Key Points
Early MRD positivity in NPM-ALK–positive ALCL correlates with a very high relapse risk and inferior survival.
Abstract
Detection of minimal disseminated disease (MDD) at diagnosis correlates with relapse risk in children with anaplastic lymphoma kinase (ALK)-positive anaplastic large-cell lymphoma (ALCL). We investigated whether minimal residual disease (MRD) positivity by qualitative reverse-transcriptase polymerase chain reaction (RT-PCR) for Nucleophosmin (NPM)-ALK during treatment identifies patients at the highest relapse risk. Blood and/or bone marrow of 180 patients with NPM-ALK–positive ALCL treated with Berlin-Frankfurt-Münster-type protocols were screened for NPM-ALK transcripts at diagnosis; 103 were found to be MDD-positive. MRD before the second therapy course could be evaluated in 52 MDD-positive patients. MRD positivity correlated with uncommon histology. The cumulative incidence of relapses (CIR) of 26 MDD-positive/MRD-positive patients (81% ± 8%) was significantly higher than the CIR of 26 MDD-positive/MRD-negative (31% ± 9%) and 77 MDD-negative patients (15% ± 5%) (P < .001). Five-year survival of MDD-negative and MDD-positive/MRD-negative patients was 91% ± 3% and 92% ± 5%, respectively, compared with 65% ± 9% of MDD-positive/MRD-positive patients (P < .001). Early evaluation of MRD in NPM-ALK–positive ALCL identifies patients with a very high relapse risk and inferior survival.
Introduction
The relapse rate of children with anaplastic large-cell lymphoma (ALCL) reaches 25% to 35% with current chemotherapy.1-4 More than 95% of childhood ALCL expresses anaplastic lymphoma kinase (ALK) fusion proteins, 90% of which carry the translocation t(2;5)(p23;q35), resulting in the specific fusion gene NPM-ALK.5,6 NPM-ALK–expressing cells can be detected in bone marrow (BM) or peripheral blood (PB) by RT-PCR in 50% to 60% of patients with NPM-ALK–positive ALCL as a sign of minimal disseminated disease (MDD).7,8 MDD turned out to be a significant prognostic factor7-9 in addition to the histologic subtype and clinical risk factors.10,11
In vivo response to chemotherapy measured by minimal residual disease (MRD) has been established as the strongest prognostic factor for children with acute lymphoblastic leukemia.12-14 In Burkitt leukemia, early detection of MRD could identify patients at the highest risk of relapse as well.15
We therefore investigated whether detection of MRD by qualitative RT-PCR for NPM-ALK in MDD-positive patients in BM or PB early during treatment allows for identification of patients at the highest risk of relapse in ALCL.
Patients and methods
Eligibility
ALCL patients treated according to the protocols Non-Hodgkin Lymphoma-Berlin-Frankfurt-Münster 95, Linfomi Non-Hodgkin 97, or ALCL 99 (Italian and German patients) between August 1998 and December 2008 were potentially eligible. Three patients with completely resected stage I disease were excluded because they received no prephase and only 3 courses of chemotherapy. Eligibility was confirmed by demonstration of NPM-ALK positivity of the tumor by either positive NPM-ALK PCR and/or positive 2-color fluorescence in situ hybridization for t(2;5) and/or nuclear and cytoplasmic staining for ALK. BM or PB had to be available at diagnosis for MDD analyses by PCR from consenting patients/parents. From patients with a positive MDD result, PB or BM before the second course of chemotherapy was requested after informed consent for MRD studies. Ethical approval of the studies was obtained from national and local review boards, and informed consent was obtained in accordance with the Declaration of Helsinki from all patients.
Patients
One-hundred eighty patients fulfilled the inclusion criteria. This cohort is an extension of the previously published series of the Associazione Italiana Ematologia Oncologia Pediatrica (AIEOP) and Berlin-Frankfurt-Münster (BFM) groups.7-9 Patients were stratified according to stage (St. Jude staging system) and the involvement of risk organs.1,16,17 Staging procedures included BM aspiration cytologic and spinal tap. BM involvement was defined by cytologically detectable ALCL cells, irrespective of numbers. The BFM-type protocols consisted of comparable cytoreductive prephase and chemotherapy courses. All patients received six 5-day chemotherapy courses over a period of 4 to 6 months.1,16,17 Twenty-seven patients received vinblastine maintenance therapy in the trial ALCL99 (11 with MRD available). The clinical characteristics of the patients are shown in supplemental Table 1, available on the Blood Web site.
Qualitative PCR for NPM-ALK
Total RNA was isolated from mononuclear or nuclear BM or PB samples by standard methods. cDNA synthesis was performed using 1 µg total RNA, random hexamers, and superscript II reverse transcriptase (Invitrogen, Carlsbad, CA). The qualitative PCR with a sensitivity of 10−5 was performed as previously described.7-9
Statistical analysis
Analysis of event-free survival (EFS) and overall survival (OS) was performed using the Kaplan-Meier method, with differences compared using the log-rank test. Cumulative incidence functions for relapse were constructed following the method of Kalbfleisch and Prentice.18 Functions were compared using the Gray test.19
The prognostic effect of MRD on treatment outcome was compared with other known prognostic factors in patients with ALCL using Cox regression analysis.20 All analyses were performed using SAS, version 9.1(SAS Institute Inc., Cary, NC). Data had been updated by June 2012.
Results and discussion
Five-year OS and EFS for the entire group of patients (n = 180) with MDD measurement were 84% ± 3% and 65% ± 4%, respectively. The cumulative incidence of relapse (CIR) was 32% ± 4%. As in our previous report with a limited patient number,8 MDD in BM and PB was highly concordant (data not shown). Therefore, both media were accepted for MDD and MRD assessment.
MDD
MDD was detected in BM, PB, or both in 103 of 180 patients (57%) with NPM-ALK–positive ALCL, which was comparable with our earlier reports.7-9 MDD positivity significantly correlated with mediastinal or visceral involvement, stage, and histologic subtype (supplemental Table 1), confirming our earlier observations in a large patient cohort.7-9
Forty-six of 103 MDD-positive patients relapsed compared with 10 of 77 patients without detectable MDD (CIR, 46% ± 5% vs 15% ± 5%; P < .001). MDD-positive patients had a 5-year EFS and OS of 51% ± 5% and 79% ± 4%, respectively, compared with 83% ± 5% and 91% ± 3%, respectively, for MDD-negative patients (P < .0001 for EFS and .016 for OS).
MRD
PB and/or BM results before the second course of chemotherapy were available for MRD measurement from 52 of 103 patients with detectable MDD; 26 were MRD-positive and 26 MRD-negative. MRD positivity among MDD-positive patients significantly correlated with uncommon histologic subtype but not with clinical characteristics (Table 1).
Association of MRD assessed before the second course of therapy by qualitative PCR for NPM-ALK in BM or PB with clinical and biological characteristics of the ALCL among MDD-positive patients
| . | . | MDD-positive . | MRD . | |||
|---|---|---|---|---|---|---|
| Negative . | Positive . | n.a. . | P‡ . | |||
| All patients | 103 | 26 | 26 | 51 | ||
| Skin | No | 81 | 21 (81%) | 20 (77%) | 40 | .73 |
| Yes | 22 | 5 (19%) | 6 (23%) | 11 | ||
| Mediastinum | No | 38 | 8 (31%) | 3 (12%) | 27 | .09 |
| Yes | 65 | 18 (69%) | 23 (88%) | 24 | ||
| Visceral organs* | No | 57 | 18 (69%) | 11 (42%) | 28 | .05 |
| Yes | 46 | 8 (31%) | 15 (58%) | 23 | ||
| CNS | No | 93 | 25 (100%) | 25 (96%) | 43 | .32 |
| Yes | 7 | 1 (4%) | 6 | |||
| n.a. | 3 | 1 | 2 | |||
| BM | Neg | 89 | 22 (85%) | 21 (81%) | 46 | .71 |
| Pos | 14 | 4 (15%) | 5 (19%) | 5 | ||
| Stage† | I | — | .31 | |||
| II | 9 | 2 (8%) | 7 | |||
| III | 70 | 19 (73%) | 19 (73%) | 32 | ||
| IV | 23 | 5 (19%) | 7 (27%) | 11 | ||
| Histologic subtype | Common | 39 | 17 (68%) | 6 (26%) | 16 | .004 |
| Not common | 42 | 8 (32%) | 17 (74%) | 17 | ||
| n.a. | 22 | 1 | 3 | 18 | ||
| ALK-AB titer | ≤1/750 | 26 | 7 (32) | 10 (59%) | 9 | .09 |
| >1/750 | 49 | 15 (68%) | 7 (41%) | 27 | ||
| n.a. | 28 | 4 | 9 | 15 | ||
| . | . | MDD-positive . | MRD . | |||
|---|---|---|---|---|---|---|
| Negative . | Positive . | n.a. . | P‡ . | |||
| All patients | 103 | 26 | 26 | 51 | ||
| Skin | No | 81 | 21 (81%) | 20 (77%) | 40 | .73 |
| Yes | 22 | 5 (19%) | 6 (23%) | 11 | ||
| Mediastinum | No | 38 | 8 (31%) | 3 (12%) | 27 | .09 |
| Yes | 65 | 18 (69%) | 23 (88%) | 24 | ||
| Visceral organs* | No | 57 | 18 (69%) | 11 (42%) | 28 | .05 |
| Yes | 46 | 8 (31%) | 15 (58%) | 23 | ||
| CNS | No | 93 | 25 (100%) | 25 (96%) | 43 | .32 |
| Yes | 7 | 1 (4%) | 6 | |||
| n.a. | 3 | 1 | 2 | |||
| BM | Neg | 89 | 22 (85%) | 21 (81%) | 46 | .71 |
| Pos | 14 | 4 (15%) | 5 (19%) | 5 | ||
| Stage† | I | — | .31 | |||
| II | 9 | 2 (8%) | 7 | |||
| III | 70 | 19 (73%) | 19 (73%) | 32 | ||
| IV | 23 | 5 (19%) | 7 (27%) | 11 | ||
| Histologic subtype | Common | 39 | 17 (68%) | 6 (26%) | 16 | .004 |
| Not common | 42 | 8 (32%) | 17 (74%) | 17 | ||
| n.a. | 22 | 1 | 3 | 18 | ||
| ALK-AB titer | ≤1/750 | 26 | 7 (32) | 10 (59%) | 9 | .09 |
| >1/750 | 49 | 15 (68%) | 7 (41%) | 27 | ||
| n.a. | 28 | 4 | 9 | 15 | ||
ALK-AB titer, ALK-antibody titer; CNS, central nervous system; n.a., not available.
Liver, spleen, lung.
St. Jude staging system.
For comparison of MRD-positive with MRD-negative patients.
For control purposes, we measured MRD from 23 MDD-negative patients. None of these patients turned MRD-positive.
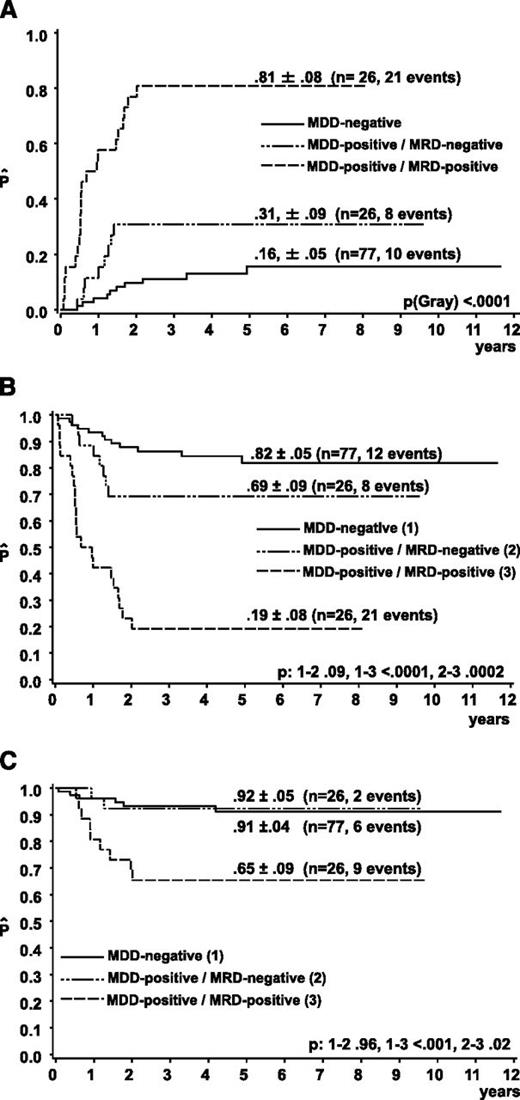
The CIR of the 26 MDD-positive/MRD-positive patients was significantly higher (81% ± 8%) than the CIR of the 26 MDD-positive/MRD-negative (31% ± 9%) and 77 MDD-negative patients (15% ± 5%) (P < .001) (Figure 1A). The CIR of the 51 MDD-positive patients without available material for MRD evaluation was 33% ± 7%. The 5-year EFS of MDD-positive/MRD-positive patients was significantly lower compared with that of the MDD-positive/MRD-negative or MDD-negative patients (Figure 1B). Five-year survival of MDD-negative and MDD-positive/MRD-negative patients was 91% ± 3% and 92% ± 5%, respectively, whereas survival of MRD-positive patients was 65% ± 9% (P < .001) (Figure 1C). In addition to MDD and MRD, clinical risk factors (ie, mediastinal, visceral, or skin involvement), uncommon histologic subtype, and ALK antibody titers ≤1/750 were associated with a significantly higher risk of relapse in univariate analysis. In multivariate analysis including the covariables MDD, MRD, clinical risk factors, histologic subtype, and ALK-antibody titers, the hazard ratio for EFS was 1.83 for MDD (95% confidence interval [CI], 0.55-6.12, P = .3), 6.00 for MRD (95% CI, 2.01-17.92, P = .001), 1.1 (95% CI, 0.28-4.33, P = .90) for clinical risk factors, 3.66 for uncommon histology (95% CI, 1.54-8.68, P = .003), and 3.25 for low ALK-antibody titers (95% CI, 1.38-7.68, P = .007).
Outcome of ALCL patients according to MRD in BM or PB measured by qualitative PCR results for NPM-ALK before the second course of chemotherapy. (A) CIR and Kaplan-Meier estimates of (B) 5-year EFS and (C) OS of the patients, with the initial qualitative PCR result for NPM-ALK in BM and/or blood (MDD).
Outcome of ALCL patients according to MRD in BM or PB measured by qualitative PCR results for NPM-ALK before the second course of chemotherapy. (A) CIR and Kaplan-Meier estimates of (B) 5-year EFS and (C) OS of the patients, with the initial qualitative PCR result for NPM-ALK in BM and/or blood (MDD).
This is the first observation of the prognostic impact of MRD measured early during treatment in ALK-positive ALCL. Clinical, pathological (histologic subtype), and biological characteristics measurable at diagnoses (MDD, ALK-antibody titers) distinguish patients with low and high relapse risk in ALCL.7-10 MRD with a strong impact in multivariate analysis allows for identification of patients with chemoresistant disease. The correlation of MRD positivity with uncommon subtypes is in line with observations that the histologic subtype is associated with other high-risk characteristics and remains an independent prognostic factor, even in studies including MDD and ALK antibody titers.8-10 The observation that MRD measured early—after only 3 weeks of chemotherapy—allowed detection of patients with a very high relapse risk is indicative of a different lymphoma biology, which is also reflected by the histologic subtype. Taking into account that the preexisting immune response against ALK, in part reflected by the titer of ALK antibodies, may play a major role in final control of ALCL,9,21,22 it is possible that the absence of MRD in ALCL may be influenced in part by host factors as well. Given the strong association of MRD positivity with relapse risk, early MRD assessment could be envisioned as a surrogate marker for the end point EFS among MDD-positive patients in future clinical trials. Early treatment response may even serve as an eligibility criterion for early-phase clinical studies.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by a grant from the Deutsche Jose Carreras Leukämie-Stiftung (DJCLS08/09) (W.W., W.K.), the Forschungshilfe Peiper (C.D.W., W.W.), Fondazione Citta’ Della Speranza, Associazione Italiana contro le Leucemie, and Camera di Commercio di Venezia (L.M., M.P., A.R.).
Authorship
Contribution: C.D.W., L.M., A. Rosolen, A. Reiter, and W.W. designed and coordinated the study; C.D.W. and L.M. performed the molecular analyses and collected samples; W.K., I.O., and E.S.G.d’A. performed the histopathological analyses; M.P. and M.Z. analyzed the data; C.D.W., L.M., A. Rosolen, and W.W. wrote the paper; and all authors approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Christine Damm-Welk, Justus-Liebig-University, Department of Pediatric Hematology and Oncology, Feulgenstr. 12, D-35392 Giessen, Germany; e-mail: christine.damm-welk@paediat.med.uni-giessen.de; or Willi Woessmann, Justus-Liebig-University, Department of Pediatric Hematology and Oncology, Feulgenstr. 12, D-35392 Giessen, Germany; e-mail: wilhelm.woessmann@paediat.med.uni-giessen.de.
References
Author notes
W.W. and A.R. are considered cosenior authors.
C.D.-W. and L.M. contributed equally to this study.