Key Points
An immunodeficiency scoring index for RSV-infected hematopoietic cell transplant recipients predicts the risk of pneumonia and death.
This scoring index would assist in the decision-making for initiating antiviral therapy in patients at high risk for poor outcomes.
Abstract
We developed an immunodeficiency scoring index for respiratory syncytial virus (ISI-RSV) infection, based on a cohort of 237 allogeneic hematopoietic cell transplant (allo-HCT) recipients, that can predict the risk of progression to lower respiratory tract infection (LRTI) and RSV-associated mortality. A weighted index was calculated using adjusted hazard ratios for immunodeficiency markers. Based on the ISI-RSV (range, 0-12), patients were stratified into low (0-2), moderate (3-6), and high (7-12) risk groups. A significant trend of increasing incidence of LRTI and RSV-associated mortality was observed as the risk increased from low to moderate to high (P < .001). Patients in the high-risk group had the greatest benefit of ribavirin-based therapy at the upper respiratory tract infection stage and the highest risk for progression to LRTI and death when antiviral therapy was not given (6.5 [95% confidence interval (CI), 1.8-23.6] and 8.1 [95% CI, 1.1-57.6], respectively). The ISI-RSV is designed to stratify allo-HCT recipients with RSV infection into groups according to their risk for progression to LRTI and RSV-associated mortality. Identification of high-risk groups using this index would distinguish patients who would benefit the most from antiviral therapy, mainly with aerosolized ribavirin. The ISI-RSV should be validated in a multi-institutional study.
Introduction
Respiratory syncytial virus (RSV) infections are common in immunocompromised patients during the cold seasons, especially those who have received allogeneic hematopoietic cell transplants (allo-HCT). RSV infections have a wide spectrum of outcomes, ranging from self-limited upper respiratory tract infection (URTI) to severe lower respiratory tract infection (LRTI) to death. Because of their highly compromised immune systems, mainly during the early phases after transplantation, allo-HCT recipients are vulnerable to respiratory viral infections, particularly RSV infections.1 Many studies have identified potential risk factors for RSV-associated LRTI or RSV-associated mortality in allo-HCT recipients, such as age, lymphocytopenia, neutropenia, myeloablative conditioning regimens, corticosteroids, graft-versus-host disease (GVHD), and preengraftment phase or recent transplantation.1-11 However, quantifiable measures that account for the magnitude and combination of the risks attributable to immunodeficiency in predicting the outcomes of these infections have yet to be established.
Although it is controversial, the effectiveness of ribavirin-based antiviral therapy in preventing LRTI and death in RSV-infected allo-HCT recipients is described in some studies.1,2,6,12-17 However, this therapy is not recommended for all patients with RSV infections, owing to concerns about safety, efficacy, and cost-effectiveness. Given the high morbidity and mortality rates associated with RSV infections in these patients, a scoring index that accounts for the effects of all these immunodeficiency indicators or risk factors and predicts the likelihood of progression to LRTI or death would be clinically useful as a guide for whether to initiate antiviral therapy. Hence we developed an immunodeficiency scoring index for RSV (ISI-RSV) infection that includes clinical and laboratory indicators that can predict the risk of progression to LRTI and/or death in allo-HCT recipients with RSV infections.
Methods
Data collection
Data for the current study were derived from a large retrospective, single-center study of all allo-HCT recipients with laboratory-confirmed RSV infections presenting to The University of Texas MD Anderson Cancer Center from January 1996 to May 2009.2 Specifically, data on patients in this cohort presenting with URTIs (n = 237) were analyzed to develop the ISI-RSV. The University of Texas MD Anderson Cancer Center institutional review board approved the study, and the waiver for informed consent was granted for the retrospective study.
Definitions
URTI was defined2 as the onset of any of the respiratory symptoms including rhinorrhea, nasal or sinus congestion, otitis media, pharyngitis, or cough, with no hypoxemia or infiltrates seen on chest radiograph or computed tomography scan of the chest at the time of presentation in patients with positive RSV from a nasal wash specimen or nasopharyngeal swab. LRTI was defined as respiratory symptoms in patients with laboratory-confirmed RSV infection on nasal washes with new or changing pulmonary infiltrates as seen on chest radiographs or computed tomography scans that were suggestive of a viral etiology (ie, interstitial infiltrates, ground glass opacities) and with or without evidence of RSV in lower respiratory tract samples (eg, endotracheal tube aspirate, sputum, bronchoalveolar lavage fluid).2,18 Death from all causes was assessed within 90 days after diagnosis of RSV infection and was attributed to RSV if the patient had persistent or progressive RSV infection with respiratory failure at the time of death. Neutropenia was defined as an absolute neutrophil count (ANC) <500/μL, and lymphocytopenia was defined as an absolute lymphocyte count (ALC) <200/μL. Detailed definitions for other variables and data collection procedures have been described previously.2
Development of the ISI-RSV
The ISI-RSV, a weighted scoring index for RSV infection accounting for the number and magnitude of immunodeficiency indicators, was developed in a cohort of 237 allo-HCT recipients with RSV URTI. Progression from URTI to LRTI and RSV-associated mortality were the outcomes of interest in developing this index. Each of the potential immunodeficiency indicators was examined individually to determine its effect on this outcome. Cox proportional hazards regression modeling with progression to LRTI as the target prediction was performed, which facilitated estimation of the magnitude of impact of individual immunodeficiency risk factors on this outcome. Adjusted hazard ratios (AHRs) calculated from a multivariable Cox regression model were used to assign weights to these immunodeficiency indicators. Thus weights were assigned for the presence of each risk factor and then summed to determine each patient’s ISI-RSV score (range, 0-12). These scores were further discretized to classify patients into low-risk (0-2), moderate-risk (3-6), and high-risk (7-12) groups. Risk of progression to LRTI, risk of RSV-associated mortality, and the impact of treatment with aerosolized ribavirin at the URTI stage on these 2 outcomes in each risk group were compared using Kaplan-Meier failure curves and log-rank tests. In addition, a nonparametric test of trend for the ranks of ordered groups of ISI-RSV scores was performed. The number of patients needed to treat (NNT) to prevent one LRTI event or RSV-associated mortality in each risk group of patients who did or did not receive antiviral therapy at the URTI stage was determined using the Bender method.19 The ISI-RSV was also applied to the remaining cohort of patients who presented with RSV LRTI to determine the mortality rate in each risk group. All statistical analyses were performed using the Stata software program (version 11.0; StataCorp, College Station, TX). All tests were 2-tailed, and an α level of .05 was considered significant.
Antiviral therapy
Aerosolized ribavirin alone (for URTI) or combined with intravenous immunoglobulins (IVIG) or palivizumab (for LRTI), was administered at 6 g per day in a continuous (20 mg/mL for 18 hours/day) or split intermittent (60 mg/mL over 2-3 hours every 8 h) schedules at the discretion of the treating physician. IVIG was administered at a dose of 500 mg/kg every other day for 5 to 7 doses, whereas palivizumab was administered as a single intravenous infusion of 15 mg/kg.2
Results
Patient characteristics
The study population was composed of patients of all ages (median, 47 years; range, 3-68) who were predominantly white (156 [66%]) and male (134 [57%]). Progression to LRTI was observed in 37 (16%) of the 237 allo-HCT recipients who presented with RSV URTI. Detailed characteristics of these 37 patients, as well as the remaining 200 patients whose infections resolved at RSV URTI stage, were described previously.2
Immunodeficiency markers
Based on Cox proportional hazards regression modeling, we identified 3 immunodeficiency indicators that were significantly and independently associated with progression to LRTI in our cohort: neutropenia (ANC <500/μL), lymphocytopenia (ALC <200/μL), and patient age of at least 40 years. In addition, we included 4 other immunodeficiency indicators, such as myeloablative conditioning regimen, acute or chronic GVHD, use of corticosteroids within 30 days of RSV infection, and performance of hematopoietic cell transplantation within the prior 30 days or in the pre-engraftment period during RSV infection, in the scoring index on the basis of their clinical significance as risk factors for worst outcomes as reported in previous studies.1,3,4,8,9 Owing to overlap between receiving an allo-HCT over the prior 30 days or lack of engraftment during RSV diagnosis, we combined these 2 variables into one variable and assigned patients a score of 1 if either, or both, of these factors was present.
Development of the ISI-RSV
We calculated AHRs for each of the 7 immunodeficiency indicators regarding progression from RSV URTI to LRTI using a multivariable Cox regression model (Table 1). We used these ratios to assign a weight for the presence of each indicator. Thus we assigned a score of 3 (AHR >2.5) for neutropenia or lymphocytopenia, a score of 2 (AHR of 2.0-2.5) for age of at least 40 years, and a score of 1 each (AHR <2) for the 4 remaining immunodeficiency indicators. The overall ISI-RSV for each patient was the sum of the scores for all of the indicators present at the time of diagnosis of RSV infection (range, 0-12). For example, for a patient with neutropenia and acute GVHD, the overall score would be 4. We discretized the cohort according to overall score into 3 risk groups: low-risk (0-2), moderate-risk (3-6), and high-risk (7-12). As is shown in Table 2, we observed a significant trend of increasing incidence of progression from URTI to LRTI as the risk of progression increased from the low (7%) to moderate (15%) to high (48%) groups (P < .001). When we stratified the patients according to whether they received ribavirin-based antiviral therapy at the URTI stage, the trend of increasing incidence of progression to LRTI with increasing ISI-RSV was maintained (P = .065 and P < .0001 for the moderate-risk and high-risk groups, respectively).
Development of the ISI-RSV for patients presenting with RSV infections (N = 237)
| . | . | No. (%) . | . | . | . | |
|---|---|---|---|---|---|---|
| Criteria . | . | Patients 237, n (%) . | Progression to LRTI 37, n (%) . | AHR* (95% CI) . | Weighing criteria . | Assigned weights (score) . |
| 1 | ANC <500/μL | 11 (5) | 7 (64) | 4.1 (1.4-11.6) | >2.5 | 3 |
| 2 | ALC <200/μL | 35 (15) | 11 (31) | 2.6 (1.02-6.4) | >2.5 | 3 |
| 3 | Age ≥40 y | 154 (65) | 28 (18) | 2.5 (1.1-5.6) | 2.0-2.5 | 2 |
| 4 | Myeloablative conditioning regimen | 98 (41) | 17 (17) | 1.2 (0.6-2.3) | <2.0 | 1 |
| 5 | GVHD (acute or chronic) | 149 (63) | 19 (13) | 1.0 (0.5-2.2) | <2.0 | 1 |
| 6 | Corticosteroids† | 117 (49) | 17 (15) | 0.89 (0.4-1.8) | <2.0 | 1 |
| 7 | Recent† or pre-engraftment allo-HSCT | 21 (9) | 5 (24) | 0.68 (0.2-2.3) | <2.0 | 1 |
| Maximum possible overall score‡ | 12 | |||||
| Low risk: 0-2 score, moderate risk 3-6 score, high risk 7-12 score | ||||||
| . | . | No. (%) . | . | . | . | |
|---|---|---|---|---|---|---|
| Criteria . | . | Patients 237, n (%) . | Progression to LRTI 37, n (%) . | AHR* (95% CI) . | Weighing criteria . | Assigned weights (score) . |
| 1 | ANC <500/μL | 11 (5) | 7 (64) | 4.1 (1.4-11.6) | >2.5 | 3 |
| 2 | ALC <200/μL | 35 (15) | 11 (31) | 2.6 (1.02-6.4) | >2.5 | 3 |
| 3 | Age ≥40 y | 154 (65) | 28 (18) | 2.5 (1.1-5.6) | 2.0-2.5 | 2 |
| 4 | Myeloablative conditioning regimen | 98 (41) | 17 (17) | 1.2 (0.6-2.3) | <2.0 | 1 |
| 5 | GVHD (acute or chronic) | 149 (63) | 19 (13) | 1.0 (0.5-2.2) | <2.0 | 1 |
| 6 | Corticosteroids† | 117 (49) | 17 (15) | 0.89 (0.4-1.8) | <2.0 | 1 |
| 7 | Recent† or pre-engraftment allo-HSCT | 21 (9) | 5 (24) | 0.68 (0.2-2.3) | <2.0 | 1 |
| Maximum possible overall score‡ | 12 | |||||
| Low risk: 0-2 score, moderate risk 3-6 score, high risk 7-12 score | ||||||
CI, confidence interval.
Adjusted for year of RSV diagnosis and ribavirin-based therapy at the URTI stage to identify the independent effect of each immunodeficiency indicator on progression to LRTI.
Within the prior 30 days.
The overall score equals the sum of the scores for the immunodeficiency indicators present at the time of diagnosis of RSV infection. For example, for a patient with an ANC <500/μL (ISI-RSV score 3) and acute GVHD (ISI-RSV score 1), the total ISI-RSV score would be 4, and the patient would be stratified into the moderate-risk group.
RSV-associated outcomes in the ISI-RSV risk groups stratified according to antiviral therapy at the URTI stage
| . | . | . | Antiviral therapy at the URTI stage . | . | |||
|---|---|---|---|---|---|---|---|
| No . | Yes . | ||||||
| Risk group . | No. . | LRTI, n (%) . | No. . | LRTI, n (%) . | No. . | LRTI, n (%) . | Risk ratio for lack of antiviral therapy (95% CI) . |
| Progression from URTI to LRTI | |||||||
| Low | 69 | 5 (7) | 25 | 4 (16) | 44 | 1 (2) | 7 (0.8-59.6) |
| Moderate | 147 | 22 (15) | 47 | 11 (23) | 100 | 11 (11) | 2.1 (0.9-4.6) |
| High | 21 | 10 (48) | 8 | 8 (100) | 13 | 2 (15) | 6.5 (1.8-23.6) |
| Death, n (%) | Death, n (%) | Death, n (%) | |||||
| Low | 69 | 0 | 25 | 0 | 44 | 0 | — |
| Moderate | 147 | 4 (3) | 47 | 2 (4) | 100 | 2 (2) | 2.1 (0.3-14.6) |
| High | 21 | 6 (29) | 8 | 5 (63) | 13 | 1 (8) | 8.1 (1.1-57.6) |
| . | . | . | Antiviral therapy at the URTI stage . | . | |||
|---|---|---|---|---|---|---|---|
| No . | Yes . | ||||||
| Risk group . | No. . | LRTI, n (%) . | No. . | LRTI, n (%) . | No. . | LRTI, n (%) . | Risk ratio for lack of antiviral therapy (95% CI) . |
| Progression from URTI to LRTI | |||||||
| Low | 69 | 5 (7) | 25 | 4 (16) | 44 | 1 (2) | 7 (0.8-59.6) |
| Moderate | 147 | 22 (15) | 47 | 11 (23) | 100 | 11 (11) | 2.1 (0.9-4.6) |
| High | 21 | 10 (48) | 8 | 8 (100) | 13 | 2 (15) | 6.5 (1.8-23.6) |
| Death, n (%) | Death, n (%) | Death, n (%) | |||||
| Low | 69 | 0 | 25 | 0 | 44 | 0 | — |
| Moderate | 147 | 4 (3) | 47 | 2 (4) | 100 | 2 (2) | 2.1 (0.3-14.6) |
| High | 21 | 6 (29) | 8 | 5 (63) | 13 | 1 (8) | 8.1 (1.1-57.6) |
We observed a significant trend of increasing incidence of progression from URTI to LRTI and RSV-associated mortality with increased risk (P < .001). These trends were maintained even when we stratified the patients according to antiviral therapy. The incidence of both outcomes decreased when antiviral therapy was administered at the URTI stage.
Prediction of RSV-associated mortality
As is shown in Table 2, we observed a significant trend of increasing incidence of RSV-associated mortality from the low (0%) to the moderate (3%) and to the high (29%) risk groups (P < .001). Stratification of the cohort based on the use of ribavirin-based antiviral therapy at the URTI stage again demonstrated this trend of increasing mortality with increasing risk groups except in the low-risk group, where no death was recorded regardless of receipt of therapy (Table 2).
Impact of therapy with aerosolized ribavirin
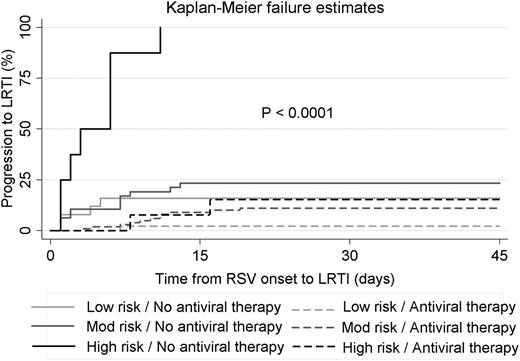
The use of antiviral therapy with aerosolized ribavirin in patients with RSV URTI was associated with a decreased incidence of LRTI, with the greatest risk reduction observed in the high-risk group (Table 2). Patients in the high-risk group who presented with URTI and did not receive aerosolized ribavirin were 6 times more likely to progress to LRTI than were those who did receive it. The impact of ribavirin-based therapy on progression to LRTI in each risk group is demonstrated by the Kaplan-Meier failure curves in Figure 1.
Kaplan-Meier failure curves for progression from RSV URTI to LRTI stratified according to ISI-RSV risk group and antiviral therapy at the URTI stage.
Kaplan-Meier failure curves for progression from RSV URTI to LRTI stratified according to ISI-RSV risk group and antiviral therapy at the URTI stage.
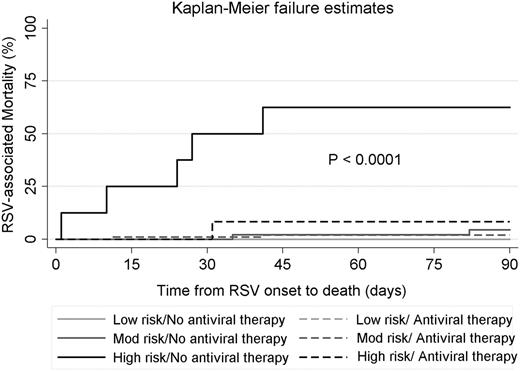
Antiviral therapy had a significant impact on RSV-associated mortality in the high-risk group, with a decreased mortality (8%) for patients who received aerosolized ribavirin when compared with patients who did not (63%). This means that patients in the high-risk group who did not receive antiviral therapy at the URTI stage were 8 times more likely to die than were those who did. We did not observe a significant difference in the mortality rate between treated or not-treated patients in the moderate-risk group (2% vs 4%, respectively), and there were no deaths reported in the low-risk group. The impact of ribavirin-based antiviral therapy on the RSV-associated mortality rate in each risk group is displayed in the Kaplan-Meier failure curves in Figure 2.
Kaplan-Meier failure curves for RSV-associated mortality stratified according to ISI-RSV risk group and antiviral therapy at the URTI stage. No deaths were observed in the low-risk group, regardless of whether they received antiviral therapy at the URTI stage; hence the 2 lines for this group overlap.
Kaplan-Meier failure curves for RSV-associated mortality stratified according to ISI-RSV risk group and antiviral therapy at the URTI stage. No deaths were observed in the low-risk group, regardless of whether they received antiviral therapy at the URTI stage; hence the 2 lines for this group overlap.
A total of only 10 patients needed intensive care unit (ICU) admission and subsequent mechanical ventilation in our cohort (6/157 [4%] who received ribavirin at the URTI stage needed ICU admission and mechanical ventilation vs 4/80 [10%] who were not treated; odds ratio, 0.75; 95% confidence interval, 0.21-2.76; P = .67). Moreover, 5 of 10 patients received ribavirin while they were intubated and 3 survived. The difference in this outcome was more pronounced for high-risk patients: 2 of 13 (15%) patients who received ribavirin at the URTI stage needed ICU admission and mechanical ventilation compared with 4 of 8 (50%) patients who were not treated (odds ratio, 0.18; 95% confidence interval, 0.02-1.41; P = .103). Overall, the need for ICU admission and mechanical ventilation increased significantly from the low- and moderate-risk to the high-risk groups (0% vs 3% vs 29%; P < .001).
Table 3 depicts the absolute risk reduction and NNT calculations for each risk group for preventing an event (1 LRTI or 1 death) in each risk group. It also compares risk reduction and NNT for the entire cohort without the application of ISI-RSV. The low-risk group had the least benefit of antiviral therapy, followed by the moderate-risk group; both groups had a risk reduction of <15% for both progression to LRTI and RSV-associated mortality. In comparison, the high-risk group shows the maximum benefit of antiviral therapy given at the URTI stage with an absolute risk reduction of 85% for LRTI and 55% for mortality. This means that the number needed to treat is 2 for high-risk patients to prevent one progression to LRTI or one RSV-associated death.
NNT to prevent one LRTI or one death and absolute risk reduction by antiviral therapy at URTI stage to prevent progression to LRTI and RSV-associated mortality
| . | Progression to LRTI . | RSV-associated mortality . | ||
|---|---|---|---|---|
| Outcome . | NNT . | Absolute risk reduction, % (95% CI) . | NNT . | Absolute risk reduction, % (95% CI) . |
| Without application of ISI-RSV | ||||
| Total cohort (n = 237) | 6 | 20 (9-31) | 15 | 7 (0-13) |
| After application of ISI-RSV | ||||
| Low-risk group | 8 | 14 (0-29) | NA | 0 |
| Moderate-risk group | 9 | 12 (0-26) | 45 | 2 (0-9) |
| High-risk group | 2 | 85 (65-104) | 2 | 55 (18-91) |
| . | Progression to LRTI . | RSV-associated mortality . | ||
|---|---|---|---|---|
| Outcome . | NNT . | Absolute risk reduction, % (95% CI) . | NNT . | Absolute risk reduction, % (95% CI) . |
| Without application of ISI-RSV | ||||
| Total cohort (n = 237) | 6 | 20 (9-31) | 15 | 7 (0-13) |
| After application of ISI-RSV | ||||
| Low-risk group | 8 | 14 (0-29) | NA | 0 |
| Moderate-risk group | 9 | 12 (0-26) | 45 | 2 (0-9) |
| High-risk group | 2 | 85 (65-104) | 2 | 55 (18-91) |
NA, not applicable due to 0 deaths in low-risk group.
Patients presenting with RSV LRTI
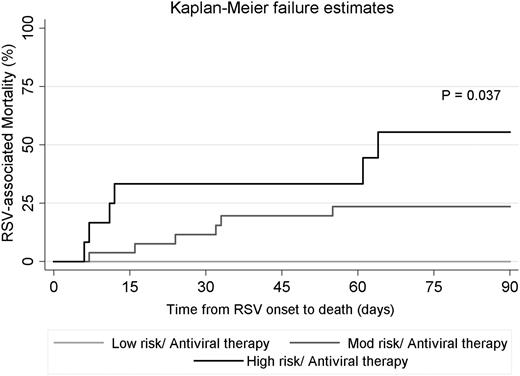
Forty-three (15%) patients presented with RSV LRTI.2 We applied the ISI-RSV to this subset of the cohort and discretized them into low-risk (5 [12%]), moderate-risk (26 [60%]), and high-risk (12 [28%]) groups (Table 4). We observed a significant trend of increasing mortality rate as the ISI-RSV increased, going from 0% in the low-risk group to 23% in the moderate-risk group to 50% in the high-risk group (P = .025). Survival probability was significantly higher in the low-risk group than in the high-risk group (P = .037; log-rank test) as shown in the Kaplan-Meier failure curves in Figure 3. We could not determine the impact of aerosolized ribavirin at the LRTI stage on mortality because all but 2 of the patients (in the low-risk group) were treated.
RSV-associated mortality in the ISI-RSV risk groups for 43 patients presenting with RSV infection at the LRTI stage stratified according to antiviral therapy
| . | . | . | Antiviral therapy at LRTI stage . | |||
|---|---|---|---|---|---|---|
| No . | Yes . | |||||
| Risk group . | No. . | Deaths, n (%) . | No. . | Deaths, n (%) . | No. . | Deaths, n (%) . |
| Low | 5 | 0 | 2 | 0 | 3 | 0 |
| Moderate | 26 | 6 (23) | 0 | NA | 26 | 6 (23) |
| High | 12 | 6 (50) | 0 | NA | 12 | 6 (50) |
| . | . | . | Antiviral therapy at LRTI stage . | |||
|---|---|---|---|---|---|---|
| No . | Yes . | |||||
| Risk group . | No. . | Deaths, n (%) . | No. . | Deaths, n (%) . | No. . | Deaths, n (%) . |
| Low | 5 | 0 | 2 | 0 | 3 | 0 |
| Moderate | 26 | 6 (23) | 0 | NA | 26 | 6 (23) |
| High | 12 | 6 (50) | 0 | NA | 12 | 6 (50) |
NA, not applicable.
Kaplan-Meier failure curves for RSV-associated mortality in patients presenting with RSV-LRTI stage stratified according to ISI-RSV risk group. Only 2 LRTI patients in the low-risk group did not receive antiviral therapy and no death was observed in this group (data not shown in the figure).
Kaplan-Meier failure curves for RSV-associated mortality in patients presenting with RSV-LRTI stage stratified according to ISI-RSV risk group. Only 2 LRTI patients in the low-risk group did not receive antiviral therapy and no death was observed in this group (data not shown in the figure).
Discussion
Many studies have identified several potential risk factors for increased morbidity and mortality in allo-HCT recipients with RSV infections.20 To our knowledge, this is the first study that developed a scoring index with the specific purpose of accounting for the frequency and magnitude of these risk factors to quantitatively predict the outcome of RSV infections in this population. The ISI-RSV is an easy-to-use, clinically applicable scoring index designed to help clinicians target allo-HCT recipients with RSV infections who are at high risk of progression to RSV LRTI and may benefit from antiviral therapy.
The ISI-RSV is based on readily available clinical and laboratory data such as age, neutropenia, lymphocytopenia, myeloablative conditioning regimen use, presence of GVHD, corticosteroid use, recent HCT, and lack of stem cell engraftment. Several findings in the present study are consistent with previous reports including the host factors that increase the risk of progression to LRTI in RSV patients: older age, lymphocytopenia, and neutropenia,3-7,9,21,22 which are significantly associated with increased risk of progression to LRTI.
On the basis of these available clinical and laboratory indicators, we stratified patients into low-, moderate-, and high-risk groups. Patients in the low-risk group had the lowest infection progression rates, and none of them died. Antiviral therapy with aerosolized ribavirin greatly reduced the incidence of progression to LRTI. However, the NNT was 8 to prevent 1 LRTI, and none of the patients in this group died, even those who experienced progression to LRTI while not receiving therapy. Therefore the low-risk group benefited the least from antiviral therapy. In comparison, in the moderate-risk group, the absolute reduction in the risk of LRTI progression and RSV-associated death was <15% after ribavirin-based therapy, with a high number of treated patients required to prevent one case of progression (n = 9) or death (n = 45). This resulted from a mortality rate <5% irrespective of antiviral therapy use at the URTI stage in this group. Finally, patients in the high-risk group had the greatest absolute reduction in the risk of progression from RSV URTI to LRTI and the lowest number of treated patients required to prevent one LRTI or one RSV-associated death (n = 2). By stratifying the patients into these 3 risk groups, we were able to identify those who had the highest morbidity and mortality rates and those who would benefit the most from ribavirin-based therapy at the URTI stage.
Despite its potential benefits, treatment with ribavirin is not routinely recommended because of inconvenient administration, high cost, and questionable efficacy owing to the lack of randomized controlled trials. Decision making regarding antiviral therapy in allo-HCT recipients with RSV infections remains controversial. Randomized clinical trials in this population have proven to be challenging23 ; thus given the high morbidity and mortality rates in these patients, identifying target populations that would benefit the most from antiviral therapy is imperative. Herein, we propose the use of ISI-RSV as a tool to help clinicians to categorize patients into risk groups that may benefit the most from antiviral therapy. About 60% of the patients in each risk group in our study underwent ribavirin-based therapy, which means that 60% of low-risk patients received treatment at the URTI stage, but 40% of high-risk patients did not. This apparent risk-treatment paradox indicates the importance of risk stratification using the ISI-RSV as a means of identifying high-risk patients ideally suited for ribavirin-based therapy. Targeting high-risk patients (<10% of the present cohort) and, arguably, moderate-risk patients holds great potential to decrease costs and thus increase the cost-effectiveness of ribavirin-based therapy.
We did not observe any effect of time delay between RSV diagnosis and initiation of ribavirin therapy for the entire cohort. The median time from RSV diagnosis to initiation of ribavirin therapy was 1 day (range, 0-13) for patients who progressed to LRTI and 1 day (range, 0-10) for those who did not (P = .08). A statistical difference was not observed after stratification by risk groups including the high-risk group (P = .5). Lack of ribavirin therapy at the URTI stage was the only significant risk factor for LRTI and mortality. Importantly, patients presenting at the LRTI stage had a high mortality rate despite receiving ribavirin-based therapy, probably owing to a lack of initiation of therapy at the URTI stage which may be an important factor in determining the outcome of these infections.2,20 Furthermore, use of the ISI-RSV identified a low-risk group of patients presenting with RSV LRTI who did not die of their infection regardless of whether they were treated.
Our study has some limitations. First, the ISI-RSV was developed using a retrospective cohort that was not specifically designed for this aim. We attempted to minimize the impact of this potential bias by ensuring that information on risk factors such as neutropenia and lymphocytopenia was collected for the day of RSV diagnosis and that the actual date of each outcome was noted. We developed this scoring index by analyzing a subset of patients presenting with RSV infections at the URTI stage to track their progression to LRTI and RSV-associated mortality. Second, this was a single-center study and the results therefore should be viewed with caution, especially given that the factors we used to develop the ISI-RSV reflect the immune status of the host but not the fitness and virulence of the virus. Most importantly, further validation of the ISI-RSV in another large but different cohort is necessary and for different respiratory viruses as well and should examine other risk factors such as smoking history and conditioning regimen with high-dose total body irradiation.24 Third, concomitant infections may increase the risk of progression to LRTI or RSV-associated mortality. However, no specific pathogen or site of coinfection has been identified as a definitive predictor for these outcomes. Thus, inclusion of this factor would add to the complexity of this simple clinical index score.
In summary, we designed the ISI-RSV to stratify allo-HCT recipients with RSV URTI into risk groups that predict progression to LRTI and RSV-associated mortality using immunodeficiency indicators that are readily available at the time of RSV diagnosis. A well-designed, multi-institutional study examining various risk factors should be undertaken to validate this scoring index. Identification of high-risk groups in particular would facilitate early targeting of these patients for antiviral therapy to prevent unfavorable outcomes. At our institution, we developed algorithms and internal guidelines and raised awareness about prompt testing and management of patients with URTI symptoms all year long.25 Finally, the ISI-RSV could be used as a prognostic tool to accurately predict progression to LRTI and RSV-associated mortality in allo-HCT recipients.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Mr Donald Norwood (Department of Scientific Publications, The University of Texas MD Anderson Cancer Center) for his editorial support.
This manuscript was supported by a grant from the National Institutes of Health, National Cancer Institute under award number P30CA016672 (Cancer Center Support Grant).
Authorship
Contribution: R.F.C. and D.P.S. conceptualized and designed the study; R.F.C., D.P.S., S.S.G., E.J.A.-H., L.N., K.K.E.T., and J.N.S. performed the clinical research and data validation; G.R., C.H., and R.E.C. helped with data acquisition; D.P.S. and P.K.S. performed the statistical analyses; D.P.S., P.K.S., and R.F.C. wrote the paper; and all authors helped critically review the manuscript and checked the final version of it.
Conflict-of-interest disclosure: R.F.C. received research grants from ADMA Biologics Inc. The remaining authors declare no competing financial interests.
Correspondence: Roy F. Chemaly, Department of Infectious Diseases, Infection Control and Employee Health, Unit 402, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030-4009; e-mail: rfchemaly@mdanderson.org.