In this issue of Blood, Rusu et al use a state-of-the-art combination of pharmacological, genetic, and molecular techniques to dissect a mechanism for von Willebrand factor (vWF) secretion from endothelial cells mediated via Gαq/11 and Gα12 that can be triggered in basal or stimulated conditions.1 Indeed, although the storage of vWF in Weibel-Palade bodies (WPBs) of endothelial cells has been known for decades,2 the molecular mechanisms governing WPB docking with plasma membrane and vWF secretion remains undefined. One of the reasons this is still a very active area of investigation is that understanding the mechanism behind WPB docking is crucial for understanding potential pharmacological targets for pathological conditions associated with platelet aggregation/thrombi (eg, stroke), where vWF levels directly correlate with severity of disease progress. The work by Rusu et al opens up a new area of investigation into G-protein-coupled receptors as triggers for WPB fusion at the plasma membrane and for vWF secretion.
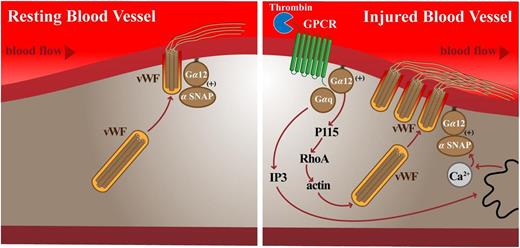
Schematic of vWF secretion from endothelial cells under basal and stimulated conditions. Note the role of Gα12 in both basal and stimulated vWF secretion acting directly through αSNAP; however, Gαq11 only appears to be active on PAR-1 activation. See Figure 7 in the article by Rusu et al that begins on page 442.
Schematic of vWF secretion from endothelial cells under basal and stimulated conditions. Note the role of Gα12 in both basal and stimulated vWF secretion acting directly through αSNAP; however, Gαq11 only appears to be active on PAR-1 activation. See Figure 7 in the article by Rusu et al that begins on page 442.
The docking of WPB in endothelial cells has been shown to be mediated in much the same manner that occurs with the docking and exocytosis of neurotransmitters, which includes the formation of soluble N-ethylmaleimide-sensitive factor (NSF) attachment protein receptor (SNARE) complexes on vesicles as they approach the plasma membrane. In conjunction with NSF, SNAREs bind WPB as they approach the endothelial cell plasma membrane. However, it is the effector α-soluble NSF attachment protein (αSNAP) that permits the fusion of WPB to the membrane to occur.3 In the end, each hexameric NSF requires 3 αSNAPs to mediate binding to the SNAREs.4 There is evidence of G-protein-coupled receptor involvement in αSNAP/SNARE complex formation (eg, Zhao et al5 ), but not in the vasculature and not in knockout mice. Overall, this intricate molecular process allows for specific targeting, and the dynamics permit the docking to occur rapidly as the cellular environment changes.
What Rusu et al demonstrate is that αSNAP is regulated by Gα12 in both unstimulated and stimulated (with thrombin, activating protease-activated receptor [PAR]) conditions (Figure 1). This was initially demonstrated using Gα12−/− mice and double-knockout mice in which Gα11 was deleted throughout the animal and Gαq was deleted only in the endothelium (EC-Gαq−/−;Gα11−/−). During baseline vWF measurements, there was significantly decreased vWF in Gα12−/− mice, which leads to the initial conclusion that basal vWF could be regulated by Gα12; the EC-Gαq−/−;Gα11−/− mice were the same as control. However, in both knockout mice, there was a significant decrease in the amount of vWF from lung perfusate after activation of PAR-1 receptors by the mimetic-peptide TFLLR with little to no response. This was a clear demarcation between the 2 G proteins, with Gα12 a component to basal vWF secretion and Gαq not involved with vWF secretion until after PAR-1 activation. The loss of vWF secretion in these mice was further demonstrated by increased blood loss after tail snip and reduced platelet thrombus formation. Taken together, the authors provide evidence at the whole-animal level for a role between GPCRs and vWF secretion.
To dissect the molecular mechanisms possibly governing these observations, the authors turned to cultured endothelial cells and a heterologous expression system. Using a genetic library of Gα12 mutants in which segments of the Gα12 sequence were replaced with Asn-Ala-Ala-Ile-Arg-Ser cassettes, they examined the ability of purified αSNAP to bind the Gα12 protein directly. They identified a unique sequence in Gα12 (amino acids 10-15) and produced a minigene construct against it, with the thought of inhibiting the Gα12-αSNAP interaction. When the construct was introduced into endothelial cells, it was able to significantly reduce the amount of vWF secreted after PAR-1 activation. When summed, the Gα12 activation in basal and stimulated conditions can regulate vWF secretion by direct interactions with αSNAP, as well as the classical Gα12 pathway after stimulated conditions; Gαq moving through its classical transduction pathway only participates in vWF secretion after stimulation (see figure).
In a broader context related to endothelial cell biology, another key component of this article relates to the authors’ discovery that the vWF secretion is tightly regulated by a series of proteins both in close proximity and with redundant functions. This observation is consistent with the current state of the field in endothelial cell biology, where an accumulation of evidence appears to indicate discreet signaling microdomains that can regulate distinct physiological functions ranging from integrin function6 to nitric oxide production7 and calcium events.8 Considering the balancing act that an endothelial cell must perform (respond to inflammatory stimuli/blood factors and regulate constriction and/or dilation), it is no wonder this cell is so clever with protein organization and use.
There is still much to do concerning mechanisms and triggers involved in WPB docking and vWF secretion. For example, where on the plasma membrane does the docking occur? It is likely that lipid raft accumulation clusters the components in particular areas of endothelial cells. In disease states with turbulent flow (eg, atherosclerosis), could that alter the dynamics of where the proteins are localized and/or function? Is there any secondary role for Gα13 in vWF secretion? Also, how does the endothelial cell confine the response to just WPB docking? Do localized calcium events prevent other physiological functions from occurring after activation of Gα? Pharmacologically, how can the Gα-initiated vWF secretion be targeted? This is an especially important question, considering that targeting α-SNAP could have several nonspecific effects (eg, neuronal function). The work described by Rusu et al provides the basis for starting to elucidate some of these exciting questions.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal