In this issue of Blood, Turesson et al study the risk of progression of monoclonal gammopathy of undetermined significance (MGUS) to lymphoplasmacellular and myeloid malignancies in a large population, validating current risk factors and adding immunoparesis as a predictor of progression.1
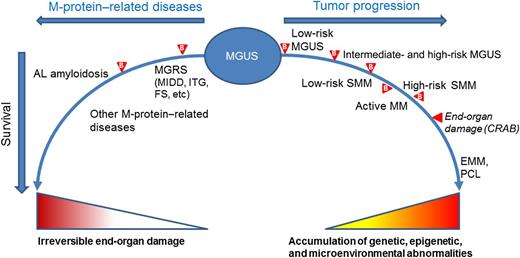
Spectrum of the possible progression of MGUS. Acquisition of somatic genetic and epigenetic abnormalities in the tumor cells and changes in the bone marrow microenvironment lead to the transformation of non-IgM MGUS into SMM, to MM, extramedullary myeloma (EMM), and plasma cell leukemia (PCL). IgM-MGUS can progress to smoldering Waldenström macroglobulinemia (WM), to WM, lymphoma, or other chronic lymphoproliferative disorders (not shown in the figure). The stages of progression are differentiated using biomarkers ( ) and imaging. Recently, the diagnosis of “active myeloma” has been proposed, a condition anticipating the occurrence of end-organ damage (CRAB).5 The clone may also produce end-organ damage through the M-protein. The protein may target the kidney in monoclonal gammopathy of renal significance (MGRS), including, among others, monoclonal immunoglobulin deposition disease (MIDD), immunotactoid glomerulopathy (ITG), and Fanconi syndrome (FS). The monoclonal light chains may deposit in tissues, causing progressive organ dysfunction; the most notable condition is AL amyloidosis. Biomarkers may help to anticipate the diagnosis of these conditions, before irreversible organ damage has occurred. Other M-protein–related conditions, such as autoimmune neuropathies and chronic cold agglutinin disease, are caused by the autoantibody activity of the M-protein.
) and imaging. Recently, the diagnosis of “active myeloma” has been proposed, a condition anticipating the occurrence of end-organ damage (CRAB).5 The clone may also produce end-organ damage through the M-protein. The protein may target the kidney in monoclonal gammopathy of renal significance (MGRS), including, among others, monoclonal immunoglobulin deposition disease (MIDD), immunotactoid glomerulopathy (ITG), and Fanconi syndrome (FS). The monoclonal light chains may deposit in tissues, causing progressive organ dysfunction; the most notable condition is AL amyloidosis. Biomarkers may help to anticipate the diagnosis of these conditions, before irreversible organ damage has occurred. Other M-protein–related conditions, such as autoimmune neuropathies and chronic cold agglutinin disease, are caused by the autoantibody activity of the M-protein.
Spectrum of the possible progression of MGUS. Acquisition of somatic genetic and epigenetic abnormalities in the tumor cells and changes in the bone marrow microenvironment lead to the transformation of non-IgM MGUS into SMM, to MM, extramedullary myeloma (EMM), and plasma cell leukemia (PCL). IgM-MGUS can progress to smoldering Waldenström macroglobulinemia (WM), to WM, lymphoma, or other chronic lymphoproliferative disorders (not shown in the figure). The stages of progression are differentiated using biomarkers ( ) and imaging. Recently, the diagnosis of “active myeloma” has been proposed, a condition anticipating the occurrence of end-organ damage (CRAB).5 The clone may also produce end-organ damage through the M-protein. The protein may target the kidney in monoclonal gammopathy of renal significance (MGRS), including, among others, monoclonal immunoglobulin deposition disease (MIDD), immunotactoid glomerulopathy (ITG), and Fanconi syndrome (FS). The monoclonal light chains may deposit in tissues, causing progressive organ dysfunction; the most notable condition is AL amyloidosis. Biomarkers may help to anticipate the diagnosis of these conditions, before irreversible organ damage has occurred. Other M-protein–related conditions, such as autoimmune neuropathies and chronic cold agglutinin disease, are caused by the autoantibody activity of the M-protein.
) and imaging. Recently, the diagnosis of “active myeloma” has been proposed, a condition anticipating the occurrence of end-organ damage (CRAB).5 The clone may also produce end-organ damage through the M-protein. The protein may target the kidney in monoclonal gammopathy of renal significance (MGRS), including, among others, monoclonal immunoglobulin deposition disease (MIDD), immunotactoid glomerulopathy (ITG), and Fanconi syndrome (FS). The monoclonal light chains may deposit in tissues, causing progressive organ dysfunction; the most notable condition is AL amyloidosis. Biomarkers may help to anticipate the diagnosis of these conditions, before irreversible organ damage has occurred. Other M-protein–related conditions, such as autoimmune neuropathies and chronic cold agglutinin disease, are caused by the autoantibody activity of the M-protein.
MGUS is one of the most common premalignant disorders in the general population, occurring in over 3% of individuals ≥50 years old. It is predominantly diagnosed incidentally and is characterized by the presence of a serum monoclonal (M) protein in the absence of symptoms, with an unrelenting annual risk of progression to multiple myeloma (MM) or related plasma cell (PC) disorders of approximately 1%.2 Although virtually all patients with MM have a previously recognized MGUS, most cases of MGUS do not progress toward malignancy. Differentiating low-risk patients, who may not need further follow-up, from high-risk patients, who warrant close monitoring, is challenging.
Multistep genetic and microenvironmental changes lead to the transformation of MGUS into smoldering myeloma (SMM), MM, and, finally, to extramedullary myeloma or related malignancies. Intense research is ongoing to identify biologically relevant markers differentially expressed throughout this progression, which could be exploited to predict evolution or suggest therapeutic strategies that would prevent or delay progression. Despite important progress, we still lack reliable biological markers to predict which patients will progress and which will not, and MGUS is currently risk stratified based on clinical variables identified through epidemiologic studies. Both the Mayo Clinic and Spanish investigators have developed models predicting the risk of MGUS progression (reviewed in Merlini and Palladini3 ). The former is centered on serum biomarkers and identifies 3 risk factors (non-IgG isotype, serum M-protein concentration ≥15 g/L, and an abnormal free light chain [FLC] ratio), whereas the latter is based mainly on multiparametric flow cytometry identification of PC populations. Given its easier applicability, the Mayo Clinic model is more widely used and is incorporated in the International Myeloma Working Group’s guidelines.4 However, neither risk model has been independently validated in large patient populations with long-term follow-up. The study by Turesson et al fills this gap, analyzing a large Swedish cohort using retrospective data collected from medical records and case ascertainment from a national cancer registry. Although the study design has limitations, many findings are relevant. One notable observation is that the annual risk of progression toward lymphoplasmacellular malignancies is only 0.5% in the Swedish cohort, unlike the ∼1% found in the population-based Mayo Clinic cohort.2 This may be due to different study designs and populations. The negative impact of serum M-protein concentration ≥15 g/L and abnormal FLC ratio is confirmed in the Swedish cohort. The latter biomarker also plays an important role in identifying patients with highest-risk SMM (or “active MM,” according to a recently proposed reclassification).5 In the Swedish study, the suppression of noninvolved immunoglobulins (immunoparesis) increases the discriminatory power of the Mayo Clinic model to identify high-risk MGUS. Interestingly, isotype-specific paresis (suppression of IgGk in IgGλ MGUS) appears to be an early predictor of progression, although this needs confirmation.6
The study by Turesson et al falls within a comprehensive effort to redefine the risk categories of monoclonal gammopathies throughout the whole spectrum, ranging from the precursor state, to smoldering and active MM (see figure). The definition of active MM, in the absence of CRAB (calcium increase, renal insufficiency, anemia, bone lesions) is ongoing, exploiting validated and novel biomarkers and modern imaging.5 The goal is to reliably intercept the disease at earlier stages of genetic and host immunologic abnormalities, when intraclonal heterogeneity is limited and the clone would be more amenable to therapeutic intervention, anticipating organ damage and prolonging survival. It is also important to identify patients with low-risk, benign MGUS who have an absolute risk of progression at 20 years, adjusting for competing causes of death, of only 2%.2 These individuals may not require baseline bone marrow examination, skeletal radiography, or annual follow-up and, particularly the elderly, could be discharged to family physicians.3 The Mayo Clinic risk model2 efficiently identifies these subjects, who represent a substantial proportion (∼40%) of MGUS cases.4 Patients with intermediate- and high-risk MGUS, however, require close monitoring, including not only biomarkers of clonal progression but also biomarkers of organ damage caused by the M protein (see figure). The 2 most common and clinically relevant target organs are the kidney, in monoclonal gammopathy of renal significance,7 and the heart, in light chain (AL) amyloidosis.8 Organ failure can develop silently in subjects with MGUS. However, urinary albumin excretion and serum creatinine, with estimated glomerular filtration rate, can efficiently detect early kidney involvement, while NT-proBNP has 100% sensitivity, although it is not specific, in detecting early, reversible, cardiac dysfunction caused by amyloidogenic light chains.8 Inclusion of these biomarkers in routine follow-up of individuals with MGUS and an abnormal FLC ratio (who are at higher risk of developing M-protein–related organ damage) may allow early detection of these insidious diseases. The underlying PC clones are usually small, indolent, and probably in an early stage of progression. Treatment of these “early clones” in patients with AL amyloidosis results in long-lasting complete responses translating into superior survival compared with that of MM patients.9
Although only a fraction of MGUS cases (21% of 70-year-olds)10 are recognized during routine clinical practice, the current consensus is still that screening for MGUS should not be performed, considering the generally low risk of progression to malignancy, related emotional burden, and lack of effective interventions. However, because it is now possible to rapidly identify low-risk individuals (thus reducing the economic and emotional burden for approximately 40% of cases), we can focus on the remaining 60%, with careful monitoring, in order to detect progression promptly and anticipate end-organ damage.4 If evidence of clinical benefit of early interventions11 is consolidated, we could likely reconsider our current practice, adopting a more proactive search for M protein in clinical practice.
Available biomarkers are enabling clinicians to risk stratify individuals with MGUS, optimizing their management. Novel, biologically relevant markers, in combination with imaging techniques, have the potential to change our clinical practice throughout the spectrum of monoclonal gammopathies.
Conflict-of-interest disclosure: The author declares no competing financial interests.