Key Points
Free light chain ratio, M-protein concentration, and immunosuppression predict progression of MGUS to lymphoid malignancies.
Abstract
In 728 Swedish cases of monoclonal gammopathy of undetermined significance (MGUS), followed up to 30 years (median, 10 years), we estimated the cumulative risk of hematologic disorders originating from lymphoid and myeloid lineages. Using Cox regression models, we examined associations of demographic and laboratory factors with progression and determined the discriminatory power of 3 prediction models for progression. Eighty-four MGUS cases developed a lymphoid disorder, representing a cumulative risk of 15.4%. Multiple myeloma (MM) occurred in 53 patients, and the 30-year cumulative risk was 10.6%; an ∼0.5% annual risk. Three factors were significantly associated with progression: abnormal free light-chain (FLC) ratio (<0.26 or >1.65), M-protein concentration (≥1.5 g/dL), and reduction of 1 or 2 noninvolved immunoglobulin isotype levels (immunoparesis). A prediction model with separate effects for these 3 factors and the M-protein isotype had higher discriminatory power than other models, although the differences were not statistically significant. The 30-year cumulative risk for myeloid malignancies was <2%. Our study confirms that abnormal FLC ratio and M-protein concentration >1.5 g/dL, factors previously considered by Mayo Clinic researchers, are predictors for MM progression and suggests that separate consideration of immunoparesis and the Mayo Clinic risk factors could improve identification of MGUS patients at high risk for progression.
Continuing Medical Education online
This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education through the joint sponsorship of Medscape, LLC and the American Society of Hematology.
Medscape, LLC is accredited by the ACCME to provide continuing medical education for physicians.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 70% minimum passing score and complete the evaluation at http://www.medscape.org/journal/blood; and (4) view/print certificate. For CME questions, see page 457.
Disclosures
The authors, Associate Editor A. Keith Stewart, and CME questions author Laurie Barclay, freelance writer and reviewer, Medscape, LLC, declare no competing financial interests.
Learning objectives
Describe the risk for hematologic disorders originating from lymphoid and myeloid lineages in patients with monoclonal gammopathy of undetermined significance (MGUS), based on a long-term follow-up study.
List factors associated with progression of MGUS to hematologic disorders originating from lymphoid and myeloid lineages.
Discuss a prediction model for progression of MGUS to hematologic disorders originating from lymphoid and myeloid lineages.
Release date: January 16, 2014; Expiration date: January 16, 2015
Introduction
Monoclonal gammopathies are disorders characterized by a homogeneous immunoglobulin (the M-protein) spike in serum or urine arising from the proliferation of an abnormal clone of a single plasma cell precursor. Multiple myeloma (MM) is the archetype of a malignant monoclonal plasma cell disorder. Benign monoclonal gammopathies, as first described by Waldenström, also occur and are much more common than MM.1 Once it was observed that a substantial proportion of benign gammopathies develop into MM, the now universally accepted term monoclonal gammopathy of undetermined significance (MGUS) was introduced to describe the disorder.2
In 2003, the International Myeloma Working Group (IMWG) defined diagnostic criteria differentiating MGUS from MM, Waldenström’s macroglobulinemia (WM), and other non-Hodgkin lymphomas (NHLs), based on the type of M-protein, its concentration, degree of bone marrow infiltration of plasma cells or lymphoplasmacytic cells, and presence of certain clinical manifestations (the CRAB criteria).3 The term smoldering MM (SMM) was introduced to distinguish a more advanced premalignant stage with a higher risk of progression but still not requiring treatment.4 There are 3 types of MGUS with distinct natural histories: non-IgM-(IgG or IgA)-MGUS, IgM-MGUS, and light-chain-MGUS.5,6 Although IgG and IgA- and light-chain-MGUS typically progress to MM or develop light chain amyloidosis, IgM-MGUS cases more often progress to WM or other NHLs.
Data from the Mayo Clinic suggest that MGUS is present in ∼3% of the general white population ≥50 years of age and is predominantly incidentally diagnosed.7,8 Although 2 independent studies indicate that MGUS always precedes MM,9,10 the majority of MGUS patients do not progress to a lymphoproliferative disorder. The average risk of progression is estimated to be 1%/yr, and the 25-year cumulative risk is 30%.11,12 However, there is considerable variation in the risk of progression, and differentiating low-risk patients, who may not need further follow-up, from high-risk patients, who may warrant close monitoring or enrolment in early intervention studies, is a challenge.
Several models have been published for risk stratification of MGUS patients.12,13 Risk factors included in a Mayo Clinic model are non-IgG isotype, M-protein concentration ≥1.5 g/dL, and an abnormal serum free light chain (FLC) ratio (normal reference: 0.26-1.65).12 Risk factors in the Spanish study group (Programa para el Estudio de la Terapéutica en Hemopatía Maligna [PETHEMA]) model are based on multiparametric flow cytometry and include the presence of >95% abnormal plasma cells and aneuploidy.13 Although a small study of 165 MGUS patients followed for 5 years found that 3 of 5 patients who progressed to MM or WM had an abnormal FLC ratio and 2 had an M-protein concentration ≥1.5 g/dL,14 no published risk model of progression has been independently validated with a large number of MGUS cases with long-term follow-up. We therefore assessed established risk factors and explored novel risk factors for progression using a large independent cohort of 728 MGUS patients followed up to 30 years.
Patients and methods
Study subjects
During the study period 1963 to 2000, there were 1202 individuals diagnosed with MGUS at Malmö University Hospital (97.8%) or another local hospital (2.2%). The study was approved by the research ethical committee of the University of Lund (Dnr 2009/400), and informed consent was obtained in accordance with the Declaration of Helsinki. Per institutional procedures, for each patient with a newly detected M-protein, 1 tube of serum was stored at the Department of Clinical Chemistry at −20°C. However, at the time of analysis, stored samples were no longer available for all subjects, as some were lost or had evaporated. After visual inspection of the serum samples, we identified 797 MGUS patients (66%) with samples of sufficient quality to be considered for analysis: 780 patients lived in Malmö and 17 in neighboring towns. We reviewed the medical records of the first 476 patients identified and confirmed the MGUS diagnosis in 466 (97.9%). The 10 unconfirmed cases were excluded. MGUS diagnosis was made according to IMWG criteria3 : M-protein concentration <3 g/dL, absence of CRAB criteria or clinical signs of other lymphoproliferative disorders, and <10% plasma cells if bone marrow examination was performed. Bone marrow examination was performed in 20% of patients with low M-protein levels, for whom examination was not mandatory. We thus further excluded 8 individuals with M-protein concentrations ≥3 g/dL (≥1.5 g/dL). Of the remaining 779 MGUS patients, we excluded 35 (4.5%) subjects with missing date of birth and/or missing date of M-protein diagnosis, 8 (1%) subjects with missing M-protein isotype information, and 8 (1%) subjects who had a lymphoproliferative disorder prior to the date of M-protein detection, leaving a final study sample of 728 MGUS cases (supplemental Table 1 on the Blood Web site).
Patient characteristics and sedimentation rate were obtained from medical records. All remaining laboratory measurements were based on analyses of stored serum samples as described below. As a quality control step, we investigated differences in demographic characteristics of registered MGUS patients by inclusion status. Although there were no significant differences in age or gender, patients who were excluded due to serum sample availability tended to be more recently diagnosed (median year of diagnosis 1993 vs 1986, P < .01).
Laboratory analyses
Freelite serum free κ assays (LK016.10H; Binding Site) and serum free λ assays (LK 018.10H; Binding Site) were conducted on a Roche Hitachi Modular P turbidimeter using the manufacturer's instructions. Using the turbidimeter and Roche immunodiagnostic kits, we measured IgG: 03507378190, IgA: 03507246190, IgM: 03507041190, C-reactive protein: 03002039122, β2 microglobulin: 11660551216, creatinine: 11875418216, albumin: 11970909216, and total protein: 11929917216. Electrophoresis was performed on an Interlab Microgel analyzer (product code SRE 601K) in the Clinical Immunology Service, a clinical pathology accreditation–accredited laboratory in the University of Birmingham. Nearly 20% of this study’s stored serum samples were >30 years old. However, in analyses comparing the concentration of protein assays, we did not find any evidence that the quality of samples varied by calendar year (data not shown).
Outcome ascertainment
MGUS cases with available serum samples were followed through June 1, 2009 for progression to a primary lymphoid disorder, primary myeloid disorder, or death. We obtained the date of diagnosis of incident cancers from 3 sources: the nationwide Swedish Cancer Registry, the nationwide Patient Registry, and the Patient Registry of Malmö University Hospital. Since 1958, all physicians and pathologists/cytologists in Sweden are obligated by law to report each incident case of cancer that they diagnose and/or treat to the centralized nationwide Swedish Cancer Registry. The registry contains information on diagnosis, gender, date of birth, date of diagnosis, region/hospital where the diagnosis was made, and data from the Swedish Cause of Death Registry.15 In a recent validation study focusing on lymphoproliferative hematologic tumors diagnosed between 1964 and 2003, we found the completeness and the diagnostic accuracy of the registry to be >90% to 95%.16 As light chain (AL) amyloidosis is not reported to the Swedish Cancer Registry, information on AL amyloidosis was obtained from the Swedish Patient Registry, which contains all information on discharge diagnoses and discharge listing from inpatient care since 1964 and has high coverage.17
Hematologic malignancies considered in this study and their disease classification codes were as follows: MM (ICD7 203.0), amyloidosis (289.2), acute myeloid leukemia (AML) (205-206, 207.0-207.3), NHL (200.1-9, 202.1-202.9, 204.1), chronic lymphocytic leukemia (CLL) (204.1), WM (200.3), acute lymphoblastic leukemia (ALL) (204), chronic myelogenous leukemia (CML) (205.1), Hodgkin lymphoma (HL) (201), myeloproliferative neoplasms (MPNs) (207.9, 208, 209), and myelodysplastic syndromes (ICD10 D460-464, D467, D469). We further grouped hematologic disorders into 2 categories: lymphoid disorders (MM, amyloidosis, NHL, HL, and ALL) and myeloid malignancies (AML, CML, MPNs, and myelodysplastic syndrome).
Statistical analysis
Descriptive statistics were used to summarize the sample and disease outcomes, stratified by MGUS subtype. All event summaries refer to the first hematologic malignancy. Crude incidence rates were calculated as the number of events divided by the total number of person-years at-risk following MGUS diagnosis, and 95% confidence intervals (CIs) were based on a Poisson distribution. One patient was diagnosed with NHL and amyloidosis on the same day. For this patient, we included both events in the cause-specific analyses but counted 1 event for analyses of the composite lymphoid outcome.
We estimated unadjusted (crude) cumulative risk,18 defined as the probability of disease within a specific number of years following MGUS diagnosis, and its 95% CI over a 30-year time period for each lymphoproliferative disease outcome.
Using Cox proportional hazards models, we examined the hazard ratio associations (HRs) with progression for selected demographic and laboratory measures and 3 risk factors previously identified by researchers at the Mayo Clinic12 (Mayo 3): FLC ratio <0.26 or >1.65, non-IgG subtype, and serum M-protein ≥1.5 g/dL. We also assessed immunoparesis, a depression of immunoglobulin protein levels for the noninvolved isotype, because it has been previously implicated in the risk of progression.13 We defined immunoparesis separately for each MGUS subtype. For subtype IgG, we considered serum IgA <0.88 g/L or IgM <0.27 g/L as indicative of immunoparesis; for subtype IgA, IgM <0.27 g/L or IgG <6.7 g/L; and for subtype IgM, IgA <0.88 g/L or IgG <6.7 g/L.
The time scale for the Cox proportional hazards models was days from MGUS diagnosis, and all models were adjusted for age (continuous) and year of diagnosis (<1991, ≥1991), to account for the period effect we found with serum sample availability. Observations with missing values were removed, and we assessed the impact of their omission in sensitivity analyses that refitted the models with a separate risk factor category for missing values. We tested the proportional hazards assumption using Schoenfeld residuals19 and examined the presence of age-covariate interactions by refitting the Cox models using age as the time scale.
We contrasted the discriminatory power of 3 different prediction models for progression. The risk factors included in the different models were (1) the count of a patient’s total number of Mayo Clinic factors (maximum of 3), (2) the count of a patient’s total number of risk factors including immunoparesis and the 3 Mayo Clinic factors, and (3) individual effects for immunoparesis and each of the 3 Mayo Clinic factors. The last model addresses the question of whether information about a patient’s specific combination of risk factors, not merely the total count, improves prediction of progression.
Discriminatory ability was measured with the area under the receiver operating characteristic curve (AUC), which represents the probability that a model correctly assigns a higher risk to a randomly selected true case vs a randomly selected non-case. A threefold Monte Carlo cross-validation was used to estimate the AUC for each model, with one-third of the sample used as the test set in each iteration of the procedure.20 Cases were defined as persons progressing within 5 years of MGUS diagnosis. To protect against bias due to competing death, non-cases were defined as persons having no event and a minimum of 5 years of follow-up. Our procedure consisted of 50 iterations of the threefold cross-validation. We used Monte Carlo summaries of the resulting 150 AUC estimates to obtain the mean and 95% CIs of each model’s discriminatory ability.
Results
The 728 patients in the study cohort were diagnosed with MGUS at a median age of 74 years (range, 28-98 years), and 21%, 46%, and 33% were diagnosed in 1963 to 1980, 1981 to 1990, and 1991 to 2000, respectively (Table 1). The most frequent MGUS subtype was IgG with 501 patients (69%). There were 107 patients with IgA (15%), 118 with IgM (16%), and 2 with IgE subtype (0.3%). Twenty-four percent of the samples had evidence of immunoparesis (Table 1). The FLC ratio was abnormal in 47% of the patients, and the M-protein concentration was ≥1.5 g/dL in 13% (median, 0.7 g/dL, range, 0.1-3.0 g/dL).
MGUS patient characteristics
| Variable . | Number (percentage) . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MGUS isotype . | Overall . | |||||||||
| IgG . | IgA . | IgM . | IgE . | |||||||
| N | 501 | 68.8 | 107 | 14.7 | 118 | 16.2 | 2 | 0.0 | 728 | 100.0 |
| Follow-up, years, median (SD) | 10.7 | (7.7) | 12.9 | (7.0) | 10.0 | (9.2) | 3.7 | (2.1) | 10.9 | (7.8) |
| Gender | ||||||||||
| Male | 251 | 50.1 | 51 | 47.7 | 60 | 50.8 | 2 | 100.0 | 364 | 50.0 |
| Female | 250 | 49.9 | 56 | 52.3 | 58 | 49.2 | 0 | 0.0 | 364 | 50.0 |
| Year diagnosed | ||||||||||
| 1963-1980 | 104 | 20.8 | 19 | 17.8 | 30 | 25.4 | 1 | 50.0 | 154 | 21.2 |
| 1981-1990 | 231 | 46.1 | 43 | 40.2 | 58 | 49.2 | 1 | 50.0 | 333 | 45.7 |
| 1991-2000 | 166 | 33.1 | 45 | 42.1 | 30 | 25.4 | 0 | 0.0 | 241 | 33.1 |
| Age at diagnosis, years | ||||||||||
| 25-60 | 92 | 18.4 | 9 | 8.4 | 15 | 12.7 | 0 | 0.0 | 116 | 15.9 |
| 61-70 | 118 | 23.6 | 24 | 22.4 | 33 | 28.0 | 1 | 50.0 | 176 | 24.2 |
| 71-80 | 177 | 35.3 | 44 | 41.1 | 39 | 33.1 | 0 | 0.0 | 260 | 35.7 |
| 81-90 | 103 | 20.6 | 27 | 25.2 | 27 | 22.9 | 1 | 50.0 | 158 | 21.7 |
| 91-100 | 11 | 2.2 | 3 | 2.8 | 4 | 3.4 | 0 | 0.0 | 18 | 2.5 |
| Median age (range) | 73 | (28-96) | 74 | (53-93) | 75 | (38-98) | 74 | (67-81) | 74 | (28-98) |
| Albumin, g/L | ||||||||||
| <35 | 115 | 23.0 | 19 | 17.8 | 31 | 26.3 | 0 | 0.0 | 165 | 22.7 |
| ≥35 | 212 | 42.3 | 41 | 38.3 | 56 | 47.5 | 2 | 100.0 | 311 | 42.7 |
| Missing | 174 | 34.7 | 47 | 43.9 | 31 | 26.3 | 0 | 0.0 | 252 | 34.6 |
| Sedimentation rate, mm/h | ||||||||||
| 0.1-40 | 105 | 21.0 | 18 | 16.8 | 0 | 0.0 | 1 | 50.0 | 124 | 17.0 |
| 41-100 | 118 | 23.6 | 24 | 22.4 | 0 | 0.0 | 0 | 0.0 | 142 | 19.5 |
| ≥101 | 19 | 3.8 | 3 | 2.8 | 0 | 0.0 | 0 | 0.0 | 22 | 3.0 |
| Missing | 259 | 51.7 | 62 | 57.9 | 118 | 100.0 | 1 | 50.0 | 440 | 60.4 |
| Creatinine, μmol/L | ||||||||||
| 0.1-50 | 48 | 9.6 | 11 | 10.3 | 13 | 11.0 | 0 | 0.0 | 72 | 9.9 |
| 51-100 | 222 | 44.3 | 50 | 46.7 | 66 | 55.9 | 2 | 100.0 | 340 | 46.7 |
| ≥101 | 146 | 29.1 | 35 | 32.7 | 39 | 33.1 | 0 | 0.0 | 220 | 30.2 |
| Missing | 85 | 17.0 | 11 | 10.3 | 0 | 0.0 | 0 | 0.0 | 96 | 13.2 |
| C-reactive protein, mg/L | ||||||||||
| 0.1-5 | 226 | 45.1 | 58 | 54.2 | 54 | 45.8 | 1 | 50.0 | 339 | 46.6 |
| 5-24 | 132 | 26.3 | 24 | 22.4 | 37 | 31.4 | 1 | 50.0 | 194 | 26.6 |
| ≥25 | 138 | 27.5 | 25 | 23.4 | 27 | 22.9 | 0 | 0.0 | 190 | 26.1 |
| Missing | 5 | 1.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 5 | 0.7 |
| β-2 microglobulin, mg/L | ||||||||||
| <3.5 | 313 | 62.5 | 72 | 67.3 | 78 | 66.1 | 0 | 0.0 | 463 | 63.6 |
| 3.5-5.5 | 118 | 23.6 | 22 | 20.6 | 23 | 19.5 | 2 | 100.0 | 165 | 22.7 |
| >5.5 | 64 | 12.8 | 13 | 12.1 | 17 | 14.4 | 0 | 0.0 | 94 | 12.9 |
| Missing | 6 | 1.2 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 6 | 0.8 |
| FLC ratio | ||||||||||
| 0.26-1.65 | 267 | 53.3 | 50 | 46.7 | 66 | 55.9 | 0 | 0.0 | 383 | 52.6 |
| <0.26 or >1.65 | 228 | 45.5 | 57 | 53.3 | 52 | 44.1 | 2 | 100.0 | 339 | 46.6 |
| Missing | 6 | 1.2 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 6 | 0.8 |
| M-protein concentration, g/dL | ||||||||||
| Median, (SD) | 0.7 | (0.6) | 0.8 | (0.5) | 0.7 | (0.6) | 0.2 | — | 0.7 | (0.6) |
| <1.5 | 436 | 87.0 | 94 | 87.9 | 103 | 87.3 | 1 | 50.0 | 634 | 87.1 |
| ≥1.5 | 63 | 12.6 | 13 | 12.1 | 15 | 12.7 | 0 | 0.0 | 91 | 12.5 |
| Missing | 2 | 0.4 | 0 | 0.0 | 0 | 0.0 | 1 | 50.0 | 3 | 0.4 |
| Immunoparesis* | ||||||||||
| No | 394 | 78.6 | 69 | 64.5 | 86 | 72.9 | 2 | 100.0 | 551 | 75.7 |
| Yes | 102 | 20.4 | 38 | 35.5 | 32 | 27.1 | 0 | 0.0 | 172 | 23.6 |
| Missing | 5 | 1.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 5 | 0.7 |
| Variable . | Number (percentage) . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MGUS isotype . | Overall . | |||||||||
| IgG . | IgA . | IgM . | IgE . | |||||||
| N | 501 | 68.8 | 107 | 14.7 | 118 | 16.2 | 2 | 0.0 | 728 | 100.0 |
| Follow-up, years, median (SD) | 10.7 | (7.7) | 12.9 | (7.0) | 10.0 | (9.2) | 3.7 | (2.1) | 10.9 | (7.8) |
| Gender | ||||||||||
| Male | 251 | 50.1 | 51 | 47.7 | 60 | 50.8 | 2 | 100.0 | 364 | 50.0 |
| Female | 250 | 49.9 | 56 | 52.3 | 58 | 49.2 | 0 | 0.0 | 364 | 50.0 |
| Year diagnosed | ||||||||||
| 1963-1980 | 104 | 20.8 | 19 | 17.8 | 30 | 25.4 | 1 | 50.0 | 154 | 21.2 |
| 1981-1990 | 231 | 46.1 | 43 | 40.2 | 58 | 49.2 | 1 | 50.0 | 333 | 45.7 |
| 1991-2000 | 166 | 33.1 | 45 | 42.1 | 30 | 25.4 | 0 | 0.0 | 241 | 33.1 |
| Age at diagnosis, years | ||||||||||
| 25-60 | 92 | 18.4 | 9 | 8.4 | 15 | 12.7 | 0 | 0.0 | 116 | 15.9 |
| 61-70 | 118 | 23.6 | 24 | 22.4 | 33 | 28.0 | 1 | 50.0 | 176 | 24.2 |
| 71-80 | 177 | 35.3 | 44 | 41.1 | 39 | 33.1 | 0 | 0.0 | 260 | 35.7 |
| 81-90 | 103 | 20.6 | 27 | 25.2 | 27 | 22.9 | 1 | 50.0 | 158 | 21.7 |
| 91-100 | 11 | 2.2 | 3 | 2.8 | 4 | 3.4 | 0 | 0.0 | 18 | 2.5 |
| Median age (range) | 73 | (28-96) | 74 | (53-93) | 75 | (38-98) | 74 | (67-81) | 74 | (28-98) |
| Albumin, g/L | ||||||||||
| <35 | 115 | 23.0 | 19 | 17.8 | 31 | 26.3 | 0 | 0.0 | 165 | 22.7 |
| ≥35 | 212 | 42.3 | 41 | 38.3 | 56 | 47.5 | 2 | 100.0 | 311 | 42.7 |
| Missing | 174 | 34.7 | 47 | 43.9 | 31 | 26.3 | 0 | 0.0 | 252 | 34.6 |
| Sedimentation rate, mm/h | ||||||||||
| 0.1-40 | 105 | 21.0 | 18 | 16.8 | 0 | 0.0 | 1 | 50.0 | 124 | 17.0 |
| 41-100 | 118 | 23.6 | 24 | 22.4 | 0 | 0.0 | 0 | 0.0 | 142 | 19.5 |
| ≥101 | 19 | 3.8 | 3 | 2.8 | 0 | 0.0 | 0 | 0.0 | 22 | 3.0 |
| Missing | 259 | 51.7 | 62 | 57.9 | 118 | 100.0 | 1 | 50.0 | 440 | 60.4 |
| Creatinine, μmol/L | ||||||||||
| 0.1-50 | 48 | 9.6 | 11 | 10.3 | 13 | 11.0 | 0 | 0.0 | 72 | 9.9 |
| 51-100 | 222 | 44.3 | 50 | 46.7 | 66 | 55.9 | 2 | 100.0 | 340 | 46.7 |
| ≥101 | 146 | 29.1 | 35 | 32.7 | 39 | 33.1 | 0 | 0.0 | 220 | 30.2 |
| Missing | 85 | 17.0 | 11 | 10.3 | 0 | 0.0 | 0 | 0.0 | 96 | 13.2 |
| C-reactive protein, mg/L | ||||||||||
| 0.1-5 | 226 | 45.1 | 58 | 54.2 | 54 | 45.8 | 1 | 50.0 | 339 | 46.6 |
| 5-24 | 132 | 26.3 | 24 | 22.4 | 37 | 31.4 | 1 | 50.0 | 194 | 26.6 |
| ≥25 | 138 | 27.5 | 25 | 23.4 | 27 | 22.9 | 0 | 0.0 | 190 | 26.1 |
| Missing | 5 | 1.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 5 | 0.7 |
| β-2 microglobulin, mg/L | ||||||||||
| <3.5 | 313 | 62.5 | 72 | 67.3 | 78 | 66.1 | 0 | 0.0 | 463 | 63.6 |
| 3.5-5.5 | 118 | 23.6 | 22 | 20.6 | 23 | 19.5 | 2 | 100.0 | 165 | 22.7 |
| >5.5 | 64 | 12.8 | 13 | 12.1 | 17 | 14.4 | 0 | 0.0 | 94 | 12.9 |
| Missing | 6 | 1.2 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 6 | 0.8 |
| FLC ratio | ||||||||||
| 0.26-1.65 | 267 | 53.3 | 50 | 46.7 | 66 | 55.9 | 0 | 0.0 | 383 | 52.6 |
| <0.26 or >1.65 | 228 | 45.5 | 57 | 53.3 | 52 | 44.1 | 2 | 100.0 | 339 | 46.6 |
| Missing | 6 | 1.2 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 6 | 0.8 |
| M-protein concentration, g/dL | ||||||||||
| Median, (SD) | 0.7 | (0.6) | 0.8 | (0.5) | 0.7 | (0.6) | 0.2 | — | 0.7 | (0.6) |
| <1.5 | 436 | 87.0 | 94 | 87.9 | 103 | 87.3 | 1 | 50.0 | 634 | 87.1 |
| ≥1.5 | 63 | 12.6 | 13 | 12.1 | 15 | 12.7 | 0 | 0.0 | 91 | 12.5 |
| Missing | 2 | 0.4 | 0 | 0.0 | 0 | 0.0 | 1 | 50.0 | 3 | 0.4 |
| Immunoparesis* | ||||||||||
| No | 394 | 78.6 | 69 | 64.5 | 86 | 72.9 | 2 | 100.0 | 551 | 75.7 |
| Yes | 102 | 20.4 | 38 | 35.5 | 32 | 27.1 | 0 | 0.0 | 172 | 23.6 |
| Missing | 5 | 1.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 5 | 0.7 |
Subtype IgG: IgA <0.88 g/L or IgM <0.27 g/L; subtype IgA: IgM <0.27 g/L or IgG <6.7 g/L; subtype IgM: IgA <0.88 g/L or IgG <6.7 g/L.
During 7590 person-years of follow-up (median, 10 years per subject), we observed 84 lymphoid and 10 myeloid malignancies in the study cohort (Table 2). As expected, the most frequent lymphoid malignancy was MM (53 events), which occurred at a rate of 6.7 per 1000 person-years. Of the 10 myeloid malignancies, 7 were AML and 3 were myelodysplastic syndrome (MDS), corresponding to incidence rates of 0.9 and 0.4 per 1000 person-years, respectively. Although lymphoid and myeloid malignancy incidence rates were similar for the IgG and IgA subtypes, no MM cases occurred among MGUS patients of the IgM isotype, but more than half of the cases of NHL (59%) were among these patients, WM being the most common NHL subtype (14/17; 82%). We observed only 3 cases of AL amyloidosis. No events of HL or ALL were observed.
Summary of lymphoid and myeloid outcomes and incidence rates (per 1000 person-years)
| Outcome . | Ig isotype* . | All Ig isotypes combined . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IgG . | IgA . | IgM . | ||||||||||
| Events . | Rate (95% CI) . | Median age (years)† . | Events . | Rate (95% CI) . | Median age (years)† . | Events . | Rate (95% CI) . | Median age (years)† . | Events . | Rate (95% CI) . | Median age (years)† . | |
| Lymphoid | 54‡ | 10.3 (7.7, 13.4) | 78 | 13 | 10.7 (5.7, 18.3) | 71 | 17 | 14.6 (8.5, 23.3) | 79 | 84c | 11.0 (8.8, 13.6) | 76 |
| MM | 42 | 7.9 (5.7, 10.6) | 77 | 11 | 8.8 (4.4, 15.8) | 72 | 0 | 0.0 (0.0, 2.8) | 53 | 6.7 (5.0, 8.8) | 75 | |
| Amyloidosis | 2 | 0.4 (0.0, 1.3) | 71 | 1 | 0.8 (0.0, 4.3) | 71 | 0 | 0.0 (0.0, 2.8) | 3 | 0.4 (0.1, 1.1) | 71 | |
| NHL | 11 | 2.0 (1.0, 3.6) | 78 | 1 | 0.8 (0.0, 4.3) | 60 | 17 | 14.6 (8.5, 23.3) | 79 | 29 | 3.7 (2.5, 5.3) | 78 |
| CLL | 1 | 0.2 (0.0, 1.0) | 49 | 0 | 0.0 (0.0, 2.8) | 3 | 2.3 (0.5, 6.7) | 75 | 1 | 0.1 (0.0, 0.7) | 49 | |
| Waldenström | 0 | 0.0 (0.0, 0.7) | 0 | 0.0 (0.0, 2.8) | 14 | 11.7 (6.4, 19.6) | 79 | 3 | 0.4 (0.1, 1.1) | 75 | ||
| Other | 10 | 1.8 (0.9, 3.4) | 78 | 1 | 0.8 (0.0, 4.3) | 60 | 0 | 0.0 (0.0, 2.8) | 25 | 3.2 (2.0, 4.7) | 79 | |
| Hodgkin | 0 | 0.0 (0.0, 0.7) | 0 | 0.0 (0.0, 2.8) | 0 | 0.0 (0.0, 2.8) | 0 | 0.0 (0.0, 0.4) | 0 | |||
| ALL | 1 | 0.2 (1.0, 3.6) | 49 | 0 | 0.0 (0.0, 2.8) | 0 | 0.0 (0.0, 2.8) | 1 | 0.1 (0.0, 0.7) | 49 | ||
| Myeloid | 8 | 1.4 (0.6, 2.9) | 81 | 1 | 0.8 (0.0, 2.8) | 90 | 1 | 0.8 (0.0, 4.3) | 64 | 10 | 1.2 (0.6, 2.3) | 81 |
| AML | 5 | 0.9 (0.3, 2.1) | 79 | 1 | 0.8 (0.0, 2.8) | 90 | 1 | 0.8 (0.0, 4.3) | 64 | 7 | 0.9 (0.3, 1.8) | 79 |
| CML | 0 | 0.0 (0.0, 0.7) | 0 | 0.0 (0.0, 2.8) | 0 | 0 | 0.0 (0.0, 2.8) | 0 | 0.0 (0.0, 0.4) | |||
| MDS | 3 | 0.5 (0.1, 1.6) | 83 | 0 | 0.0 (0.0, 2.8) | 0 | 0 | 0.0 (0.0, 2.8) | 3 | 0.4 (0.1, 1.1) | 83 | |
| MPD | 0 | 0.0 (0.0, 0.7) | 0 | 0.0 (0.0, 2.8) | 0 | 0 | 0.0 (0.0, 2.8) | 0 | 0.0 (0.0, 0.4) | |||
| Mortality | 291 | 52.8 (46.9, 59.2) | 84 | 55 | 42.6 (32.1, 55.5) | 84 | 63 | 48.5 (37.3, 62.1) | 82 | 411 | 50.7 (45.9, 55.8) | 82 |
| Outcome . | Ig isotype* . | All Ig isotypes combined . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IgG . | IgA . | IgM . | ||||||||||
| Events . | Rate (95% CI) . | Median age (years)† . | Events . | Rate (95% CI) . | Median age (years)† . | Events . | Rate (95% CI) . | Median age (years)† . | Events . | Rate (95% CI) . | Median age (years)† . | |
| Lymphoid | 54‡ | 10.3 (7.7, 13.4) | 78 | 13 | 10.7 (5.7, 18.3) | 71 | 17 | 14.6 (8.5, 23.3) | 79 | 84c | 11.0 (8.8, 13.6) | 76 |
| MM | 42 | 7.9 (5.7, 10.6) | 77 | 11 | 8.8 (4.4, 15.8) | 72 | 0 | 0.0 (0.0, 2.8) | 53 | 6.7 (5.0, 8.8) | 75 | |
| Amyloidosis | 2 | 0.4 (0.0, 1.3) | 71 | 1 | 0.8 (0.0, 4.3) | 71 | 0 | 0.0 (0.0, 2.8) | 3 | 0.4 (0.1, 1.1) | 71 | |
| NHL | 11 | 2.0 (1.0, 3.6) | 78 | 1 | 0.8 (0.0, 4.3) | 60 | 17 | 14.6 (8.5, 23.3) | 79 | 29 | 3.7 (2.5, 5.3) | 78 |
| CLL | 1 | 0.2 (0.0, 1.0) | 49 | 0 | 0.0 (0.0, 2.8) | 3 | 2.3 (0.5, 6.7) | 75 | 1 | 0.1 (0.0, 0.7) | 49 | |
| Waldenström | 0 | 0.0 (0.0, 0.7) | 0 | 0.0 (0.0, 2.8) | 14 | 11.7 (6.4, 19.6) | 79 | 3 | 0.4 (0.1, 1.1) | 75 | ||
| Other | 10 | 1.8 (0.9, 3.4) | 78 | 1 | 0.8 (0.0, 4.3) | 60 | 0 | 0.0 (0.0, 2.8) | 25 | 3.2 (2.0, 4.7) | 79 | |
| Hodgkin | 0 | 0.0 (0.0, 0.7) | 0 | 0.0 (0.0, 2.8) | 0 | 0.0 (0.0, 2.8) | 0 | 0.0 (0.0, 0.4) | 0 | |||
| ALL | 1 | 0.2 (1.0, 3.6) | 49 | 0 | 0.0 (0.0, 2.8) | 0 | 0.0 (0.0, 2.8) | 1 | 0.1 (0.0, 0.7) | 49 | ||
| Myeloid | 8 | 1.4 (0.6, 2.9) | 81 | 1 | 0.8 (0.0, 2.8) | 90 | 1 | 0.8 (0.0, 4.3) | 64 | 10 | 1.2 (0.6, 2.3) | 81 |
| AML | 5 | 0.9 (0.3, 2.1) | 79 | 1 | 0.8 (0.0, 2.8) | 90 | 1 | 0.8 (0.0, 4.3) | 64 | 7 | 0.9 (0.3, 1.8) | 79 |
| CML | 0 | 0.0 (0.0, 0.7) | 0 | 0.0 (0.0, 2.8) | 0 | 0 | 0.0 (0.0, 2.8) | 0 | 0.0 (0.0, 0.4) | |||
| MDS | 3 | 0.5 (0.1, 1.6) | 83 | 0 | 0.0 (0.0, 2.8) | 0 | 0 | 0.0 (0.0, 2.8) | 3 | 0.4 (0.1, 1.1) | 83 | |
| MPD | 0 | 0.0 (0.0, 0.7) | 0 | 0.0 (0.0, 2.8) | 0 | 0 | 0.0 (0.0, 2.8) | 0 | 0.0 (0.0, 0.4) | |||
| Mortality | 291 | 52.8 (46.9, 59.2) | 84 | 55 | 42.6 (32.1, 55.5) | 84 | 63 | 48.5 (37.3, 62.1) | 82 | 411 | 50.7 (45.9, 55.8) | 82 |
MPD, myeloproliferative disorders.
Summaries for the 2 patients with MGUS subtype E have been omitted, as neither experienced a lymphoproliferative event.
Age of cases at disease diagnosis or death.
Total is not the sum of the subtypes because 2 persons had diagnoses on the same date. One person was diagnosed with NHL and amyloidosis on the same date and another person with NHL and ALL on the same date. Both diagnoses are included in the NHL and amyloidosis counts but are counted as 1 event for the composite lymphoid outcome.
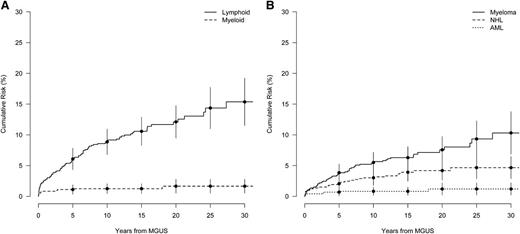
There were 411 (56%) of the MGUS patients who died during follow-up, a rate of 51 per 1000 person-years (Table 2). The median age at death was 82 years. During up to 30 years of follow-up after MGUS diagnosis, the cumulative risk of a lymphoid disorder was 15.4% (Figure 1A), and the cumulative risk for MM was 10.6%, an ∼0.5% annual risk (Figure 1B). The cumulative risk for any lymphoid malignancy was steepest in the first 10 years following diagnosis, showing a 8.9% cumulative risk increase between 0 and 10 years and a 3.3% absolute increase in risk between 20 and 30 years (Figure 1A). A similar but less pronounced pattern was observed for the cumulative risk for MM, with a 5.6% and 2.9% absolute risk increase for the first and last 10 years of observation, respectively (Figure 1B). However, for patients diagnosed with MGUS before age 65, the cumulative risk for progression to any lymphoid disorder was closer to linear (supplemental Figure 1). During up to 30 years of follow-up after MGUS diagnosis, the cumulative risk for any myeloid malignancy was <2% (Figure 1A).
Cumulative risk of a lymphoproliferative event in years from MGUS diagnosis. (A) Cumulative risk for the grouped outcomes of lymphoid (MM, amyloidosis, NHL, Hodgkin lymphoma, ALL) and myeloid (AML, CML, MDS, myeloproliferative disorder) events. (B) Event-specific cumulative risk for MM, NHL, and AML. Bars denote the 95% CI.
Cumulative risk of a lymphoproliferative event in years from MGUS diagnosis. (A) Cumulative risk for the grouped outcomes of lymphoid (MM, amyloidosis, NHL, Hodgkin lymphoma, ALL) and myeloid (AML, CML, MDS, myeloproliferative disorder) events. (B) Event-specific cumulative risk for MM, NHL, and AML. Bars denote the 95% CI.
Table 3 shows the adjusted HRs for selected clinical variables in relation to risk of hematologic malignancies. No evidence of violation of the proportionality assumption for any Cox regression model in Table 3 was found. Immunoparesis, M-protein concentration, and abnormal FLC ratio were significantly associated with progression to any lymphoid event and progression to MM specifically. Although the evidence was weaker, there was some suggestion that high levels of β2-microglobulin were associated with higher risk, whereas having high levels of C-reactive protein (≥25 mg/L) was associated with a lower risk of progression to a lymphoid malignancy. None of the clinical variables we investigated were significantly associated with progression to a myeloid malignancy.
HR associations for progression following MGUS diagnosis
| Factor . | Lymphoid . | MM . | Myeloid . | ||||
|---|---|---|---|---|---|---|---|
| HR . | P value . | HR . | P value . | HR . | P value . | ||
| Albumin | |||||||
| <35 | 1.00 | 1.00 | 1.00 | ||||
| ≥35 | 1.50 | .240 | 1.40 | .430 | 1.30 | .808 | |
| Sedimentation rate | |||||||
| 0.1-40 | 1.00 | 1.00 | 1.00 | ||||
| 41-100 | 1.12 | 1.15 | 0.92 | ||||
| ≥101 | 0.89 | .916 | 0.99 | .927 | <0.01 | .805 | |
| C-reactive protein | |||||||
| 0.1-5 | 1.00 | 1.00 | 1.00 | ||||
| 5-24 | 1.50 | 1.12 | 0.35 | ||||
| ≥25 | 0.60 | .024 | 0.33 | .038 | 1.03 | .526 | |
| β-2 microglobulin, mg/L | |||||||
| <3.5 | 1.00 | 1.00 | 1.00 | ||||
| 3.5-5.5 | 1.57 | 1.27 | 0.40 | ||||
| >5.5 | 2.06 | .082 | 1.77 | .474 | 1.41 | .521 | |
| Immunoparesis* | |||||||
| No | 1.00 | 1.00 | 1.00 | ||||
| Yes | 2.80 | <.001 | 2.77 | <.001 | 1.00 | .996 | |
| Mayo Clinic model | |||||||
| FLC ratio | |||||||
| 0.26-1.65 | 1.00 | 1.00 | 1.00 | ||||
| <0.26 or >1.65 | 2.32 | <.001 | 3.03 | <.001 | 0.60 | .468 | |
| M-protein, g/dL | |||||||
| <1.5 | 1.00 | 1.00 | 1.00 | ||||
| ≥1.5 | 3.68 | <.001 | 3.64 | <.001 | 0.80 | .823 | |
| Isotype | |||||||
| IgG | 1.00 | 1.00 | 1.00 | ||||
| non-IgG | 1.19 | .458 | 0.54 | .055 | 0.49 | .336 | |
| Factor . | Lymphoid . | MM . | Myeloid . | ||||
|---|---|---|---|---|---|---|---|
| HR . | P value . | HR . | P value . | HR . | P value . | ||
| Albumin | |||||||
| <35 | 1.00 | 1.00 | 1.00 | ||||
| ≥35 | 1.50 | .240 | 1.40 | .430 | 1.30 | .808 | |
| Sedimentation rate | |||||||
| 0.1-40 | 1.00 | 1.00 | 1.00 | ||||
| 41-100 | 1.12 | 1.15 | 0.92 | ||||
| ≥101 | 0.89 | .916 | 0.99 | .927 | <0.01 | .805 | |
| C-reactive protein | |||||||
| 0.1-5 | 1.00 | 1.00 | 1.00 | ||||
| 5-24 | 1.50 | 1.12 | 0.35 | ||||
| ≥25 | 0.60 | .024 | 0.33 | .038 | 1.03 | .526 | |
| β-2 microglobulin, mg/L | |||||||
| <3.5 | 1.00 | 1.00 | 1.00 | ||||
| 3.5-5.5 | 1.57 | 1.27 | 0.40 | ||||
| >5.5 | 2.06 | .082 | 1.77 | .474 | 1.41 | .521 | |
| Immunoparesis* | |||||||
| No | 1.00 | 1.00 | 1.00 | ||||
| Yes | 2.80 | <.001 | 2.77 | <.001 | 1.00 | .996 | |
| Mayo Clinic model | |||||||
| FLC ratio | |||||||
| 0.26-1.65 | 1.00 | 1.00 | 1.00 | ||||
| <0.26 or >1.65 | 2.32 | <.001 | 3.03 | <.001 | 0.60 | .468 | |
| M-protein, g/dL | |||||||
| <1.5 | 1.00 | 1.00 | 1.00 | ||||
| ≥1.5 | 3.68 | <.001 | 3.64 | <.001 | 0.80 | .823 | |
| Isotype | |||||||
| IgG | 1.00 | 1.00 | 1.00 | ||||
| non-IgG | 1.19 | .458 | 0.54 | .055 | 0.49 | .336 | |
Proportional hazards models were adjusted for age and year at diagnosis.
Isotype IgG: IgA <0.88 g/L or IgM <0.27 g/L; isotype IgA: IgM <0.27 g/L or IgG <6.7 g/L; isotype IgM: IgA <0.88 g/L or IgG <6.7 g/L.
Of the 172 subjects with immunoparesis in the study cohort, 48 (28%) had depression of both noninvolved isotypes. To address whether there was a dose effect for immunoparesis, we split individuals into those with no depression of a noninvolved isotype, a depression of only 1 noninvolved isotype, and those with depression of both noninvolved isotypes. For progression to any lymphoid disorder, the HR association with depression of 1 noninvolved isotype was HR = 2.79 (95% CI = 1.73, 4.52), and for immunoparesis involving 2 isotypes, HR = 2.82 (95% CI = 1.45, 5.46), indicating no dose effect.
We conducted several additional analyses to evaluate the robustness of our findings. When we included missing data as a separate variable category, we found similar HR associations for all studied risk factors as for the complete-case analysis. We also obtained similar associations when the time scale for the Cox models was age rather than time from MGUS diagnosis.
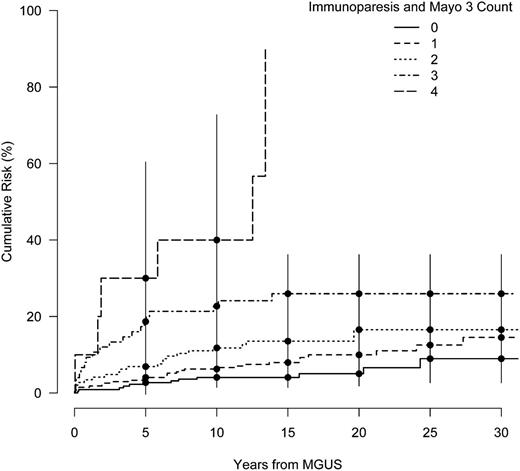
Our findings in Table 3 suggest that immunoparesis and the Mayo Clinic 3 factors may be useful in identifying MGUS patients at higher risk of progressing to a lymphoid malignancy. Figure 2 shows crude cumulative risk curves by the subgroups defined by the total count of immunoparesis and the Mayo Clinic 3 risk factors. This plot suggests an increasing risk with the number of factors, with a striking difference in the risk curves between patients with ≥3 risk factors compared with those with ≤2, although we did not have sufficient follow-up data to estimate risk beyond 10 years for individuals with all 4 risk factors. Within the first 10 years, the addition of 1 risk factor for persons with ≥1 risk factor approximately doubled the cumulative risk of progression (Figure 2).
Cumulative risk of a lymphoid event in years from MGUS diagnosis stratified by the count of total risk factors among the Mayo 3 and immunoparesis factors. Bars denote the 95% CI.
Cumulative risk of a lymphoid event in years from MGUS diagnosis stratified by the count of total risk factors among the Mayo 3 and immunoparesis factors. Bars denote the 95% CI.
To formally assess the comparative performance of risk factors for prediction of progression, we compared the cross-validated AUCs of risk models with (1) the total count among the 3 Mayo Clinic factors, (2) the total count of immunoparesis and the 3 Mayo Clinic factors, and (3) a multivariable model with separate effects for immunoparesis and the 3 Mayo Clinic factors. The multivariable model best predicted the risk of a lymphoid disorder (cross-validated AUC = 0.73, 95% CI = 0.59, 0.85; supplemental Figure 2A) and MM specifically (cross-validated AUC = 0.81, 95% CI = 0.67, 0.94; supplemental Figure 2B). However, this AUC was not statistically significantly higher than the AUC of a model with the count of the 3 Mayo Clinic factors (cross-validated AUC [lymphoid malignancy] = 0.71, 95% CI = 0.58, 0.85; and cross-validated AUC [myeloid malignancy] = 0.74, 95% CI = 0.54, 0.89).
Discussion
In this study of 728 MGUS cases followed up to 30 years, we estimated the risk of lymphoid disorders and myeloid malignancies. We assessed established risk factors and explored novel factors for progression. Based on 84 patients who progressed to a lymphoid disorder, the 30-year cumulative risk was 15.4% overall and 10.6% for MM. The absolute risk of progression to any lymphoid disorder or MM specifically increased most steeply during the first 10 years following MGUS diagnosis. Consistent with earlier reports,21,22 cause-specific risks among lymphoid disorders highly depended on M-protein isotype. No MM cases occurred among patients diagnosed with IgM MGUS, whereas the rate of NHL was much higher in patients with IgM MGUS compared with the IgG or IgA subtypes (14.6 vs 2.0 and 0.8 per 1000 person-years). Also as previously observed,6 progression to any myeloid malignancy was comparatively infrequent, having a 30-year cumulative risk <2%.
We found 3 clinical variables to be significantly associated with progression to any lymphoid malignancy or MM: M-protein concentration ≥1.5 g/dL, abnormal FLC ratio, and presence of immunoparesis. In 2002, in a long-term study of prognosis in MGUS patients at the Mayo Clinic, the size of the M-protein at the time of MGUS diagnosis was the most important predictor of progression, and this observation has been confirmed in several studies.11 The negative impact of an abnormal FLC ratio was first reported in 2005,12 and the present study is the first to confirm the role of an abnormal FLC ratio in a large independent cohort of MGUS patients with long-term follow-up. Regarding immunoparesis, conflicting findings have been reported. In the Mayo Clinic series, the concentration of uninvolved immunoglobulins was reduced in 38% of patients but was not predictive of progression to MM. In the Spanish PETHEMA series, immunoparesis was significantly associated with progression to MM among both MGUS and SMM patients when assessed in univariate analyses; however, in a multivariate analysis, a significant association remained only in SMM.13
In contrast to previous reports,11,12,23 we did not observe a difference in the risk of progression in non-IgG vs IgG MGUS. No association was found between M-protein isotype and risk of progression in the PETHEMA study.13 The reason for these differences is not clear. The interpretation of the association of isotype with progression is complicated by the different types of lymphoid malignancies in IgM vs IgG/IgA MGUS. Also, due to the higher catabolic rate of IgA and IgM compared with IgG,24 at a given M-protein concentration in serum, there is on the average more clonal cells in MGUS of these isotypes than in IgG-MGUS.
A model that considered the separate effects of each Mayo Clinic risk factor (FLC ratio, M-protein concentration, and isotype) and immunoparesis better predicted the risk of progression than a model using only the count of present factors, although the difference was not statistically significant. This finding suggests that a patient’s particular combination of risk factors could improve the prediction of his or her risk of progression. On a clinical note, in our study, patients with all 4 risk factors had a very high rate (40%) of progression within 10 years and constitute a group who should be followed carefully and recommended for interventional studies. A small group of patients with a high rate of progression (46% after 5 years) was also identified in the model proposed by the Spanish PETHEMA group.13
Strengths of our study were the large sample size and 30 years of follow-up. However, the use of retrospective data collection from medical records and case ascertainment from a national cancer registry had several limitations. There were 390 patients (after excluding 15 SMM cases) who did not have adequate serum samples to be included in the risk factor analyses. Although their exclusion reduced our study’s power (which was especially limited for the study of myeloid malignancies), we do not believe it introduced a selection bias because sample availability was not associated with patient demographics, and there was no difference in the risk of progression between excluded and included patients (age-adjusted lymphoproliferative disease HR = 0.91, 95% CI = 0.64, 1.29). We attempted to collect information on several additional variables (haptoglobin, hemoglobin, and white blood cell count), but the information available from medical records was too incomplete to be analyzed. Other risk factors based on flow cytometry13 and genetic factors25 were also not available for analysis. Moreover, because bone marrow examination was not performed in a large proportion of patients with low M-protein concentrations, we could not determine whether some patients in the sample would have fulfilled criteria for SMM. Neither could we exclude light-chain MGUS cases, as serum FLC assays were not available during the study period. Also, the reported incidence of AL amyloidosis is likely underestimated, because it is based on in-patient registry data that only includes cases needing hospitalization.
Since 1978,2 varying rates of progression to MM and other lymphoid malignancies have been reported for MGUS patients, with the majority of published rates being based on small patient series and short-term follow-up.21,26,27 In a large long-term follow-up study of 1148 MGUS patients treated at the Mayo Clinic, the 25-year cumulative risk of MM was 30% (1% annual risk), a substantially higher rate of progression than the 0.5% annual risk observed in our study.12 Although the median age at MGUS diagnosis, MGUS isotype distribution, and M-protein concentration were very similar for both cohorts, the percentage of MGUS patients with an abnormal FLC ratio was higher in our study (47% vs 33%). However, the higher prevalence of abnormal FLC ratio is in contrast with the lower risk of progression we found.
Owing to possible differences in the intensity of monitoring and case completeness between registry- and clinically based cohorts, the differences between progression rates in the Swedish and Mayo Clinic cohorts (0.5% vs 1% annual risk of progression, respectively) may not reflect true population heterogeneity but rather differences in study design. Although the ascertainment rate of the Swedish Cancer Registry is high,16 there could still be underreporting of hematologic malignancies. We therefore performed case verification for lymphoid disorders in the inpatient registry of Malmö University Hospital using a detailed medical record review. Due to limited resources, only records for the first 476 patients in our study could be reviewed. Among these patients, we found 63 cases of a lymphoproliferative disorder during follow-up, and of these, 3 (2 cases of MM and 1 case of WM; 4.7%) were not reported to the Cancer Registry but were subsequently included in our study. Based on this finding, we expect 1.5 cases of a lymphoproliferative disease in the Swedish cohort might have been missed due to underreporting. Thus, underreporting cannot fully account for the differences between the Swedish and Mayo Clinic cohort progression rates. However, Mayo Clinic MGUS patients may have been more intensively monitored than the Swedish cohort, as suggested by the high prevalence of SMM reported in these patients. It is unclear to what extent heightened surveillance could explain the comparatively higher rate of progression reported by the Mayo Clinic investigators.
In conclusion, in an independent, large cohort of MGUS patients with long-term follow-up, we confirmed that an abnormal FLC ratio predicts risk for progression of MGUS. This is an important observation as serum FLC assays are commonly used in the clinical setting while no replication of the Mayo Clinic risk model12 has been available until date. Furthermore, our results confirm the predictive value of a high M-protein concentration. A novel observation in our study is that the addition of immunoparesis to a multivariable model that includes independent effects for the factors of the Mayo Clinic risk model increases the discriminatory power to identify high-risk (vs low-risk) MGUS patients.
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Jean Giles and Ramesh Ramnatsing for excellent technical assistance with the laboratory analyses. The Department of Clinical Chemistry at Skane University Hospital provided stored serum samples.
Authorship
Contribution: I.T., O.L., and M.T.D. designed the study; I.T. and S.Y.K. collected data on outcome; M.T.D. performed all laboratory analyses; S.A.K., R.M.P., and L.R.G. performed all statistical analyses; and I.T., S.A.K., R.M.P., and O.L. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ingemar Turesson, Department of Hematology and Coagulation Disorders, Skane University Hospital, S-20502 Malmö, Sweden; e-mail: Ingemar.turesson@med.lu.se.
References
Author notes
I.T. and S.A.K. contributed equally to this work.