 (39.4) | 4 (21.1) |
| After second course | 10 (11.9) | 8 (19.5) | 9 (15.0) | 5 (17.2) | 6 (18.2) | 2 (10.5) |
| Consolidation therapy (responders only) | ||||||
| None | 15 (26.8) | 3 (14.3) | 10 (26.3) | 2 (14.3) | 5 (26.3) | 0 |
| Chemotherapy only: 1 course | 15 (26.8) | 5 (23.8) | 12 (31.6) | 4 (28.6) | 6 (31.6) | 1 (16.7) |
| Chemotherapy only: 2 courses | 13 (23.2) | 8 (38.1) | 7 (18.4) | 5 (35.7) | 2 (10.5) | 3 (50.0) |
| Transplantation only | 4 (7.1) | 3 (14.3) | 4 (10.5) | 1 (7.1) | 2 (10.5) | 1 (16.7) |
| Chemotherapy followed by transplantation | 9 (16.1) | 2 (9.5) | 5 (13.2) | 2 (14.3) | 4 (21.1) | 1 (16.7) |
| Salvage therapy | ||||||
| Any chemotherapy | 39 (45.9) | 20 (48.8) | 23 (37.7) | 12 (41.4) | 10 (30.3) | 7 (36.8) |
| Patient crossover | — | 10 (24.4) | — | 6 (20.7) | — | 6 (31.5) |
| Transplant | 1 | 5‡ | 1 | 3§ | 1 | 2¶ |
Values are n (%). WBC, white blood cell.
High-risk = ≥70 years of age or sAML or adverse cytogenetics.
One patient was removed from the CPX-351 group before response assessment to be treated with imatinib.
Three patients were transplanted after crossover.
Two patients were transplanted after crossover.
Both patients were transplanted after crossover.
Treatment outcomes
| . | CR + Cri* . | Time to event† . | ||
|---|---|---|---|---|
| CR (%) . | CRi (%) . | EFS (median, mo.) . | OS (median, mo.) . | |
| All | ||||
| CPX-351 | 41/84 (48.8) | 15/84 (17.9) | 6.5 | 14.7 |
| 7+3 | 20/41 (48.8) | 1/41 (2.4) | 2.0 | 12.9¶ |
| High-risk‡ | ||||
| CPX-351 | 27/60 (45.0) | 11/60 (18.3) | 5.6 | 11.0 |
| 7+3 | 14/29 (48.3) | 0 | 1.8 | 7.6¶ |
| Cytogenetics | ||||
| Adverse | ||||
| CPX-351 | 11/22 (50.0) | 6/22 (27.3) | 7.8 | 10.6 |
| 7+3 | 5/13 (38.5) | 0 | 1.1 | 12.2¶ |
| Intermediate§ | ||||
| CPX-351 | 30/62 (48.4) | 9/62 (14.5) | 6.3 | 17.5 |
| 7+3 | 15/28 (53.4) | 1/28 (3.6) | 6.7 | 14.7¶ |
| sAML | ||||
| CPX-351 | 12/33 (36.7) | 7/33 (21.2) | 4.5 | 12.1TF2-5 |
| 7+3 | 6/19 (31.6) | 0 | 1.3 | 6.1¶ |
| . | CR + Cri* . | Time to event† . | ||
|---|---|---|---|---|
| CR (%) . | CRi (%) . | EFS (median, mo.) . | OS (median, mo.) . | |
| All | ||||
| CPX-351 | 41/84 (48.8) | 15/84 (17.9) | 6.5 | 14.7 |
| 7+3 | 20/41 (48.8) | 1/41 (2.4) | 2.0 | 12.9¶ |
| High-risk‡ | ||||
| CPX-351 | 27/60 (45.0) | 11/60 (18.3) | 5.6 | 11.0 |
| 7+3 | 14/29 (48.3) | 0 | 1.8 | 7.6¶ |
| Cytogenetics | ||||
| Adverse | ||||
| CPX-351 | 11/22 (50.0) | 6/22 (27.3) | 7.8 | 10.6 |
| 7+3 | 5/13 (38.5) | 0 | 1.1 | 12.2¶ |
| Intermediate§ | ||||
| CPX-351 | 30/62 (48.4) | 9/62 (14.5) | 6.3 | 17.5 |
| 7+3 | 15/28 (53.4) | 1/28 (3.6) | 6.7 | 14.7¶ |
| sAML | ||||
| CPX-351 | 12/33 (36.7) | 7/33 (21.2) | 4.5 | 12.1TF2-5 |
| 7+3 | 6/19 (31.6) | 0 | 1.3 | 6.1¶ |
One patient was removed from the CPX-351 group before response assessment to be treated with imatinib.
Twenty-four-month data.
High-risk defined as sAML or adverse cytogenetics or ≥70 years of age.
Includes unknown and favorable cytogenetics.
Includes patients that crossed over to CPX-351.
Efficacy
CPX-351 treatment was associated with a higher CR rate (41 CR + 15 CRi/84 [66.7%] vs 20 CR + 1 CRi/41 [51.2%]; P = .07), with CR occurring in 48.8% of patients in both arms and CRi responses favoring CPX-351 (17.9% vs 2.4%). Two CRi patients in the CPX-351 arm had incomplete recovery of neutrophil counts. The remaining 13 CRi patients in the CPX-351 arm and the 1 CRi patient in the control arm had incomplete platelet count recovery. The median time to response was longer for CPX-351 (48 vs 42 days), whereas the median duration of response was similar (8.9 vs 8.6 months). Higher CR rates favoring CPX-351 also occurred in patients with adverse cytogenetics (11 CR + 6 CRi [17/22, 77.3%] vs 5 CR + 0 CRi [5/13, 38.5%]; P = .03) and sAML (12 CR + 7 CRi/33 [57.6%] vs 6 CR + 0 CRi/19 [31.6%]; P = .06).
Ten patients with persistent AML after induction treatment with 7+3 were crossed over (9 after 1 induction course) to receive CPX-351 as salvage therapy, with 4 achieving response (3 CR + 1 CRi). These 10 patients were counted as control arm induction failures; none were counted as responders to CPX-351. Before crossover, these patients had little or no change in bone marrow cellularity, percent blast count, or both after 7+3 treatment and were considered by the investigator as unlikely to respond to additional courses of 7+3. Details of the 10 patients crossed over to CPX-351 have been presented previously13 (see supplemental Table 1, available on the Blood Web site).
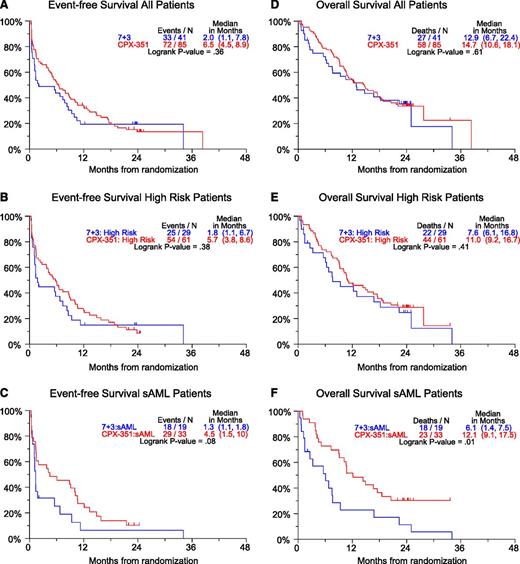
Twenty-four months of follow-up was attempted in all patients; median OS was 14.7 vs 12.9 months and median EFS was 6.5 vs 2.0 months for the CPX-351 and 7+3 arms, respectively (Figure 1A,D). Similar differences in OS and EFS were found in the high-risk population (n = 90) (Figure 1B,E). Although the aforementioned observations were not statistically significant, a planned analysis of sAML patients (n = 52) found a statistically significant improvement in OS (median 12.1 vs 6.1 months, HR = 0.46, P = .01) and EFS (median 4.5 vs 1.3 months, HR = 0.59, P = .08) in favor of the CPX-351 cohort (Figure 1C,F). A log-rank test for heterogeneity was performed to look for overall differences in 4 AML subgroups: CPX-351 primary AML, CPX-351 secondary AML, 7+3 primary AML, and 7+3 secondary AML. Heterogeneity testing was significant for OS (P = .0003) and for EFS (P = .03). A 2-month landmarked analysis of survival based on 35 CR patients and 12 CRi patients in the CPX-351 arm demonstrated no significant difference in survival between CR and CRi patients (supplemental Figure 1).14
Twenty-four-month OS and EFS curves. Hazard ratios: (A) 0.83, (B) 0.81, (C) 0.59, (D) 0.88, (E) 0.81, (F) 0.46. High-risk defined as sAML or adverse cytogenetics or ≥70 years of age. sAML: Secondary AML: a history of antecedent hematologic disorder, usually with MDS, MPN, or history of cytotoxic treatment for non-hematologic malignancy.
Twenty-four-month OS and EFS curves. Hazard ratios: (A) 0.83, (B) 0.81, (C) 0.59, (D) 0.88, (E) 0.81, (F) 0.46. High-risk defined as sAML or adverse cytogenetics or ≥70 years of age. sAML: Secondary AML: a history of antecedent hematologic disorder, usually with MDS, MPN, or history of cytotoxic treatment for non-hematologic malignancy.
CONSORT diagram. 7+ 3 = cytarabine and daunorubicin; OS = overall survival.
Transplants were permitted in responding patients. Among responding CPX-351 patients: 10 of 41 CR (24.4%) and 3 of 15 CRi (20%) patients had allogeneic transplants. Among control patients, 4 of 20 (20%) CR and 1 of 1 (100%) CRi patients had transplants. In addition, a small number of nonresponding patients received transplants as salvage therapy: 1 of 29 (3.4%) of CPX-351 and 5 of 20 (25%) of control patients. Overall, slightly more control arm patients were transplanted (14/85 [16.5%] vs 10/41 [24.4%]). The sAML subset was well balanced with respect to demographic characteristics, type of antecedent hematologic disorder, and proportion of patients with prior hypomethylating (5-azacitidine or decitabine) agent therapy (39% vs 37%). CPX-351–treated patients with prior hypomethylating agent therapy had higher response rates than did control arm patients with similar histories of prior treatment (3 CR + 4 CRi/13 [54%] vs 2 CR/7 [29%]). Similarly, CPX-351–treated patients without prior hypomethylating agent exposure also appeared to have higher response rates than did control patients (9 CR + 3 CRi/20 [60%] vs 4 CR/12 [33%]).
The contribution of older patients was similar to that of younger patients to study outcomes. Older patients (age >70) constituted similar proportions of patients in both study arms (40% vs 36.6%), with more males (58.8% vs 66.7%) and similar performance status (11.8% vs 13.3%). Outcomes among older patients also reflected the study as a whole with 60-day mortality (5.9% vs 21.4%), response (CR 47.1% vs 46.7%, CRi 17.6% vs 0%), and median EFS (6.0 vs 1.4 months) favoring the CPX-351 arm of the study.
Safety
By day 30, 3 of 85 CPX-351 (3.5%) and 3 of 41 control (7.3%) patients had died. By day 60, 4 of 85 CPX-351 (4.7%) and 6 of 41 control (14.6%) patients had died, indicating a trend for lower mortality in the CPX-351 arm (P = .053) (Table 3). All deaths by day 60 occurred in high-risk patients (4/52 [7.7%] vs 6/25 [24%]), particularly among high-risk patients with secondary AML (2/32 [6.3%] vs 6/19 [31.6%]). Of the 10 early deaths, 4 (2 in each arm) were a result of sepsis in patients with no day 14 bone marrow assessment, and 3 (2 on CPX-351 and 1 on 7+3) occurred in the setting of persistent AML. Three control arm patients died during marrow hypoplasia, 2 patients died from infection, and 1 patient died from cardiogenic pulmonary edema. Nonhematologic toxicities of grades 3 to 5 severity are shown in Table 4. Common adverse events included febrile neutropenia, infection, rash, diarrhea, nausea, edema, and constipation, with minimal differences between the treatment arms. CPX-351 was associated with increased incidence of grades 3 to 4 infection (60/85 [70.6%] vs 18/41 [43.9%]) but not infection-related deaths (3/85 [3.5%] vs 3/41 [7.3%]). Median time to neutrophil recovery to ≥1000/μL (36 vs 32 days) and platelet recovery to ≥100 000/μL (37 vs 28 days) appeared to be longer in the CPX-351 arm. Two patients died during CPX-351 consolidation of intracranial hemorrhage, one of which was associated with head trauma and relapsed AML and the other a result of chemotherapy-induced thrombocytopenia.
Induction mortality in all patients, high-risk patients, and sAML patients
| . | CPX-351 . | 7+3 . | . |
|---|---|---|---|
| All patients | n = 85 | n = 41 | |
| 30-d mortality | 3 (3.5) | 3 (7.3) | P = .053 |
| 60-d mortality | 4 (4.7) | 6 (14.6) | |
| High-risk | n = 52 | n = 25 | |
| 30-d mortality | 3 (5.8) | 3 (12.0) | |
| 60-d mortality | 4 (7.7) | 6 (24.0) | |
| sAML | n = 33 | n = 19 | |
| 30-d mortality | 1 (3.0) | 3 (15.8) | |
| 60-d mortality | 2 (6.1) | 6 (31.6) |
| . | CPX-351 . | 7+3 . | . |
|---|---|---|---|
| All patients | n = 85 | n = 41 | |
| 30-d mortality | 3 (3.5) | 3 (7.3) | P = .053 |
| 60-d mortality | 4 (4.7) | 6 (14.6) | |
| High-risk | n = 52 | n = 25 | |
| 30-d mortality | 3 (5.8) | 3 (12.0) | |
| 60-d mortality | 4 (7.7) | 6 (24.0) | |
| sAML | n = 33 | n = 19 | |
| 30-d mortality | 1 (3.0) | 3 (15.8) | |
| 60-d mortality | 2 (6.1) | 6 (31.6) |
Values are n (%) unless otherwise indicated.
Toxicities, grades 3-5, ≥5% incidence
| . | Grade 3 . | Grade 4 . | Grade 5 . | Grade 3-5 . | Grade 3-5 . | ||||
|---|---|---|---|---|---|---|---|---|---|
| . | CPX-351 . | 7+3 . | CPX-351 . | 7+3 . | CPX-351 . | 7+3 . | CPX-351 . | 7+3 . | All patients . |
| Febrile neutropenia | 52 (61.2) | 20 (48.8) | 2 (2.4) | 1 (2.4) | 0 (0.0) | 0 (0.0) | 54 (63.5) | 21 (51.2) | 75 (59.5) |
| Bacteremia | 30 (35.3) | 8 (19.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 30 (35.3) | 8 (19.5) | 38 (30.2) |
| Pneumonia | 12 (14.1) | 7 (17.1) | 0 (0.0) | 0 (0.0) | 1 (1.2) | 1 (2.4) | 13 (15.3) | 8 (19.5) | 21 (16.7) |
| Hypokalemia | 12 (14.1) | 5 (12.2) | 1 (1.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 13 (15.3) | 5 (12.2) | 18 (14.3) |
| Sepsis | 4 (4.7) | 2 (4.9) | 4 (4.7) | 0 (0.0) | 2 (2.4) | 3 (7.3) | 10 (11.8) | 5 (12.2) | 15 (11.9) |
| Fungal infection | 11 (12.9) | 1 (2.4) | 1 (1.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 12 (14.1) | 1 (2.4) | 13 (10.3) |
| Neutropenia | 0 (0.0) | 3 (7.3) | 6 (7.1) | 3 (7.3) | 0 (0.0) | 0 (0.0) | 6 (7.1) | 6 (14.6) | 12 (9.5) |
| Thrombocytopenia | 1 (1.2) | 0 (0.0) | 7 (8.2) | 4 (9.8) | 0 (0.0) | 0 (0.0) | 8 (9.4) | 4 (9.8) | 12 (9.5) |
| Diarrhea | 8 (9.4) | 3 (7.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 8 (9.4) | 3 (7.3) | 11 (8.7) |
| Fatigue | 7 (8.2) | 2 (4.9) | 1 (1.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 8 (9.4) | 2 (4.9) | 10 (7.9) |
| Acute renal failure | 5 (5.9) | 2 (4.9) | 3 (3.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 8 (9.4) | 2 (4.9) | 10 (7.9) |
| Urinary tract infection | 6 (7.1) | 3 (7.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 6 (7.1) | 3 (7.3) | 9 (7.1) |
| Dyspnea | 3 (3.5) | 3 (7.3) | 2 (2.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 5 (5.9) | 3 (7.3) | 8 (6.3) |
| Syncope | 6 (7.1) | 2 (4.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 6 (7.1) | 2 (4.9) | 8 (6.3) |
| Hypoxia | 5 (5.9) | 1 (2.4) | 1 (1.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 6 (7.1) | 1 (2.4) | 7 (5.6) |
| Mental status changes | 4 (4.7) | 3 (7.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 4 (4.7) | 3 (7.3) | 7 (5.6) |
| Rash | 7 (8.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 7 (8.2) | 0 (0.0) | 7 (5.6) |
| Renal failure | 2 (2.4) | 3 (7.3) | 1 (1.2) | 1 (2.4) | 0 (0.0) | 0 (0.0) | 3 (3.5) | 4 (9.8) | 7 (5.6) |
| . | Grade 3 . | Grade 4 . | Grade 5 . | Grade 3-5 . | Grade 3-5 . | ||||
|---|---|---|---|---|---|---|---|---|---|
| . | CPX-351 . | 7+3 . | CPX-351 . | 7+3 . | CPX-351 . | 7+3 . | CPX-351 . | 7+3 . | All patients . |
| Febrile neutropenia | 52 (61.2) | 20 (48.8) | 2 (2.4) | 1 (2.4) | 0 (0.0) | 0 (0.0) | 54 (63.5) | 21 (51.2) | 75 (59.5) |
| Bacteremia | 30 (35.3) | 8 (19.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 30 (35.3) | 8 (19.5) | 38 (30.2) |
| Pneumonia | 12 (14.1) | 7 (17.1) | 0 (0.0) | 0 (0.0) | 1 (1.2) | 1 (2.4) | 13 (15.3) | 8 (19.5) | 21 (16.7) |
| Hypokalemia | 12 (14.1) | 5 (12.2) | 1 (1.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 13 (15.3) | 5 (12.2) | 18 (14.3) |
| Sepsis | 4 (4.7) | 2 (4.9) | 4 (4.7) | 0 (0.0) | 2 (2.4) | 3 (7.3) | 10 (11.8) | 5 (12.2) | 15 (11.9) |
| Fungal infection | 11 (12.9) | 1 (2.4) | 1 (1.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 12 (14.1) | 1 (2.4) | 13 (10.3) |
| Neutropenia | 0 (0.0) | 3 (7.3) | 6 (7.1) | 3 (7.3) | 0 (0.0) | 0 (0.0) | 6 (7.1) | 6 (14.6) | 12 (9.5) |
| Thrombocytopenia | 1 (1.2) | 0 (0.0) | 7 (8.2) | 4 (9.8) | 0 (0.0) | 0 (0.0) | 8 (9.4) | 4 (9.8) | 12 (9.5) |
| Diarrhea | 8 (9.4) | 3 (7.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 8 (9.4) | 3 (7.3) | 11 (8.7) |
| Fatigue | 7 (8.2) | 2 (4.9) | 1 (1.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 8 (9.4) | 2 (4.9) | 10 (7.9) |
| Acute renal failure | 5 (5.9) | 2 (4.9) | 3 (3.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 8 (9.4) | 2 (4.9) | 10 (7.9) |
| Urinary tract infection | 6 (7.1) | 3 (7.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 6 (7.1) | 3 (7.3) | 9 (7.1) |
| Dyspnea | 3 (3.5) | 3 (7.3) | 2 (2.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 5 (5.9) | 3 (7.3) | 8 (6.3) |
| Syncope | 6 (7.1) | 2 (4.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 6 (7.1) | 2 (4.9) | 8 (6.3) |
| Hypoxia | 5 (5.9) | 1 (2.4) | 1 (1.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 6 (7.1) | 1 (2.4) | 7 (5.6) |
| Mental status changes | 4 (4.7) | 3 (7.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 4 (4.7) | 3 (7.3) | 7 (5.6) |
| Rash | 7 (8.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 7 (8.2) | 0 (0.0) | 7 (5.6) |
| Renal failure | 2 (2.4) | 3 (7.3) | 1 (1.2) | 1 (2.4) | 0 (0.0) | 0 (0.0) | 3 (3.5) | 4 (9.8) | 7 (5.6) |
Values are n (%).
Discussion
Numerous attempts have been made to improve on the conventional 7+3 regimen of cytarabine and daunorubicin, including manipulation of treatment intensity as well as duration of treatment, with few reports of success.15,16 The benefit of high-dose cytarabine and anthracycline has been controversial.17,18 Few attempts have been made to improve outcomes by exploiting cytarabine and daunorubicin drug interactions. In vitro studies have demonstrated that molar ratio–dependent drug-drug interactions between cytarabine and daunorubicin can be used to maximize cytotoxic synergy and minimize antagonism. Preclinical leukemia model studies and early clinical observations suggest promise for this approach. In theory, delivery of the 5:1 molar ratio of cytarabine and daunorubicin may prevent antagonistic drug-drug interactions, which may occur when daunorubicin concentrations are sufficient to lead to G2/M phase arrest,19 thereby limiting cytarabine action because of its S-phase selectivity.20 This clinical trial is the first of 2 randomized phase 2 studies intended to estimate the activity of CPX-351 and identify patient subsets that may benefit from treatment.
This randomized phase 2 study performed in older patients with previously untreated AML observed an equal rate of CR with a higher rate of CRi response after CPX-351 compared with 7+3 treatment. Increased CR+CRi response was also observed among patients with adverse cytogenetics and secondary AML. The study achieved its prespecified objective of demonstrating a difference in CR+CRi response favoring CPX-351 (P < .10). For the entire cohort of patients, although response rates were improved, given the size of this phase 2 study, statistically significant improvements in EFS and OS were not observed. CPX-351–treated patients with secondary AML appeared to have a consistent signal for efficacy with an improved response rate (CR+CRi 57.5% vs 31.6%), EFS (HR = 0.59, P = .08), and OS (HR = 0.46, P = .01).
The explanation for the disparity between CR rate and OS is not clear but may relate to effective salvage therapy after failure of 7+3 treatment. Interestingly, 4 patients who crossed over to receive CPX-351 as salvage therapy after 7+3 induction failure achieved CR and each survived >1 year, potentially confounding a possible survival advantage for CPX-351, although some patients given a second course of conventional induction may have responded.
Both a lower rate of 60-day mortality and a higher response rate after CPX-351 are likely contributors to the improvement in OS and EFS observed among secondary AML patients.
CPX-351 liposomes appear to selectively accumulate within leukemic cells in the marrow, enabling delivery of the 5:1 molar ratio of cytarabine and daunorubicin.8,21 Previous studies have shown that multidrug resistance expression is significantly increased in older patients, especially in the setting of sAML.22,23 Observations that CPX-351 may directly enter leukemic cells as intact liposomes suggest a possible method for overcoming drug resistance mechanisms such as multidrug resistance phenotype (eg, plasma membrane–localized P-glycoprotein) and rapid cytarabine inactivation by cytidine deaminase, thereby providing potential explanations for improved efficacy in patients with otherwise poor-risk AML. Finally, the persistence of CPX-351 liposomes in the plasma, resulting in greatly prolonged in vivo drug exposure, could conceivably lengthen the duration of cytotoxic action and lead to further increases in leukemic cell kill, particularly within intrinsically resistant tumor populations that are more quiescent or functionally p53 deficient.12,24,25
Highly relevant to the issue of sAML, which represents as much as 10% to 30% of all AML patients26,27 (and in this study as much as 40% to 50% of older AML patients), are recent observations suggesting that patients with MDS who transform to AML after having received prior hypomethylating agent therapy (eg, 5-azacitidine or decitabine) have very poor outcome after conventional chemotherapy.28 In this study, response to CPX-351 was not reduced among patients with prior exposure to hypomethylating agents.
CPX-351 was well tolerated. Mortality at both 30 and 60 days after CPX-351 compares favorably with historic data.1,2,29 The somewhat high rate of mortality at 60 days in the control arm patients with high-risk or sAML (24% and 31.6%, respectively) may reflect disease-related events (as evidenced by lower remission rates) more so than treatment-related mortality, although attribution of cause of early death in AML is quite challenging, considering all the competing factors in this population. In addition, the very small numbers of patients in these subsets make accurate determination of mortality difficult. The overall observation of a low rate of early mortality with CPX-351, however, if confirmed in a larger study, will provide evidence of its acceptable safety in this group of older AML patients. Time to hematologic recovery was greater after CPX-351 than with 7+3, possibly accounting for the increased risk of grades 3 to 4 infections associated with the CPX-351 arm. However, the increased infection rate after CPX-351 did not translate into a higher risk of infection-related mortality, possibly reflecting effective antiinfective therapy or discordance in the practice of diagnosing infections between the 2 arms. The incidence of nonhematologic adverse events was similar between the 2 study arms.
The rate of CRi was higher in the CPX-351 arm. Given the pharmacokinetic profile of this agent and the evidence of prolonged survival among CPX-351–treated patients with sAML, it may be reasonable to postulate that the CRi effect is more a manifestation of CPX-351 persistence in the marrow with prolonged myelosuppression than a sign of impending treatment failure with minimal residual disease. It should be noted that historically, patients with CRp (CR with incomplete recovery of platelets) typically have shorter survival than patients with CR, CRi patients have better survival than those who show no response.10 Importantly in this study, the survival of patients with CR and those with CRi was similar.
The randomized phase 2 design used in this study provides a strong starting point for planning a future confirmatory controlled trial, because few assumptions have to be made about the relative safety and efficacy of study treatments. Although eliminating all risk in the design and powering of the next study is not possible, we did observe trends favoring CPX-351 for response and EFS, significant improvement in OS in the secondary AML group, and potential improved safety. These findings are similar to efficacy observations in the poor-risk strata of the companion phase 2 study of first-relapse AML. Improved efficacy in newly diagnosed secondary AML patients will be tested in an appropriately powered phase 3 study.
In conclusion, this study met its predefined goal of an increased rate of CR+CRi response, with especially promising activity in the subset of older patients with previously untreated secondary AML, a historically poor-risk patient cohort. These data have been instrumental in guiding the design of a randomized phase 3 study, which has been initiated, to confirm improved survival in the secondary AML population.
Presented in part in abstract form at the 52nd annual meeting of the American Society of Hematology, Orlando, FL December 4-7, 2010.
There is an Inside Blood Commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank all the patients and their families who contributed to this study as well as the participating site study nurses and data coordinators. The study was sponsored by Celator Pharmaceuticals, Inc.
Authorship
Contribution: J.E.L. designed the research, wrote the paper, and contributed patients; J.E.C. wrote the paper and contributed patients; D.E.H., M.S.T., T.J.K., L.E.D., R.K., S.R.S., J.E.K., M.C., and A.M.Y. contributed patients; A.C.L. designed the research, analyzed the data, and wrote the paper; and E.J.F. designed the research and contributed patients.
Conflict-of-interest disclosure: J.E.L. is a consultant for Celator Pharmaceuticals; A.C.L. is employed by and has stock option ownership in Celator Pharmaceuticals. The remaining authors declare no competing financial interest.
Correspondence: Jeffrey E. Lancet, H. Lee Moffitt Cancer Center and Research Institute, 12902 Magnolia Ave, Tampa, FL 33612; e-mail: jeffrey.lancet@moffitt.org.