Abstract
Endoscopic gastrointestinal workup fails to establish the cause of iron deficiency anemia (IDA) in a substantial proportion of patients. In patients referred for hematologic evaluation with unexplained or refractory IDA, screening for celiac disease, autoimmune gastritis, Helicobacter pylori, and hereditary forms of IDA is recommended. About 4% to 6% of patients with obscure refractory IDA have celiac disease, and autoimmune gastritis is encountered in 20% to 27% of patients. Stratification by age cohorts in autoimmune gastritis implies a disease presenting as IDA many years before the establishment of clinical cobalamin deficiency. Over 50% of patients with unexplained refractory IDA have active H pylori infection and, after excluding all other causes of IDA, 64% to 75% of such patients are permanently cured by H pylori eradication. In young patients with a history suggestive of hereditary iron deficiency with serum ferritin higher than expected for IDA, mutations involving iron trafficking and regulation should be considered. Recognition of the respective roles of H pylori, autoimmune gastritis, celiac disease, and genetic defects in the pathogenesis of iron deficiency should have a strong impact on the current diagnostic workup and management of unexplained, or refractory, IDA.
Introduction
Iron deficiency is one of the most common nutritional problems of the human race. It is associated with serious health risks including abnormal mental and motor development in infancy, impaired work capacity, increased risk of premature delivery, and, in severe anemia, increased maternal and infant mortality.1 The development of iron deficiency is the consequence of an interaction of 3 distinct risk factors: increased host requirements, limited supply, and increased blood loss.
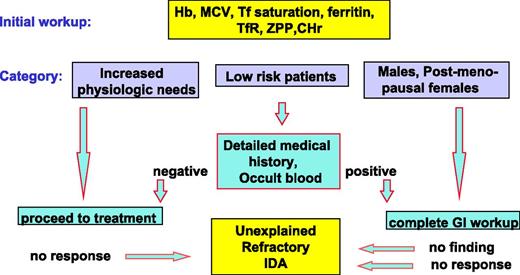
The principles of diagnosis and management in iron deficiency anemia (IDA) are well established and have been defined in a number of detailed guidelines and recommendations.2-5 Because increased requirements are the outcome of increased physiologic needs associated with normal development, this category of iron deficiency is often designated physiological or nutritional. By contrast, pathological iron deficiency is most often the result of gastrointestinal disease associated with abnormal blood loss or malabsorption. The intensity of diagnostic efforts to identify underlying pathology depends on the likelihood of encountering such pathology (Figure 1). In subjects with increased requirements and in low-risk patients such as the majority of fertile women, detailed anatomical studies are rarely necessary. By contrast, in grown men and postmenopausal women, the risk of underlying gastrointestinal disease is high and intensive gastrointestinal work up is mandatory. Once the diagnostic procedure is completed, iron deficiency should be treated, preferably by oral medications.
Diagnostic workup preceding the recognition of unexplained refractory IDA. Diagnostic workup preceding the recognition of subjects with unexplained refractory IDA: in men and postmenopausal women detailed gastrointestinal studies are mandatory. By contrast, in patients with increased physiological needs and in the majority of low-risk patients such as women of child-bearing age, endoscopic gastrointestinal studies preceding oral iron treatment are rarely necessary. The limitations of this diagnostic approach are that (1) complete gastrointestinal workup fails to identify the cause of IDA in a substantial proportion of subjects and (2) such patients, and patients in whom IDA was assumed to be physiological, may be refractory to oral iron therapy. CHr, reticulocyte Hb content; sTfR, soluble transferrin receptor; Tf, transferrin; ZPP, zinc protoporphyrin.
Diagnostic workup preceding the recognition of unexplained refractory IDA. Diagnostic workup preceding the recognition of subjects with unexplained refractory IDA: in men and postmenopausal women detailed gastrointestinal studies are mandatory. By contrast, in patients with increased physiological needs and in the majority of low-risk patients such as women of child-bearing age, endoscopic gastrointestinal studies preceding oral iron treatment are rarely necessary. The limitations of this diagnostic approach are that (1) complete gastrointestinal workup fails to identify the cause of IDA in a substantial proportion of subjects and (2) such patients, and patients in whom IDA was assumed to be physiological, may be refractory to oral iron therapy. CHr, reticulocyte Hb content; sTfR, soluble transferrin receptor; Tf, transferrin; ZPP, zinc protoporphyrin.
However, this general design may fail in several respects: first, conventional endoscopic and radiographic methods are unable to identify the source of gastrointestinal blood loss in a substantial proportion of patients even after using capsule endoscopy.6-9 Second, failure to respond to oral iron occurs in a significant proportion of patients regardless of risk category. The prevalence of refractoriness to iron is difficult to define because it is also affected by patient compliance, and the experience and inclination of the physician to use parenteral iron.
In the present article, refractoriness to oral iron is defined as failure to respond to treatment at a dose of at least 100 mg of elemental iron per day after 4 to 6 weeks of therapy. Subjects in whom IDA is unresponsive to standard oral iron treatment or in whom anemia persists despite a negative gastrointestinal workup represent an important subgroup of patients referred for hematologic evaluation.
Studies in the late 90s have established celiac disease as a possible cause of IDA refractory to oral iron treatment, without other apparent manifestations of malabsorption syndrome.10 In addition, Helicobacter pylori has been implicated in several earlier studies as a cause of IDA refractory to oral iron treatment, with a favorable response to H pylori eradication.11,12 Likewise, autoimmune atrophic gastritis, a condition associated with chronic idiopathic iron deficiency, has been shown to be responsible for refractory IDA in over 20% of patients with no evidence of gastrointestinal blood loss.13,14
The availability of convenient, noninvasive screening methods for identifying celiac disease (anti-tissue transglutaminase [TTG] antibodies), autoimmune atrophic gastritis (serum gastrin, parietal cell, or intrinsic factor antibodies), and H pylori infection (antibody screening or fecal antigen and urease breath test), and the recent recognition of rare inherited forms of iron deficiency greatly facilitated the diagnosis of these entities, resulting in an increased awareness of these conditions and their possible role in the causation of IDA. The aim of the present communication is to focus on the role of the above entities in the pathogenesis of refractory IDA, but it is not intended to represent a detailed general strategy for the diagnosis and management of iron deficiency.
Prevalence of H pylori infection, autoimmune gastritis, and celiac disease in obscure or refractory IDA
Results of a prospective study of 300 consecutive IDA patients referred to a hematology outpatient clinic with obscure or refractory IDA15 are shown in Table 1. Refractoriness to oral iron treatment was defined as a hemoglobin (Hb) increment of <1 g/dL after 4 to 6 weeks of therapy at a daily dose of at least 100 mg of elemental iron. The mean age of all subjects was 39 ± 18 years, and 84% were women of reproductive age. As the population studied was confined to age 14 years and above, the findings described may not apply to the pediatric age group. Adult celiac disease has been identified in 5%. Autoimmune atrophic gastritis was found in 26% and about half of these (51%) had coexistent H pylori infection. H pylori infection was the only finding in 19%, but it was also a common coexisting finding in 55% of the entire group. Not listed in Table 1 are 4 patients with gastroplasty. Use of aspirin or nonsteroidal anti-inflammatory drugs (NSAIDs) is listed as additional information but is not counted separately in the total number of patients. Refractoriness to oral iron treatment was found in 100% of patients with celiac disease, 69% with autoimmune atrophic gastritis, 68% with H pylori infection, but only 10% of subjects with no underlying abnormality. In the following sections, we wish to discuss the implications of the above findings to the pathogenesis and management of unexplained or refractory IDA.
Main diagnostic categories and coexistent findings in 300 consecutive IDA patients
| Diagnosis . | Autoimmune gastritis . | H pylori* . | Menorrhagia . | Gastrointestinal lesions . | Celiac . | Negative . |
|---|---|---|---|---|---|---|
| n (%) | 77 (26) | 57 (19) | 96 (32) | 31 (10) | 14 (5) | 21 (7) |
| Mean age ± 1 SD, y | 41 ± 16 | 37 ± 19 | 39 ± 10 | 60 ± 14 | 39 ± 14 | 33 ± 13 |
| Gender, M/F | 14/63 | 17/40 | 0/96 | 13/18 | 3/15 | 2/21 |
| Main diagnosis alone | 26 | 57 | 39 | 21 | 11 | 21 |
| H pylori | 39 | — | 57 | 10 | 2 | 0 |
| Menorrhagia | 11 | 0 | — | 0 | 1 | 0 |
| Gastrointestinal lesions | 1 | 0 | 0 | — | 0 | 0 |
| Aspirin or NSAID | 9 | 3 | 1 | 7 | 0 | 1 |
| Refractory to oral iron, % | 69 | 68 | 38 | 47 | 100 | 10 |
| Diagnosis . | Autoimmune gastritis . | H pylori* . | Menorrhagia . | Gastrointestinal lesions . | Celiac . | Negative . |
|---|---|---|---|---|---|---|
| n (%) | 77 (26) | 57 (19) | 96 (32) | 31 (10) | 14 (5) | 21 (7) |
| Mean age ± 1 SD, y | 41 ± 16 | 37 ± 19 | 39 ± 10 | 60 ± 14 | 39 ± 14 | 33 ± 13 |
| Gender, M/F | 14/63 | 17/40 | 0/96 | 13/18 | 3/15 | 2/21 |
| Main diagnosis alone | 26 | 57 | 39 | 21 | 11 | 21 |
| H pylori | 39 | — | 57 | 10 | 2 | 0 |
| Menorrhagia | 11 | 0 | — | 0 | 1 | 0 |
| Gastrointestinal lesions | 1 | 0 | 0 | — | 0 | 0 |
| Aspirin or NSAID | 9 | 3 | 1 | 7 | 0 | 1 |
| Refractory to oral iron, % | 69 | 68 | 38 | 47 | 100 | 10 |
Main diagnostic categories and coexistent findings in 300 consecutive IDA patients referred for hematologic evaluation (Hershko et al15 ).
One hundred sixty-five total H pylori.
H pylori gastritis
The role of H pylori in the causation of IDA is of considerable current interest. Major population surveys conducted over diverse geographic areas16-23 indicate that H pylori positivity is associated with an increased prevalence of iron deficiency, and meta- analysis of 19 observational epidemiologic studies and 6 interventional trials revealed an increased risk for IDA with a pooled odds ratio (OR) of 2.8 among H pylori-infected subjects.24
Meta-analysis of 16 randomized trials involving 956 patients with documented H pylori infection and IDA showed that the increments from baseline to end point in Hb, serum iron, and serum ferritin were significantly higher with anti-H pylori treatment plus oral iron compared with oral iron alone.25 The beneficial effect of H pylori eradication was more apparent and the results more stable in patients with moderate to severe anemia, that is, pretreatment Hb levels <9 g/dL. Similar findings were reported in another meta-analysis of randomized controlled trials involving 450 participants.26
Cure of anemia by H pylori eradication is the strongest evidence supporting a cause and effect relationship. In a group of 25 male patients with active H pylori infection with (n = 10) or without (n = 15) coexistent autoimmune gastritis,27 following H pylori eradication all previously refractory patients achieved normal Hb levels with follow-up periods ranging from 4 to 69 months (38 ± 15 months, mean ± 1 SD). This was accompanied by a significant decrease in H pylori immunoglobulin G (IgG) antibodies and in serum gastrin levels. Sixteen patients discontinued iron treatment, maintaining normal Hb and ferritin, and may be considered cured, that is, a cure rate of 64%. Remarkably, 4 of the 16 achieved normal Hb without ever having received oral iron after H pylori eradication.
Similar results were obtained in a recent study of 84 adult patients in whom the effect of H pylori eradication on iron-refractory or iron-dependent anemia of unknown origin was studied.28 Cure rates in men and postmenopausal women (75%) were much higher than in premenopausal women (23%, P < .0001) underlining the compounding effect of increased menstrual blood loss.
In view of the accumulated evidence supporting the significant role of H pylori infection in the pathogenesis of IDA, H pylori eradication is now recommended for the treatment of unexplained IDA in the guidelines for the management of H pylori infection published by a great number of national and international expert committees.2,29-35
With regard to the pathogenesis of H pylori–associated IDA, a number of possible mechanisms have been proposed to explain the relation between H pylori gastritis and IDA including occult gastrointestinal bleeding and competition for dietary iron by the bacteria. In addition, iron absorption in H pylori–infected subjects is impaired because of the effect of H pylori on the composition of gastric juice. Studies in H pylori–positive subjects have shown correction of impaired oral iron absorption after H pylori eradication.36 Likewise, studies by Annibale et al and others37 have shown that gastric acidity and ascorbate content, both of which are critical for normal iron absorption, are adversely effected by H pylori infection and that H pylori eradication results in normalization of intragastric pH and ascorbate content.
The reason why some patients are resistant to long-term H pylori colonization, and why damage to the gastric mucosa is highly variable, is at present unknown. Recent observations indicating that genetic variability of host factors, as well as the composition of iron-repressible outer membrane proteins of H pylori, may determine the clinical expression of disease may represent important new insights into the mechanism and severity of H pylori infection.38,39
Autoimmune atrophic gastritis
Achylia gastrica as a cause of IDA has first been described as a clinical entity by Faber in 190940 ; achlorhydric gastric atrophy, a synonym for the same entity, has long been recognized as a major cause of IDA41 but has been completely ignored in subsequent major surveys of gastrointestinal causes of IDA. More recently, achlorhydric gastric atrophy was rediscovered by Dickey et al,14 and implicated in 20% of IDA patients with no evidence of gastrointestinal blood loss. This observation was confirmed and greatly extended in a series of important studies by Annibale et al13 who found 27% of patients with refractory IDA without gastrointestinal symptoms to have atrophic body gastritis, a percentage identical with the proportion of subjects with autoimmune atrophic body gastritis found subsequently in our own studies.15,42 Iron absorption is severely impaired in achylia gastrica43 as normal gastric secretion and acidity are essential for solubilizing and reducing dietary iron.44,45
Relation between IDA associated with autoimmune gastritis and PA
With an intent to define the relation between IDA associated with autoimmune gastritis and pernicious anemia (PA), we have studied 160 patients with autoimmune gastritis of whom 83 presented with IDA, 48 with normocytic anemia, and 29 with macrocytic anemia46 (Table 2).
Autoimmune gastritis
| . | Macrocytic . | Normocytic . | Microcytic . |
|---|---|---|---|
| n | 29 | 48 | 83 |
| Mean age ± 1 SD, y | 62 ± 15 | 58 ± 17 | 41 ± 15 |
| Gender, M/F | 17/12 | 18/30 | 18/65 |
| Anemic, n (%) | 18 (62) | 19 (40) | 83 (100) |
| Cobalamin deficiency, n (%) | 29 (100) | 44 (92) | 38 (46) |
| Iron deficiency, n (%) | 3 (10) | 24 (50) | 83 (100) |
| Thyroid disease, n (%) | 3 (10) | 14 (29) | 15 (18) |
| Hypothyroid | 3 | 12 | 12 |
| Graves | 0 | 1 | 2 |
| Hashimoto | 0 | 1 | 1 |
| Intrinsic factor antibodies, % | 20 | 40 | 38 |
| Vitiligo | 2 | 0 | 0 |
| Diabetes mellitus, n (%) | 1 (3) | 4 (8) | 7 (8) |
| Neurologic complications, n (%) | 5 (17) | 2 (4) | 0 (0) |
| Gastric histology, n | 13 | 24 | 32 |
| Atrophic gastritis, n (%) | 9 (69) | 13 (54) | 13 (41) |
| Chronic gastritis, n (%) | 2 (15) | 9 (38) | 18 (56) |
| MALT, n (%) | 1 (8) | 1 (4) | 0 (0) |
| GI neoplasia, n (%) | 1 adeno Ca (8) | 1 polyp (4) | 1 polyp (3) |
| . | Macrocytic . | Normocytic . | Microcytic . |
|---|---|---|---|
| n | 29 | 48 | 83 |
| Mean age ± 1 SD, y | 62 ± 15 | 58 ± 17 | 41 ± 15 |
| Gender, M/F | 17/12 | 18/30 | 18/65 |
| Anemic, n (%) | 18 (62) | 19 (40) | 83 (100) |
| Cobalamin deficiency, n (%) | 29 (100) | 44 (92) | 38 (46) |
| Iron deficiency, n (%) | 3 (10) | 24 (50) | 83 (100) |
| Thyroid disease, n (%) | 3 (10) | 14 (29) | 15 (18) |
| Hypothyroid | 3 | 12 | 12 |
| Graves | 0 | 1 | 2 |
| Hashimoto | 0 | 1 | 1 |
| Intrinsic factor antibodies, % | 20 | 40 | 38 |
| Vitiligo | 2 | 0 | 0 |
| Diabetes mellitus, n (%) | 1 (3) | 4 (8) | 7 (8) |
| Neurologic complications, n (%) | 5 (17) | 2 (4) | 0 (0) |
| Gastric histology, n | 13 | 24 | 32 |
| Atrophic gastritis, n (%) | 9 (69) | 13 (54) | 13 (41) |
| Chronic gastritis, n (%) | 2 (15) | 9 (38) | 18 (56) |
| MALT, n (%) | 1 (8) | 1 (4) | 0 (0) |
| GI neoplasia, n (%) | 1 adeno Ca (8) | 1 polyp (4) | 1 polyp (3) |
Autoimmune gastritis: mode of presentation, associated findings, and results of endoscopy in 160 patients (Hershko et al46 ).
GI, gastrointestinal; MALT, mucosa-associated lymphoma.
Patients presenting with IDA were distinguished from those presenting with classical PA by a number of features: they were about 20 years younger, predominantly female, and were more likely to have active H pylori infection as evidenced by positive serology and urease breath test. Histologically, although patients presenting with IDA had a high proportion of atrophic gastritis, active chronic inflammation was nearly 4 times more common in IDA patients than in patients presenting with macrocytosis, likely reflecting their higher rate of active H pylori infection.
On the other hand, patients with autoimmune gastritis presenting with IDA had many features overlapping or identical with those of classical PA. They had an 18% prevalence of thyroid disease and 8% prevalence of diabetes mellitus, diseases well known for their association with PA as part of the autoimmune polyendocrine syndromes.47 Patients with IDA and autoimmune gastritis had a 46% prevalence of abnormal serum cobalamin. Finally, seropositivity for intrinsic factor blocking antibodies, a low sensitivity but high specificity test for PA was 38% in IDA patients, similar to the other 2 subgroups in the present study.
In view of these findings, the question should be raised as to whether we may be witnessing an earlier phase in the evolution of the same disease.48 A definitive answer to this question would require a 20-year follow up of the IDA cohort with autoimmune gastritis. Alternatively, we could stratify the entire population of 160 patients by age (Table 3). Such stratification reveals a remarkable, regular correlation between age and the hematological complications of autoimmune gastritis. With advancing age of presentation, there was a progressive increase in mean corpuscular volume (MCV), and an increase in the severity of hypergastrinemia and the severity of cobalamin deficiency. Iron deficiency and active H pylori infection, both of which were very common in the youngest age group, were gradually replaced by cobalamin deficiency and H pylori seronegativity in the oldest age group.
Effect of age on presenting features of autoimmune gastritis
| Age, y . | n . | Gender, M/F . | Hb,* g/dL . | MCV,* fL . | Serum iron,* μg/dL . | TIBC,* μg/dL . | Ferritin,* μg/L . | Cobalamin,* pg/mL . | Gastrin,* U/mL . | H pylori positive, % . |
|---|---|---|---|---|---|---|---|---|---|---|
| <20 | 8 | 2/6 | 9.6 | 67.6 | 33.8 | 317.0 | 3.5 | 391.9 | 349.3 | 87.5 |
| 1.8 | 9.0 | 18.1 | 20.2 | 2.2 | 178.9 | 247.4 | ||||
| 20-40 | 40 | 7/33 | 10.1 | 78.1 | 38.6 | 337.0 | 9.9 | 189.6 | 467.1 | 47.1 |
| 2.1 | 15.2 | 35.2 | 47.7 | 16.6 | 118.4 | 327.5 | ||||
| 41-60 | 58 | 15/43 | 10.6 | 81.8 | 48.4 | 333.8 | 17.2 | 158.3 | 650.7 | 37.5 |
| 2.4 | 16.9 | 36.3 | 50.9 | 22.3 | 79.2 | 470.2 | ||||
| >61 | 54 | 28/26 | 11.5 | 95.0 | 69.8 | 303.6 | 36.8 | 108.3 | 800.0 | 12.5 |
| 2.5 | 16.0 | 35.1 | 59.0 | 40.5 | 65.4 | 627.3 |
| Age, y . | n . | Gender, M/F . | Hb,* g/dL . | MCV,* fL . | Serum iron,* μg/dL . | TIBC,* μg/dL . | Ferritin,* μg/L . | Cobalamin,* pg/mL . | Gastrin,* U/mL . | H pylori positive, % . |
|---|---|---|---|---|---|---|---|---|---|---|
| <20 | 8 | 2/6 | 9.6 | 67.6 | 33.8 | 317.0 | 3.5 | 391.9 | 349.3 | 87.5 |
| 1.8 | 9.0 | 18.1 | 20.2 | 2.2 | 178.9 | 247.4 | ||||
| 20-40 | 40 | 7/33 | 10.1 | 78.1 | 38.6 | 337.0 | 9.9 | 189.6 | 467.1 | 47.1 |
| 2.1 | 15.2 | 35.2 | 47.7 | 16.6 | 118.4 | 327.5 | ||||
| 41-60 | 58 | 15/43 | 10.6 | 81.8 | 48.4 | 333.8 | 17.2 | 158.3 | 650.7 | 37.5 |
| 2.4 | 16.9 | 36.3 | 50.9 | 22.3 | 79.2 | 470.2 | ||||
| >61 | 54 | 28/26 | 11.5 | 95.0 | 69.8 | 303.6 | 36.8 | 108.3 | 800.0 | 12.5 |
| 2.5 | 16.0 | 35.1 | 59.0 | 40.5 | 65.4 | 627.3 |
Mean ± 1 SD (Hershko et al46 ).
If autoimmune atrophic gastritis impairs both dietary iron and cobalamin absorption, what are the factors determining its clinical presentation in the form of microcytic IDA or macrocytic megaloblastic anemia? Age, gender, duration, and severity of disease may all be important in this respect. Although atrophic gastritis may impair both cobalamin and iron absorption simultaneously, in fertile women, iron deficiency will develop many years before the depletion of cobalamin stores.
Relation between autoimmune gastritis and H pylori infection
The high prevalence of H pylori positivity in young patients with autoimmune gastritis and its almost total absence in elderly patients with PA raises the question of whether H pylori gastritis may represent an early phase of disease in which an infectious process is gradually replaced by an autoimmune disease terminating in burned-out infection and the irreversible destruction of gastric body mucosa. Although this question has long intrigued investigators, the relation between H pylori and the pathogenesis of PA is still unsettled.49,50 H pylori–infected subjects have circulating IgG antibodies directed against epitopes on gastric mucosal cells. Of these, H+K+-ATPase, the most common autoantigen in PA, is a possible target of an autoimmune mechanism triggered by H pylori by means of antigenic mimicry.51-55 Conversely, H pylori eradication in patients with autoimmune atrophic gastritis is followed by improved gastric acid and ascorbate secretion in many, and partial or complete remission of atrophic gastritis in a variable proportion of patients.56-60 In our experience, H pylori eradication is followed by a significant decrease in serum gastrin levels in almost all patients, but complete remission accompanied by disappearance of circulating antiparietal cell antibodies (APCAs) is only observed in a limited number of patients. Failure to achieve complete remission by H pylori eradication in the majority of patients does not necessarily argue against the role of H pylori in the pathogenesis of autoimmune gastritis but more likely indicates that a point of no return may be reached beyond which the autoimmune process may no longer require the continued presence of the inducing pathogen.
Celiac disease
IDA without overt clinical evidence of intestinal malabsorption is one of the most common extraintestinal manifestations of celiac disease.61,62 Conversely, among patients presenting with unexplained IDA, celiac disease is responsible for the anemia in 5% to 6% of cases.63-65 Demonstration of histological changes in the small bowel mucosa is still regarded as the gold standard of diagnosis in celiac disease.66,67 However, the discovery of antiendomysial antibodies in patients with celiac disease had a major impact on the screening and diagnostic algorithms for recognizing celiac disease.68 Its specificity and sensitivity are estimated at 99% and over 90%, respectively. More recently, an enzyme-linked immunoabsorbent assay has been developed to measure anti-TTG IgA antibody activity and is more convenient, is cheaper, and yields comparable sensitivity and specificity to the older antiendomysial indirect fluorescence test.67 Additional diagnostic criteria include identification of HLA-DQ2 or DQ8 genotypes, and response to the gluten-free diet.69,70
The 14 celiac patients in our series were indistinguishable from the rest of the anemic patients by their age, severity of IDA, or serum albumin. Their most consistent clinical feature was their complete refractoriness to oral iron treatment. The most obvious cause of anemia in celiac disease is impaired absorption of iron and other nutrients including folate and cobalamin. Occult gastrointestinal blood loss has been reported in 1 study,71 but does not appear to play a significant role in the causation of anemia. Nutritional deficiency alone does not explain anemia in all cases and inflammation appears to contribute in some patients, as evidenced by the presence of markers of anemia of chronic disease (ACD) in about 17% of celiac patients.72,73
A high proportion of seronegative clinically mild celiac disease has been reported among patients with unexplained IDA fulfilling all other criteria of celiac disease including HLA-DQ2/DQ8 genotype, distal duodenal biopsies, and response to specific therapy.74 Future studies of seronegative celiac disease may shed light on the contribution of genetic as opposed to environmental factors to the phenotypic expression of this disease.
Hereditary microcytic anemias
A discussion of unexplained refractory IDA would be incomplete without mention of the rare hereditary forms of IDA. These novel forms of microcytic anemia have been identified following the remarkable recent progress made in the understanding of molecular mechanisms involved in iron trafficking and regulation and include defects in iron absorption, transport, utilization, and recycling.75 Among these rare microcytic anemias, iron refractory IDA (IRIDA) is considered as the most ”frequent”76 and will be discussed in some detail.
Hereditary iron-refractory iron deficiency syndrome (IRIDA)
IRIDA is an autosomal-recessive disorder caused by mutations on the transmembrane serine protease 6 (TMPRSS6) gene encoding Matriptase-2 (MT-2).77 MT-2 is a transmembrane serine protease essential for downregulating hepcidin, the master regulator of iron homeostasis. In vitro studies in transfected hepatic cell lines suggest that MT-2 cleaves hemojuvelin, an activator of hepcidin expression.78 This function is impaired in mutations involving TMPRSS6 resulting in IRIDA. Hence, serum hepcidin is inappropriately high and patients are unable to respond to iron deprivation by suppressing hepcidin expression.79-81
Patients with hereditary IRIDA have hypochromic microcytic anemia with very low serum iron and transferrin saturation. However, unlike nutritional iron deficiency, their serum ferritin is normal or inappropriately high for the degree of iron deficiency. The severity of anemia is variable, usually moderate; it is not present at birth and develops after the neonatal period. Although the anemia tends to be more severe in childhood than later, there is no correlation between age at diagnosis and the severity of anemia.
Although its prevalence is presently unknown, IRIDA should be considered, especially in children and young adults, when other known causes of refractory IDA are excluded.76 Recognition of affected siblings may suggest an inherited condition, but sporadic cases may occur due to the recessive mode of transmission or when the number of siblings is limited. Hepcidin levels in IRIDA are normal or increased in contrast to nutritional iron deficiency in which hepcidin is low or undetectable.82
ACD may resemble IRIDA in many respects, as proinflammatory cytokines stimulate hepcidin synthesis and are associated with anemia, low serum iron, and increased serum ferritin. Differential diagnosis with IRIDA is based on the mild normocytic or moderately microcytic nature of the anemia in ACD, combined with clinical and laboratory evidence of inflammation.
Diagnosis of IRIDA requires identification of genetic lesions by sequencing the exons and exon-intron boundaries of the TMPRSS6 gene.83,84 Causal mutations are nucleotide changes that interfere with the protein synthesis or amino acid substitutions that are spread over all the extracellular domains of the protein. Although some mutants fully suppress the proteolytic activity, others maintain a residual activity in in vitro studies using the hepcidin promoter.85 Up to the present, over 50 patients from 34 families have been reported with a wide range of ethnic origins representing >40 different TMPRSS6 mutations.76,86,87
TMPRSS6 polymorphisms have been associated with variation in iron parameters88 and with increased risk of iron deficiency.89 Interestingly, TMPRSS6 variants were associated with IDA in a small series of autoimmune gastritis patients with polyendocrine autoimmune syndrome90 suggesting an interplay between genetic and acquired factors in refractory IDA.
As implied by the term IRIDA, patients are unable to respond satisfactorily to oral iron treatment. However, not all IRIDA patient are fully refractory to oral iron treatment. Long-term administration of oral iron has been able to induce partial correction of anemia in patients from 4 unrelated families.87,91-93 However, even with IV iron administration, response to treatment is slow and correction of anemia partial. By contrast, serum ferritin increases with IV iron therapy in a dose-dependent manner. All of these correlations are in keeping with the known hepcidin-ferroportin interactions and the suppressive effect of inappropriately high hepcidin production on iron absorption and recycling. In general, the need for iron treatment is highest in children and modest in adults who have limited iron requirements. In some cases, with many years of follow-up, adults correct anemia although severe microcytosis and low serum iron persist.94
Diagnostic workup and treatment of unexplained refractory IDA
Diagnosis
Because the methods for diagnosing IDA are well established,2-5 the following text is restricted to the specific requirements of patients with refractory anemia after all standard diagnostic requirements have already been fulfilled. As stated earlier, our definition of refractory anemia is failure to respond to oral iron treatment with 100 mg of elemental iron per day for 4 to 6 weeks by an increase in Hb of at least 1 g/dL. Other causes of treatment failure should be excluded first. Admittedly, this is not an easy task as even experienced clinicians may be misled by low compliance, inaccurate history, false diagnosis, and occasional factitious anemia. Care should be taken to exclude ACD using C-reactive protein and other acute-phase reacting proteins, chronic renal failure, use of proton pump inhibitors impairing gastric acidity, and gastrointestinal bleeding induced by drugs or by hereditary bleeding disorders.
Apart from a history of chronic IDA failing to respond to oral iron, the clinical history and physical examination are of limited value in identifying the specific cause of refractoriness to iron. Coexistent cobalamin deficiency may be encountered in some patients with autoimmune gastritis. Refractory IDA starting in early childhood with a positive family history is found in some, but not all, IRIDA patients. The probability of H pylori–related IDA is particularly high in men and postmenopausal women with no anatomic gastrointestinal findings.
It is proposed that all patients studied for unexplained refractory anemia should be tested by serology for celiac disease, H pylori IgG antibodies or fecal antigen, serum gastrin, and antiparietal and/or intrinsic factor antibodies67,95,96 (Table 4).
Proposed diagnostic workup for unexplained or refractory IDA
| . | H pylori . | Autoimmune gastritis . | Celiac disease . | IRIDA . |
|---|---|---|---|---|
| Screening | H pylori IgG antibodies or fecal antigen | Serum gastrin anti-parietal Abs anti-intrinsic factor Abs | TTG IgA Abs | Suggestive history and clinical assessment |
| Advanced | Urease breath test gastroscopy and biopsies (optional) | Gastroscopy and biopsies (recommended) | Duodenal biopsy, HLA screening for DQ2 or DQ8 genotypes | Sequencing of the TMPRSS6 gene |
| Response to specific treatment | H pylori eradication | NA | Gluten-free diet | NA |
| . | H pylori . | Autoimmune gastritis . | Celiac disease . | IRIDA . |
|---|---|---|---|---|
| Screening | H pylori IgG antibodies or fecal antigen | Serum gastrin anti-parietal Abs anti-intrinsic factor Abs | TTG IgA Abs | Suggestive history and clinical assessment |
| Advanced | Urease breath test gastroscopy and biopsies (optional) | Gastroscopy and biopsies (recommended) | Duodenal biopsy, HLA screening for DQ2 or DQ8 genotypes | Sequencing of the TMPRSS6 gene |
| Response to specific treatment | H pylori eradication | NA | Gluten-free diet | NA |
NA, not applicable.
Patients with positive H pylori serology or fecal antigen should be referred for urease breath test to confirm H pylori infection. Endoscopic confirmation of H pylori gastritis is not mandatory. Patients with autoimmune gastritis as indicated by the combined presence of increased serum gastrin and parietal cell or intrinsic factor antibodies should be screened for thyroid disease and for cobalamin depletion. Upper gastrointestinal endoscopy with mucosal biopsy is recommended for all new autoimmune gastritis patients at diagnosis and thereafter at 5- to 10-year intervals. Patients with positive celiac serology should be referred for duodenal biopsy and tested for the HLA-DQ2 and -DQ8 genotypes.
In patients with a history suggestive of hereditary iron deficiency with serum ferritin higher than expected, a genetic workup is recommended.75 For suspected IRIDA, the National Center for Biotechnology Information (NCBI) site of available laboratories for TMPRSS6 sequencing is listed in the Appendix.
Specific treatment
Standard triple therapy for H pylori eradication consists of a proton pump inhibitor, clarithromycin, and amoxycillin for 10 to 14 days or a bismuth-based protocol.29-35 To confirm eradication, the urease breath test should be repeated 1 month after completing triple therapy.
There is no specific treatment of autoimmune gastritis. Serum cobalamin levels should be monitored to determine the need for cobalamin supplementation. In autoimmune gastritis patients with coexistent active H pylori infection, H pylori eradication may be useful for arresting the progress of disease and improving response to oral iron.56-60 Patients with celiac disease should be started on a gluten-free diet with regular follow-up to ensure response and compliance.67
Oral iron therapy
A great number of iron compounds are available for oral use, with significant differences in iron bioavailability. Ferrous iron salts have superior bioavailability compared with ferric iron salts. Because of relatively low cost and reasonable bioavailability, ferrous sulfate is the iron compound used most often for oral iron therapy. A number of preparations with slow iron-release properties have been designed, but for the most part bioavailability does not equal that of standard ferrous sulfate tablets.4
In general, an erythropoietic response to iron therapy should be seen within 4 to 6 weeks of iron replacement therapy. Hb levels are expected to rise by ∼0.1 g/dL per day after starting iron therapy. In addition, reticulocyte Hb content (CHr) is an early, sensitive indicator of a favorable response to iron administration.97-99
The concomitant administration of ascorbic acid enhances iron absorption. In some of our patients, serine ferrous sulfate and ferrous glycine sulfate elicited good responses when other iron preparations failed, but the relative advantage of these preparations still awaits objective evaluation by prospective randomized trials.100
Intravenous iron therapy
Intravenous iron replacement is indicated for patients failing to respond to oral iron treatment. Several IV iron preparations are now available.101 They consist of a core of iron oxohydroxide with a protective carbohydrate shell of sugar polymers.102 Following injection, iron is slowly released and is picked up by plasma transferrin. The rate of dissociation of iron from the complex is variable. Iron gluconate is the most labile with the most rapid release of iron and, consequently, doses of 62.5 to 125 mg are the highest that can be tolerated at a single infusion. Iron sucrose is more stable and higher doses, usually 200 mg of iron, may be given safely. The most stable iron preparations are the iron dextrans tightly bound to their iron oxohydroxide cores. There are high-molecular-weight and low-molecular-weight iron dextrans.
According to recent pharmacovigilance reports from North America and Europe, the OR of reported total absolute rates of adverse life-threatening events with parenteral iron is 38 per million doses: the risk of anaphylaxis with iron dextran compared with iron sucrose is 17.7- to 16.9-fold.103 In view of the increased risk of life-threatening adverse effects and the availability of much safer IV iron compounds, the use of high-molecular iron dextran is no longer justified.
Several additional iron preparations for IV use became available recently including ferric carboxymaltose, iron isomaltoside 1000, and ferumoxitol. These preparations allow the administration of much higher doses of iron IV in a shorter time.104 They can be used safely and effectively to restore body iron, possibly even in a single treatment episode. Presently, there is a need for well-designed comparative studies to allow judgment of the relative advantage in safety and efficacy of all newly available compounds. In addition, there is still uncertainty regarding the long-term safety of IV iron regarding its potential to increase susceptibility to infections and promote oxidative stress.3
Parenteral iron preparations can be used for the correction of iron deficiency or the maintenance of iron stores. Approximately 170 mg of iron is required to obtain a 10 g/L rise in Hb in a 70-kg man, based on a total blood volume of 4.9 L. The iron status of patients taking iron therapy, whether oral or IV, should be monitored intermittently to establish whether iron stores are adequately replenished and to avoid iron overload that may occur with unmonitored long-term iron replacement.
Conclusions
In high-risk IDA patients, complete endoscopic workup is mandatory. By contrast, low-risk patients, representing the vast majority of subjects with refractory IDA, remain the responsibility of the hematologist. The hematologist, in turn, is now equipped with powerful noninvasive tools to identify IDA patients requiring special attention.
Recognition of the respective roles of H pylori, autoimmune gastritis, celiac disease, and inherited disease in the pathogenesis of iron deficiency should have a strong impact on the current diagnostic workup and management of refractory IDA and a sharp decrease in the proportion of IDA patients still labeled “unexplained.”
Authorship
Contribution: C.H. and C.C. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Chaim Hershko, Division of Quality Assurance, Israel Ministry of Health, Habirah Towers, Rm 317 Jeremia St 39, PO Box 1176, Jerusalem, Israel; e-mail: hershkoc@netvision.net.il.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal