Key Points
CD33 expression levels in AML correlate with specific disease characteristics.
Potent cytotoxicity against primary AML blasts is mediated by a CD33/CD3-bispecific antibody.
Abstract
Antibody-based immunotherapy represents a promising strategy to target and eliminate chemoresistant leukemic cells. Here, we evaluated the CD33/CD3-bispecific T cell engaging (BiTE) antibody (AMG 330) for its suitability as a therapeutic agent in acute myeloid leukemia (AML). We first assessed CD33 expression levels by flow cytometry and found expression in >99% of patient samples (n = 621). CD33 was highest expressed in AMLs with NPM1 mutations (P < .001) and lower in AMLs with complex karyotypes and t(8;21) translocations (P < .001). Furthermore, leukemic stem cells within the CD34+/CD38− compartment displayed CD33 at higher levels than healthy donor stem cells (P = .047). In MS-5 feeder cell-based long-term cultures that supported the growth of primary AML blasts for up to 36 days, AMG 330 efficiently recruited and expanded residual CD3+/CD45RA−/CCR7+ memory T cells within the patient sample. Even at low effector to target ratios, the recruited T cells lysed autologous blasts completely in the majority of samples and substantially in the remaining samples in a time-dependent manner. This study provides the first correlation of CD33 expression levels with AML genotype in a comprehensive analysis of adult patients. Targeting CD33 ex vivo using AMG 330 in primary AML samples led to T cell recruitment and expansion and remarkable antibody-mediated cytotoxicity, suggesting efficient therapeutic potential in vivo.
Introduction
Overall survival of patients with acute myeloid leukemia (AML) is poor and has not significantly improved over the past decade. Successful treatment, particularly of AML with adverse chromosomal or molecular aberrations, strongly depends on intensive combination chemotherapy followed by allogeneic stem cell transplantation. The latter has proven to provide a potent antileukemic effect that can lead to elimination of chemoresistant leukemic cells, albeit at the expense of high morbidity and mortality associated with graft-versus-host disease. For patients with a nonfavorable prognosis who are not suitable for this treatment, novel therapeutic options, including immunotherapeutic approaches, are urgently sought after.1 A promising immunotherapeutic strategy devoid of graft-versus-host disease reactions is to recruit in vivo the patient’s own T cells and retarget them directly at leukemic cells. This approach became feasible by a novel class of bispecific T cell engaging antibodies (BiTEs), which direct cytotoxic T lymphocytes at predefined surface antigens on tumor cells.2 Clinical proof of concept was provided by blinatumomab, a BiTE antibody directed at both the CD19 B-cell surface antigen and the CD3ε component of the T-cell receptor complex. Its therapeutic efficacy was shown for patients with B-cell lymphomas and B-precursor acute lymphoblastic leukemia.3-6
The antigen that is most commonly targeted in AML is CD33, a sialic acid-dependent cytoadhesion molecule. Although the intact humanized CD33 antibody lintuzumab induced complete remission in individual patients in a single-agent phase I and II trial, no survival benefit was found in a phase III trial when it was combined with a triple chemotherapy regimen.7 This might have been caused by rapid CD33 internalization induced by the bivalent antibody.8 An antibody-toxin conjugate (gemtuzumab ozogamicin [GO], Mylotarg; Pfizer Inc., New York, NY), which depends on endocytic uptake, showed therapeutic efficacy against AML cells.9 However, in 2010, GO was voluntarily withdrawn from the market due to failure to verify clinical benefit and concerns about increased side effects.10 Besides, a common problem of toxin-conjugated antibodies may be their limited effect on resting, low proliferative cells, potentially sparing quiescent leukemic stem cells (LSCs).11
In contrast, novel bispecific antibody constructs are able to effectively recruit antigen-experienced T cells irrespective of their antigen specificity without the requirement of pre- or co-stimulatory signals.12-14 AMG 330 is a human BiTE directed at CD33 and CD3 developed for treatment of AML. A prerequisite for successful immunotherapeutic approaches using this molecule is the expression of CD33 on AML blasts, including LSCs, as the supposed source of relapse. We here quantified CD33 expression by flow cytometry and correlated expression intensity with cytogenetic and molecular disease characteristics in order to identify patient cohorts suited for CD33-targeted therapy. A major hurdle for measuring cytotoxic effects on AML blasts has long been that primary AML patient samples show progressive cell death within a few days ex vivo as also has been demonstrated in a previous study using AMG 330 in an ex vivo short-term culture system.15 To simulate as closely as possible the natural setting of target and T cells in AML patients and the prolonged drug exposure typically used for BiTE treatment cycles,5 we here developed a long-term culture system for AML blasts that allowed us to observe co-cultures for up to 5 weeks. Thereby, we were able to show effective elimination of AML blasts within primary samples by AMG 330-activated residual T lymphocytes at very low effector to target (E:T) ratios. Taken together, the results obtained in this study strongly encourage further preclinical and clinical development of the CD33/CD3 BiTE antibody AMG 330.
Methods
Patients
After written informed consent in accordance with the Declaration of Helsinki and approval by the Institutional Review Board of the Ludwig-Maximilians-University (Munich, Germany), peripheral blood (PB) or bone marrow (BM) samples were collected from healthy donors (HDs) and patients with AML at primary diagnosis or relapse. Additional PB samples were collected from a single AML patient in complete remission. Patient characteristics are summarized in Table 1. Further information is provided in supplemental Methods (available on the Blood Web site). CD33 expression levels on primary AML samples were analyzed by flow cytometry in the Laboratory of Leukemia Diagnostics of the Department of Internal Medicine III between 2000 and 2012.
Patient characteristics
| PT . | Gender . | Age, y . | Disease phase . | Material . | Blasts, % . | CD33MFI . | CD33 Tercile . | CD3, % . | FAB . | NPM1mut . | FLT3-ITD . | Karyotype . | ELN genetic group . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 60 | FD | BM | 95 | 5.3 | 1 | 1.9 | M4 | + | − | 46,XX | Favorable |
| 2 | F | 45 | FD | BM | 84 | 8.6 | 1 | 2.7 | M1 | + | + | 46,XX | Intermediate-I |
| 3 | M | 40 | FD | PB | 60 | 8.2 | 1 | 7.3 | M2 | − | − | 46,X,-Y,+8,t(8;21)(q22;q22) | Favorable |
| 4 | F | 43 | FD | PB | 96 | 22.9 | 1 | 1.2 | M3 | − | + | 46,XX,t(15;17)(q22;q21) | Favorable |
| 5 | M | 61 | FD | BM | 68 | 44.3 | 2 | 11.6 | M1 | − | − | inv(16)(p13),inv(16)(q22), del(7)(q22), +22 | Favorable |
| 6 | F | 54 | FD | PB | 61 | 58.4 | 2 | 12.7 | M1 | − | + | 46,XX | Intermediate-I |
| 7 | F | 69 | FD | PB | 88 | 31.8 | 2 | 5.6 | M1 | + | + | 46,XX,del(1) | Intermediate-II |
| 8 | F | 77 | Relapse | BM | 82 | 117.1 | 3 | 7.9 | M2 | + | − | 47,XX,+8 [4] / 46,XX [4] | Intermediate-II |
| 9 | F | 59 | FD | BM | 34 | 172.6 | 3 | 9.9 | M1 | − | − | 46,XX | Intermediate-I |
| 10 | F | 40 | FD | PB | 84 | 83.0 | 2 | 10.2 | M4 | + | + | 46,XX | Intermediate-I |
| 11 | M | 24 | FD | PB | 76 | 47.6 | 2 | 9.6 | M2 | − | − | 47,XY,+4[17]/46,XY[17] | Intermediate-II |
| 12 | M | 81 | FD | PB | 47 | 77.5 | 2 | 1.6 | M5 | + | − | 46,XY,der(10)t(3;10)(?;p1?) [3] / 47,sl,+8[4] | Adverse |
| 13 | F | 63 | Relapse | PB | 64 | 151.9 | 3 | 3.7 | M4 | + | + | 46,XX | Intermediate-I |
| PT . | Gender . | Age, y . | Disease phase . | Material . | Blasts, % . | CD33MFI . | CD33 Tercile . | CD3, % . | FAB . | NPM1mut . | FLT3-ITD . | Karyotype . | ELN genetic group . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 60 | FD | BM | 95 | 5.3 | 1 | 1.9 | M4 | + | − | 46,XX | Favorable |
| 2 | F | 45 | FD | BM | 84 | 8.6 | 1 | 2.7 | M1 | + | + | 46,XX | Intermediate-I |
| 3 | M | 40 | FD | PB | 60 | 8.2 | 1 | 7.3 | M2 | − | − | 46,X,-Y,+8,t(8;21)(q22;q22) | Favorable |
| 4 | F | 43 | FD | PB | 96 | 22.9 | 1 | 1.2 | M3 | − | + | 46,XX,t(15;17)(q22;q21) | Favorable |
| 5 | M | 61 | FD | BM | 68 | 44.3 | 2 | 11.6 | M1 | − | − | inv(16)(p13),inv(16)(q22), del(7)(q22), +22 | Favorable |
| 6 | F | 54 | FD | PB | 61 | 58.4 | 2 | 12.7 | M1 | − | + | 46,XX | Intermediate-I |
| 7 | F | 69 | FD | PB | 88 | 31.8 | 2 | 5.6 | M1 | + | + | 46,XX,del(1) | Intermediate-II |
| 8 | F | 77 | Relapse | BM | 82 | 117.1 | 3 | 7.9 | M2 | + | − | 47,XX,+8 [4] / 46,XX [4] | Intermediate-II |
| 9 | F | 59 | FD | BM | 34 | 172.6 | 3 | 9.9 | M1 | − | − | 46,XX | Intermediate-I |
| 10 | F | 40 | FD | PB | 84 | 83.0 | 2 | 10.2 | M4 | + | + | 46,XX | Intermediate-I |
| 11 | M | 24 | FD | PB | 76 | 47.6 | 2 | 9.6 | M2 | − | − | 47,XY,+4[17]/46,XY[17] | Intermediate-II |
| 12 | M | 81 | FD | PB | 47 | 77.5 | 2 | 1.6 | M5 | + | − | 46,XY,der(10)t(3;10)(?;p1?) [3] / 47,sl,+8[4] | Adverse |
| 13 | F | 63 | Relapse | PB | 64 | 151.9 | 3 | 3.7 | M4 | + | + | 46,XX | Intermediate-I |
FD, first diagnosis; PB, peripheral blood; BM, bone marrow.
Flow cytometry
Cytotoxicity analysis was performed by flow cytometry (FACSCalibur, Becton Dickinson, Heidelberg, Germany). A list of all fluorochrome-labeled antibodies is given in supplemental Methods.
CD33 expression analysis
The expression of CD33 was analyzed by flow cytometry (FACSCalibur) using a fluorochrome-labeled CD33 antibody (D3HL60.251). CD33 expression on CD34+/CD38− cells from BM of AML patients and HDs was analyzed (Navios, Beckman Coulter, Krefeld, Germany) using the following antibodies: CD45 (J33), CD34 (581), CD38 (LS198.4.3), and CD33 (D3HL60.251). Corresponding isotype controls were used. All antibodies were purchased from Beckman Coulter. A CD45DIM/SSCLOW gate was used to limit the analysis to myeloid progenitor cells.
Mean fluorescence intensity (MFI) values were determined using FlowJo (Version 9.6) (Tree Star Inc., Ashland, Oregon), and the MFI ratio (MFI sample/MFI isotype) was calculated as a measure of expression intensity. Samples with CD33 MFI ratios ≥1.5 were regarded as positive.
BiTE antibodies
AMG 330 is a bispecific tandem single-chain antibody construct. The N-terminal single-chain antibody is specific for human and cynomolgus CD33, whereas the C-terminal single-chain antibody is specific for the human and cynomolgus CD3ε chain of the T-cell receptor complex. AMG 330 was constructed by recombinant DNA technology and expressed in a CHO cell line. Purification of the monomeric protein was done in a 2-step process using Ni-chelate chromatography followed by gel filtration. For the control BiTE antibody, the N-terminal single-chain antibody was exchanged for a single-chain antibody specific for an herbicide. Generation and production of these constructs were performed as described for other BiTE antibodies.16,17
Ex vivo co-cultures using primary AML patient samples
All ex vivo cytotoxicity assays using AML blasts were performed in 12-well flat-bottom culture plates in α-MEM supplemented with 12.5% fetal calf serum (FCS), 12.5% horse serum, and 1% penicillin/streptomycin/glutamine. The medium was supplemented with recombinant human granulocyte-colony stimulating factor (rhG-CSF), rhu interleukin (IL)-3 and rhu thrombopoietin (TPO) (Peprotech, Hamburg, Germany) and 57.4 µM 2-mercaptoethanol (Sigma-Aldrich, Munich, Germany) as previously described.18 To improve the viability of AML blasts in a long-term culture system, irradiated MS-5 cells were used.19-21
For conventional suspension culture systems, AML blasts were cultured in RPMI1640 supplemented with 10% fetal calf serum, 1% penicillin/streptomycin/glutamine, 0.005 mM 2-mercaptoethanol, and the following cytokines: stem-cell-factor (SCF), IL-3, transferrin, granulocyte macrophage-colony stimulating factor (GM-CSF), granulocyte (G)-CSF, and erythropoietin. All cytokines were purchased from Immunotools (Friesoythe, Germany). All cultures were analyzed by cell counting and flow cytometry at regular intervals.
Ex vivo cytotoxic effects on primary AML blasts
Lysis of AML blasts mediated by AMG 330 was tested as follows: AML samples from primary diagnosis or relapse were used within the MS-5 based ex vivo co-culture system without the addition of any other cells. Hence, the E:T ratio was determined by the number of residual T cells within the primary AML sample. Either AMG 330 or control antibody was added at 5 ng/mL at the beginning of the experiment. In a 4-day interval, antibody was replenished according to the volume needed for medium exchange. All cultures were analyzed by cell counting and flow cytometry at regular intervals. The percentage of the respective cell population determined by flow cytometry was used to determine the final cell count. The cell count of control BiTE antibody-treated relative to AMG 330-treated cultures at the end of the experiment was used to determine the percentage of lysis.
Repetitive cytotoxic effects of AMG 330-stimulated T cells on primary AML blasts
A primary AML patient sample was co-incubated with autologous PB mononuclear cells (PBMCs) from complete remission (E:T ratio 1:3) and 5 ng/mL of either AMG 330 or control antibody for 11 days. Primary AML blasts were added to the existing co-culture twice a week. AMG 330 or control antibody was added to the culture according to the exchanged medium.
Statistical analysis
The significance of differences between terciles was determined by Pearson's χ-squared test, except for differences in age, which were determined by Kruskal-Wallis one-way analysis of variance by ranks. Differences in colony-forming units were assessed by the Mann-Whitney U test. All calculations were performed in IBM SPSS Statistics for Windows (Version 21.0, Armonk, New York, NY), and statistical significance was considered for P < .05.
Additional methods are provided in supplemental Methods.
Results
CD33 expression correlates with disease characteristics of AML
The intensity of CD33 expression was evaluated in 621 samples of newly diagnosed AML patients. In >99% of samples, positivity for CD33 could be demonstrated with substantial inter-patient heterogeneity in expression levels. For further analysis, we therefore divided the patient cohort into terciles based on CD33 MFI ratios (Figure 1A; Table 2). We then correlated CD33 expression with different disease characteristics. High CD33 expression levels (T3) correlated with French American British (FAB) groups M4 and M5 (P < .001 and P = .001, respectively). In contrast, FAB M0, M2, and M6/M7 were associated with lower CD33 expression (T1) (P = .02, < .001, and .009, respectively). FAB M1 showed no significant prevalence among the terciles.
Association of CD33 expression with cytogenetic and molecular markers. (A) CD33 expression of 621 AML patient samples, subgrouped into 3 terciles. (B) Two representative examples of CD33 expression on primary AML blasts (tercile 1 [T1], left; tercile 3 [T3], right). (C-D) CD33 expression intensity correlated to specific cytogenetic/molecular markers. *Including abn(3q), inv(3)(q21q26)/t(3;3)(q21;q26), add(5q), −5/5q-, −7, add(7q)/del(7q), t(v;11)(v;q23), t(9;22)(q34;q11), −17/abn(17p), but excluding t(3;5)(q21∼25;q31∼35), t(9;11)(p21-22;q23) and t(11;19)(q23;p13). †Including t(9;11)(p21-22;q23), t(11;19)(q23;p13) and all other abnormalities not otherwise classified.
Association of CD33 expression with cytogenetic and molecular markers. (A) CD33 expression of 621 AML patient samples, subgrouped into 3 terciles. (B) Two representative examples of CD33 expression on primary AML blasts (tercile 1 [T1], left; tercile 3 [T3], right). (C-D) CD33 expression intensity correlated to specific cytogenetic/molecular markers. *Including abn(3q), inv(3)(q21q26)/t(3;3)(q21;q26), add(5q), −5/5q-, −7, add(7q)/del(7q), t(v;11)(v;q23), t(9;22)(q34;q11), −17/abn(17p), but excluding t(3;5)(q21∼25;q31∼35), t(9;11)(p21-22;q23) and t(11;19)(q23;p13). †Including t(9;11)(p21-22;q23), t(11;19)(q23;p13) and all other abnormalities not otherwise classified.
Correlation of disease characteristics with CD33 expression levels
| Category . | All cases . | CD33 MFI ratio . | |||
|---|---|---|---|---|---|
| n = 621 . | Lower third . | Middle third . | Upper third . | P . | |
| CD 33 MFI ratio | |||||
| Median (range) | 57 (1-456) | 11 (1-30) | 57 (30-94) | 157 (94-456) | |
| Age, y | .01 | ||||
| Median (range) | 57 (17-99) | 57 (19-99) | 59 (17-82) | 53 (18-89) | |
| Gender, n (%) | .13 | ||||
| Male | 337 (54%) | 124 (60%) | 108 (52%) | 105 (51%) | |
| Female | 284 (46%) | 83 (40%) | 99 (48%) | 102 (49%) | |
| FAB, n (%) | <.001 | ||||
| M0 | 29 (5%) | 15 (8%) | 11 (6%) | 3 (2%) | |
| M1 | 114 (21%) | 41 (22%) | 40 (22%) | 33 (18%) | |
| M2 | 174 (32%) | 80 (44%) | 53 (30%) | 41 (22%) | |
| M4 | 153 (28%) | 26 (14%) | 53 (30%) | 74 (40%) | |
| M5 | 61 (11%) | 10 (6%) | 19 (11%) | 32 (17%) | |
| M6 / M7 | 16 (3%) | 11 (6%) | 3 (2%) | 2 (1%) | |
| Unknown | 74 | 24 | 28 | 22 | |
| Cytogenetics, n (%) | |||||
| t(8;21) | 31 (5%) | 26 (13%) | 4 (2%) | 1 (<1%) | <.001 |
| inv(16)/t(16;16)(p13;q22) | 40 (7%) | 11 (5%) | 18 (9%) | 11 (5%) | .24 |
| Normal karyotype | 312 (52%) | 83 (41%) | 108 (54%) | 121 (60%) | <.001 |
| t(9;11)(p21-22;q23) or t(11;19)(q23;p13) | 22 (4%) | 2 (1%) | 7 (4%) | 13 (6%) | .01 |
| Abnormalities not classified as favorable or adverse | 102 (17%) | 39 (19%) | 26 (13%) | 37 (18%) | .21 |
| Complex karyotype | 55 (9%) | 30 (15%) | 19 (10%) | 6 (3%) | <.001 |
| Other adverse risk abnormalities* | 43 (7%) | 13 (6%) | 17 (9%) | 13 (6%) | .63 |
| Unknown | 16 | 3 | 8 | 5 | |
| Cytogenetic risk group, n (%) | <.001 | ||||
| Favorable | 71 (12%) | 37 (18%) | 22 (11%) | 12 (6%) | |
| Intermediate | 436 (72%) | 124 (61%) | 141 (71%) | 171 (85%) | |
| Adverse | 98 (16%) | 43 (21%) | 36 (18%) | 19 (9%) | |
| Mutations, n (%) | |||||
| NPM1+/FLT3-ITD− (normal karyotype) | 97 (16%) | 16 (8%) | 34 (17%) | 47 (23%) | <.001 |
| NPM1+/FLT3-ITD+ (normal karyotype) | 69 (11%) | 12 (6%) | 27 (14%) | 30 (15%) | .009 |
| NPM1−/FLT3-ITD+ (normal karyotype) | 34 (6%) | 9 (4%) | 11 (6%) | 14 (7%) | .54 |
| NPM1−/FLT3-ITD− (normal karyotype) | 112 (19%) | 46 (23%) | 36 (18%) | 30 (15%) | .13 |
| ELN, n (%) | .14 | ||||
| Favorable | 181 (32%) | 64 (33%) | 57 (31%) | 60 (31%) | |
| Intermediate-I | 169 (30%) | 48 (24%) | 59 (32%) | 62 (32%) | |
| Intermediate-II | 115 (20%) | 40 (20%) | 31 (17%) | 44 (23%) | |
| Adverse | 107 (19%) | 44 (22%) | 38 (21%) | 25 (13%) | |
| Unknown | 49 | 11 | 22 | 16 | |
| Category . | All cases . | CD33 MFI ratio . | |||
|---|---|---|---|---|---|
| n = 621 . | Lower third . | Middle third . | Upper third . | P . | |
| CD 33 MFI ratio | |||||
| Median (range) | 57 (1-456) | 11 (1-30) | 57 (30-94) | 157 (94-456) | |
| Age, y | .01 | ||||
| Median (range) | 57 (17-99) | 57 (19-99) | 59 (17-82) | 53 (18-89) | |
| Gender, n (%) | .13 | ||||
| Male | 337 (54%) | 124 (60%) | 108 (52%) | 105 (51%) | |
| Female | 284 (46%) | 83 (40%) | 99 (48%) | 102 (49%) | |
| FAB, n (%) | <.001 | ||||
| M0 | 29 (5%) | 15 (8%) | 11 (6%) | 3 (2%) | |
| M1 | 114 (21%) | 41 (22%) | 40 (22%) | 33 (18%) | |
| M2 | 174 (32%) | 80 (44%) | 53 (30%) | 41 (22%) | |
| M4 | 153 (28%) | 26 (14%) | 53 (30%) | 74 (40%) | |
| M5 | 61 (11%) | 10 (6%) | 19 (11%) | 32 (17%) | |
| M6 / M7 | 16 (3%) | 11 (6%) | 3 (2%) | 2 (1%) | |
| Unknown | 74 | 24 | 28 | 22 | |
| Cytogenetics, n (%) | |||||
| t(8;21) | 31 (5%) | 26 (13%) | 4 (2%) | 1 (<1%) | <.001 |
| inv(16)/t(16;16)(p13;q22) | 40 (7%) | 11 (5%) | 18 (9%) | 11 (5%) | .24 |
| Normal karyotype | 312 (52%) | 83 (41%) | 108 (54%) | 121 (60%) | <.001 |
| t(9;11)(p21-22;q23) or t(11;19)(q23;p13) | 22 (4%) | 2 (1%) | 7 (4%) | 13 (6%) | .01 |
| Abnormalities not classified as favorable or adverse | 102 (17%) | 39 (19%) | 26 (13%) | 37 (18%) | .21 |
| Complex karyotype | 55 (9%) | 30 (15%) | 19 (10%) | 6 (3%) | <.001 |
| Other adverse risk abnormalities* | 43 (7%) | 13 (6%) | 17 (9%) | 13 (6%) | .63 |
| Unknown | 16 | 3 | 8 | 5 | |
| Cytogenetic risk group, n (%) | <.001 | ||||
| Favorable | 71 (12%) | 37 (18%) | 22 (11%) | 12 (6%) | |
| Intermediate | 436 (72%) | 124 (61%) | 141 (71%) | 171 (85%) | |
| Adverse | 98 (16%) | 43 (21%) | 36 (18%) | 19 (9%) | |
| Mutations, n (%) | |||||
| NPM1+/FLT3-ITD− (normal karyotype) | 97 (16%) | 16 (8%) | 34 (17%) | 47 (23%) | <.001 |
| NPM1+/FLT3-ITD+ (normal karyotype) | 69 (11%) | 12 (6%) | 27 (14%) | 30 (15%) | .009 |
| NPM1−/FLT3-ITD+ (normal karyotype) | 34 (6%) | 9 (4%) | 11 (6%) | 14 (7%) | .54 |
| NPM1−/FLT3-ITD− (normal karyotype) | 112 (19%) | 46 (23%) | 36 (18%) | 30 (15%) | .13 |
| ELN, n (%) | .14 | ||||
| Favorable | 181 (32%) | 64 (33%) | 57 (31%) | 60 (31%) | |
| Intermediate-I | 169 (30%) | 48 (24%) | 59 (32%) | 62 (32%) | |
| Intermediate-II | 115 (20%) | 40 (20%) | 31 (17%) | 44 (23%) | |
| Adverse | 107 (19%) | 44 (22%) | 38 (21%) | 25 (13%) | |
| Unknown | 49 | 11 | 22 | 16 | |
Including abn(3q), inv(3)(q21q26)/t(3;3)(q21;q26), add(5q), −5/5q-, −7, add(7q)/del(7q), t(v;11)(v;q23), t(9;22)(q34;q11), −17/abn(17p), but excluding t(3;5)(q21∼25;q31∼35), t(9;11)(p21-22;q23) and t(11;19)(q23;p13).
Cytogenetic and molecular data were available for 605 and 511 patients, respectively. Complex karyotypes and t(8;21) translocations were more prevalent in the groups with lower CD33 expression intensity (P < .001 each) (Fig. 1C; Table 2). In contrast, cytogenetic markers classified within the intermediate risk group according to refined MRC criteria correlated with high CD33 expression, eg, normal karyotype (P < .001), t(9;11)(p21-22;q23), or t(11;19)(q23;p13) (P = .01) (Fig. 1C; Table 2). Within the normal karyotype group, NPM1 mutations were significantly associated with high CD33 expression levels, especially in the prognostically favorable group of patients without FLT3-ITD (P < .001), but also in the prognostically intermediate group of patients with FLT3-ITD (P = .009). No definite trend in prevalence among terciles was determined for patients with wild-type NPM1 and FLT3-ITD (P = .54) (Fig. 1D; Table 2).
CD33 expression on leukemic bulk and leukemic stem cells
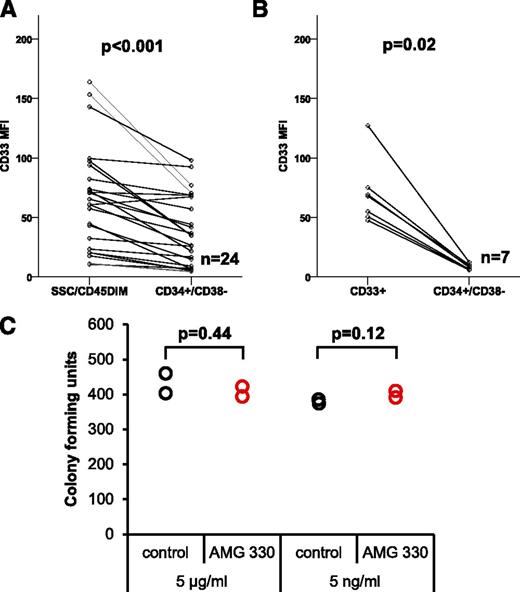
As LSCs, most frequently found within the CD34+/CD38− cell compartment, are supposed to be the source of relapse, we next analyzed the expression level of CD33 on CD34+/CD38− cells of newly diagnosed AML patients and in BM samples of HDs. Comparing the expression of CD33 on the leukemic bulk (CD45DIM) with that of CD34+/CD38− cells, we found increased CD33 expression on the former (median MFI of 59.1), whereas the latter expressed CD33 to a significantly lower degree (median MFI of 30.7; P < .001) (Fig. 2A). For HD samples, we compared the CD33 expression level on all CD33+ cells vs CD34+/CD38− cells. Myeloid precursors and differentiated monocytes within the BM fraction are known to strongly express CD33.22 Consistent with this observation, we observed high CD33 expression levels within the CD33+ bulk population (median MFI of 67.9). In contrast, median CD33 expression on CD34+/CD38− cells of HDs was much lower (median MFI of 8.1; P = .02) (Fig. 2B). Importantly, the CD33 expression intensity on CD34+/CD38− cells from HDs was significantly lower compared with CD34+/CD38− LSCs from AML patients (P = .047).
CD33 is frequently expressed on CD34+/CD38−LSCs from AML patients with significantly higher expression levels compared with HSCs from HDs. (A) CD33 expression on CD34+/CD38− LSCs compared with leukemic bulk cells (n = 24). (B) Expression of CD33 on CD34+/CD38− HSC compared with CD33+ bulk cells on BM samples from HDs (n = 7). (C) Colony-forming unit assay evaluating the unwanted on-target toxicity of AMG 330 on healthy human CD34+/Lin− BM cells.
CD33 is frequently expressed on CD34+/CD38−LSCs from AML patients with significantly higher expression levels compared with HSCs from HDs. (A) CD33 expression on CD34+/CD38− LSCs compared with leukemic bulk cells (n = 24). (B) Expression of CD33 on CD34+/CD38− HSC compared with CD33+ bulk cells on BM samples from HDs (n = 7). (C) Colony-forming unit assay evaluating the unwanted on-target toxicity of AMG 330 on healthy human CD34+/Lin− BM cells.
To detect potential toxic effects of an anti-CD33 antibody on BM cells from HDs, we examined colony formation after treatment with AMG 330 or a control BiTE. No significant difference in colony-forming units was detected between BM samples treated with AMG 330 and control BiTE antibody at 2 concentrations tested (P = .44 for 5 µg/mL, P = .12 for 5 ng/mL) (Fig. 2C).
CD33BRIGHT cells are lysed faster by AMG 330-redirected T cells than CD33DIM cells
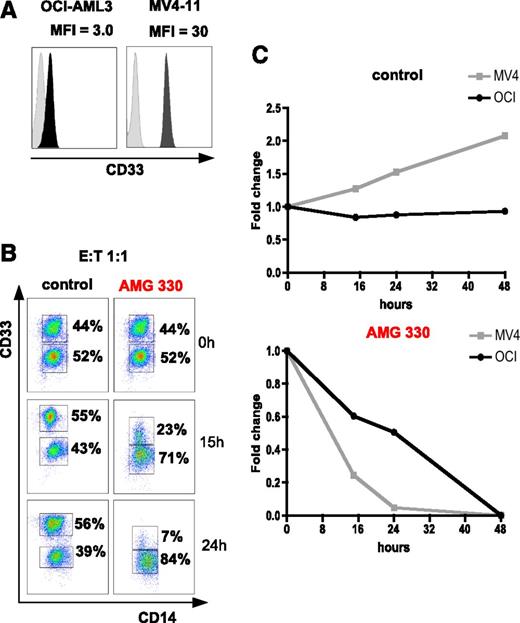
To establish adequate effector cell concentrations for in vitro experiments, we co-incubated the human AML cell line OCI-AML3 with PBMCs from HDs at E:T ratios between 5:1 and 1:20. At E:T ratios between 5:1 and 1:2, target cells were efficiently lysed within 4 days of culture (data not shown). Hence, we used E:T ratios of 1:1, 1:2, 1:3, and 1:4 to examine if cells with high CD33 surface expression are better lysed in the presence of AMG 330 than those with low expression. For this experiment, we chose human AML cell lines MV4-11 and OCI-AML3 with CD33 expression levels differing by a factor of 10 (MFI ratio for MV4-11: 30; for OCI-AML3: 3). Both populations could thus be differentiated by flow cytometry (Figure 3A). MV4-11 cells (CD33BRIGHT) were mixed with OCI-AML3 cells (CD33DIM) at a 1:1 ratio and co-incubated with HD PBMCs for 48 hours. Within 24 hours, a clear preference for lysis of CD33BRIGHT cells was observed for all 4 E:T ratios in the AMG 330-treated samples. After 48 hours, both cell lines were lysed equally well. In co-cultures using a control BiTE antibody sharing the anti-CD3 binding domain with AMG 330, no lysis of AML lines was observed (n = 2). Figure 3B-C shows representative data for an E:T ratio of 1:1.
AMG 330-mediated lysis of AML cell lines is dependent on CD33 expression intensity. (A) CD33 expression intensity (MFI ratio) of AML cell lines OCI-AML3 and MV4-11. (B-C) Preferential killing of MV4-11 cells (CD33BRIGHT) compared with OCI-AML3 cells (CD33DIM) using AMG 330 and PBMCs from a HD. In control wells (C, top) MV4-11 cells showed stronger proliferation compared with OCI-AML3. A representative example for an E:T ratio of 1:1 is shown.
AMG 330-mediated lysis of AML cell lines is dependent on CD33 expression intensity. (A) CD33 expression intensity (MFI ratio) of AML cell lines OCI-AML3 and MV4-11. (B-C) Preferential killing of MV4-11 cells (CD33BRIGHT) compared with OCI-AML3 cells (CD33DIM) using AMG 330 and PBMCs from a HD. In control wells (C, top) MV4-11 cells showed stronger proliferation compared with OCI-AML3. A representative example for an E:T ratio of 1:1 is shown.
A long-term culture system based on MS-5 feeder cells and cytokines supports the growth of primary AML blasts ex vivo for up to 36 days
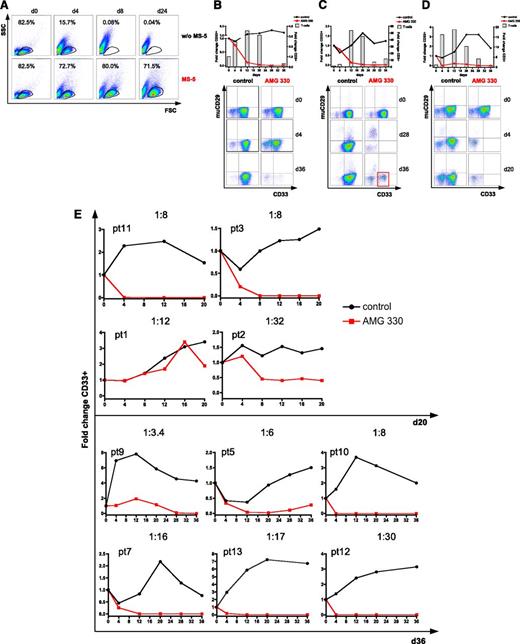
To test the cytotoxicity of AMG 330 in a clinically more relevant setting, we established an ex vivo long-term culture system. To support the growth of AML blasts, we used a combination of irradiated MS-5 feeder cells and a specific cytokine cocktail. In comparison with a conventional AML blast suspension culture, we found that the MS-5 feeder cell-based system supported the growth of AML blasts for at least 24 days in all patient samples tested (n = 5). Conversely, viable cells could not be detected after 8 days of culture using a conventional suspension system (example shown in Fig. 4A). Our blast culture system was later extended to 36 days by transferring cultured AML blasts onto fresh MS-5 feeder cells after 20 days, which further optimized growth conditions.
The MS-5–based culture system allows long-term proliferation of AML blasts and demonstration of effective AMG 330-mediated lysis, even at low E:T ratios. (A) AML blast viability over 24 days using the MS-5 feeder layer-based long-term culture system compared with a conventional suspension culture system. (B-E) Cytotoxicity assays based on flow cytometry analysis of AML blasts from primary diagnosis or relapse co-incubated with autologous T cells natively contained in the sample and AMG 330 or control antibody. Dot plots are gated on CD3− cells. T-cell expansion is shown in the graph by columns. CD33+ AML blast expansion/lysis is demonstrated in the graph by lines. Murine (mu) CD29 represents MS-5 feeder cells within the culture. (B) Example of a cytotoxicity assay (PT8; E:T ratio = 1:11) with delayed lysis of AML blasts. (C) Example of a cytotoxicity assay (PT4; E:T ratio = 1:79) with demonstration of CD33+ AML blast regrowth on day 36 of culture after a decline of viable CD3+ T cells. (D) Example of a cytotoxicity assay (PT6; E:T ratio = 1:5) with complete lysis of primary AML blasts. Continuous blast viability and proliferation over 36 days is shown for the control antibody. (E) Summary of the cytotoxicity assays with 10 more AML samples. High AMG 330-mediated cytotoxicity is demonstrated compared with continuous blast viability and proliferation in control cultures. FSC, forward scatter; SSC, side scatter.
The MS-5–based culture system allows long-term proliferation of AML blasts and demonstration of effective AMG 330-mediated lysis, even at low E:T ratios. (A) AML blast viability over 24 days using the MS-5 feeder layer-based long-term culture system compared with a conventional suspension culture system. (B-E) Cytotoxicity assays based on flow cytometry analysis of AML blasts from primary diagnosis or relapse co-incubated with autologous T cells natively contained in the sample and AMG 330 or control antibody. Dot plots are gated on CD3− cells. T-cell expansion is shown in the graph by columns. CD33+ AML blast expansion/lysis is demonstrated in the graph by lines. Murine (mu) CD29 represents MS-5 feeder cells within the culture. (B) Example of a cytotoxicity assay (PT8; E:T ratio = 1:11) with delayed lysis of AML blasts. (C) Example of a cytotoxicity assay (PT4; E:T ratio = 1:79) with demonstration of CD33+ AML blast regrowth on day 36 of culture after a decline of viable CD3+ T cells. (D) Example of a cytotoxicity assay (PT6; E:T ratio = 1:5) with complete lysis of primary AML blasts. Continuous blast viability and proliferation over 36 days is shown for the control antibody. (E) Summary of the cytotoxicity assays with 10 more AML samples. High AMG 330-mediated cytotoxicity is demonstrated compared with continuous blast viability and proliferation in control cultures. FSC, forward scatter; SSC, side scatter.
AMG 330 shows high cytotoxic potential against AML blasts from primary diagnosis at low E:T ratios using autologous T cells
Using our long-term culture system, we tested the cytotoxicity of AMG 330-activated autologous T cells on AML samples from initial diagnosis or relapse. Effector T cells consisted of residual T cells within the sample, as no further cells were added. We found that AML patient samples contained very low E:T ratios ranging between 1:3.4 and 1:79, corresponding to T-cell frequencies in the range of 1% to 13% of total cells in the samples. The importance of using a long-term culture system for AMG 330 cytotoxicity assays is exemplified by patient (PT) 8 (Figure 4B). Whereas CD33+ cells were reduced by only 16% on day 4, a close to complete elimination was achieved within 28–36 days. The quality of our long-time culture system is underlined by PT4. This AML sample had the lowest relative T-cell number leading to an E:T ratio of 1:79 at the start of the culture. No CD33+ AML blasts could be detected by day 28. However, on day 36 of culture, we observed a limited regrowth of CD33+ AML blasts (Figure 4C).
The high cytotoxic activity of AMG 330 was clearly evident from PT6, who was refractory to standard chemotherapy. Complete CD33+ blast elimination was achieved in our ex vivo cytotoxicity assay by day 4 (Figure 4D). Summarizing the remaining assays using the MS-5–based long-term culture system, we found a complete elimination (100%) of CD33+ AML blasts in 7 of 10 samples (70%). In 3 of 10 patient samples (30%), AML blasts were reduced by 44% to 81%. In control cultures, for all patient samples, either persistence or expansion of blast populations was observed during the experiments (Figure 4E).
Repeated challenge with AML blasts leads to continuous expansion of T cells and repeated cytotoxic elimination of target cells
In an attempt to simulate ex vivo the high proliferation rate of AML cells, we repeatedly added AML blasts to co-cultures containing autologous T cells (shown for PT2). We then evaluated whether AMG 330 retained its cytotoxic activity against newly added blasts (Figure 5A). After 3 days, we found that virtually all blasts were eliminated. We replenished the antibody only according to the volume needed for medium exchange. After 5 more days (day 8), no surviving CD33+ blasts could be detected. This procedure was repeated one more time (day 8 until 11), and complete AML blast lysis was seen again. This demonstrates that T cells are continuously stimulated and expanded as long as CD33+ target cells are present.
AMG 330 recruits memory T cells for target cell lysis. (A) Continuous lysis of repeatedly added AML blasts by autologous T cells from complete remission (CR) using AMG 330. Black arrows indicate AML blast addition. CD3+ T-cell expansion is represented in the graph by columns. (B) Expansion of CD3+ T cells from AML patients at primary diagnosis in a co-culture using AMG 330 compared with absence of proliferation with control antibody. One representative experiment (PT2) is shown (n = 14). CD3+ T cells are given in absolute numbers/well. (C) FACS analysis of the expanding T-cell population differentiating between CD4+ and CD8+ T cells and (D) between central memory (CD45RA−/CCR7+) and naïve (CD45RA+/CCR7+) T cells. All dot plots are gated on CD3+ cells. (E) Percentage of regulatory T cells (CD4+/CD25+/FoxP3+) in the expanding T-cell population after 3 and 9 days of culture. Dot plots are gated on CD3+/CD4+ T cells. (F) Expression of T cell activation markers Tim-3, LAG3 (n = 1), and PD-1 (n = 3) during co-culture. All histograms are gated on CD3+ cells.
AMG 330 recruits memory T cells for target cell lysis. (A) Continuous lysis of repeatedly added AML blasts by autologous T cells from complete remission (CR) using AMG 330. Black arrows indicate AML blast addition. CD3+ T-cell expansion is represented in the graph by columns. (B) Expansion of CD3+ T cells from AML patients at primary diagnosis in a co-culture using AMG 330 compared with absence of proliferation with control antibody. One representative experiment (PT2) is shown (n = 14). CD3+ T cells are given in absolute numbers/well. (C) FACS analysis of the expanding T-cell population differentiating between CD4+ and CD8+ T cells and (D) between central memory (CD45RA−/CCR7+) and naïve (CD45RA+/CCR7+) T cells. All dot plots are gated on CD3+ cells. (E) Percentage of regulatory T cells (CD4+/CD25+/FoxP3+) in the expanding T-cell population after 3 and 9 days of culture. Dot plots are gated on CD3+/CD4+ T cells. (F) Expression of T cell activation markers Tim-3, LAG3 (n = 1), and PD-1 (n = 3) during co-culture. All histograms are gated on CD3+ cells.
AMG 330 efficiently expands memory T cells for target cell lysis
To further characterize AMG 330-mediated target cell lysis, we quantitatively and phenotypically evaluated T-cell responses during long-term cultures. T-cell monitoring was performed in all cytotoxicity experiments, and we consistently observed an expansion of autologous CD3+ T cells in the presence of AMG 330, but not under control conditions (n = 14). Figure 5B shows a representative example for an E:T ratio of 1:32. After 3 and 9 days of co-culture with primary blasts, both CD4+ and CD8+ T cells were equally increased, whereas no increase was found in the presence of the control BiTE (Figure 5C). After 9 days of co-culturing, AMG 330 induced a robust shift toward a memory T-cell phenotype (CD45RA−/CCR7+) that did not occur with the control BiTE (90% vs 32%). Furthermore, in contrast to control cultures, expansion of CD45DIM/CCR7DIM/CD27DIM cells could be detected after 3 and 9 days of culture with AMG 330, representing a more differentiated effector memory T cell population (supplemental Figure 1A). Naïve T cells (CD45RA+/CCR7+) were not expanded by AMG 330; neither were terminally differentiated T cells (CD45RA+/CCR7−), probably due to their poor proliferative capacity23 (Figure 5D). Compared with control cultures, an up-regulation of the activation marker CD25 was observed in the presence of AMG 330 on day 3, which partially declined on day 9 coincident with the elimination of all target cells.
We did not observe an increase in percentage of CD3+/CD4+/CD25+/FoxP3+ regulatory T cells in the presence of AMG 330, suggesting that these cells may not have impacted AMG 330-mediated T cell activity in our experiments (Figure 5E). Finally, we found that AMG 330 induced the up-regulation of T cell activation markers Lymphocyte Activation Gene 3 (LAG3), T cell immunoglobulin mucin 3 (Tim3), and Programmed cell death protein 1 (PD-1) on day 3, which was partially reversible on day 9 of co-culture (Figure 5F). No up-regulation of these markers was observed in control cultures. Cytokine secretion analysis in 2 patient samples (PT9 and PT13) revealed high levels of interferon-γ, tumor necrosis factor, IL-2, and IL-17A using AMG 330. In contrast, only low levels or no cytokine secretion could be detected in control cultures (supplemental Figure 1B).
Discussion
In the present study, we evaluated the CD33/CD3-bispecific BiTE antibody AMG 330 for its suitability as immunotherapy in AML. We first performed a comprehensive CD33 expression analysis of 621 adult AML patient samples. Instead of assessing the percentage of CD33+ cells,24 we quantified CD33 surface expression levels by measuring MFI ratios. CD33 was detected in >99% of AML samples. Interestingly, CD33 expression levels correlated with prognostically relevant disease characteristics. Our data confirmed a previous study showing that NPM1 mutations are significantly associated with high CD33 expression, indicating a particular benefit of patients with mutated NPM1 for CD33-targeted therapies.25 Low CD33 expression was significantly associated with complex karyotypes. This may help to explain why patients of the adverse risk group had reduced response rates with GO in a phase III clinical trial.24 The effect of this antibody-drug conjugate is dependent on the rate of its internalization, which is positively correlated with CD33 surface expression.26 In contrast, BiTE antibodies do not rely on target internalization, and low CD33 expression levels seem to only slow down the kinetics of T-cell–mediated lysis, as shown in our study by faster killing of a CD33BRIGHT cell line. Therefore, in contrast to GO, adverse risk patients might also benefit from an AMG 330 therapy, especially when the antibody is continuously applied for several weeks, which is the standard schedule for the BiTE antibody blinatumumab.5 If efficient anti-AML activity in adverse risk patients with low CD33 expression could be confirmed in further studies, AMG 330 may be capable of avoiding fragmentation of AML into various subindications.
Another extensive analysis using MFI ratio for assessment of CD33 expression level has been performed in childhood AML.27 In accordance with our data, NPM1+/FLT3-ITD− as well as normal karyotype and chromosome 11 abnormalities were reported to correlate with high expression, whereas t(8;21) translocations correlated with low CD33 expression. However, in contrast to our results, the childhood AML study demonstrated a significant correlation between the favorable genetic risk group and low CD33 expression. The differential findings in correlation of genetic risk group and CD33 expression level can most likely be explained by considerable differences in frequency and classification of the various molecular and genetic markers in adult vs childhood AML.28
Notably, we showed that CD34+/CD38− LSCs express CD33 at a significantly higher level than CD34+/CD38− BM cells from HDs. This is in accordance with a previous study demonstrating that especially CD33+ cells from AML patients showed stem cell function and permitted engraftment of leukemia into conditioned SCID mice. The same study demonstrated that HSCs from HDs are divided into a CD33+ and a CD33− subset, both capable of engrafting in conditioned SCID mice.29 In combination with our data on CD33 expression on LSCs and HSCs and the expression-dependent killing kinetics, this suggests that LSCs will be preferentially eliminated using AMG 330 compared with CD33DIM HSCs. Additionally, the fraction of HSCs that are CD33− will be spared from AMG 330-mediated T-cell killing and allow for restoration of normal hematopoiesis. Accordingly, colony formation of CD34+/Lin− cells from HDs was not affected by pre-incubation with AMG 330 and T cells even at extremely high AMG 330 concentrations (5 µg/mL). Similar findings were recently reported with a different CD33/CD3 bispecific antibody construct.30 The ability to eliminate LSCs might be a general advantage of AMG 330 over antibody-drug conjugates like GO, as the latter are dependent on proliferating cells.11 Nevertheless, AML is a heterogeneous disease that may also arise from CD33− precursors and would thus be resistant to CD33-targeted immunotherapies.31
As AML blasts show progressive cell death under regular cell culture conditions within a few days,32 we established a long-term culture system for functional analysis of AMG 330 that allowed us to culture AML blasts for up to 36 days. We consider long-term cell culture experiments essential for studying AMG 330 activities for 3 major reasons. First, it is pivotal to have long-term viable blast populations within controlled cell culture conditions to clearly distinguish AMG 330-mediated from cell culture-mediated cell death. Second, T cells were shown to respond to continuous blinatumomab exposure over several weeks involving phases of redistribution, activation, and expansion.33 Especially at low E:T ratios, prolonged AMG 330 exposure is needed to follow the fate of AMG 330-activated, previously unstimulated T cells. Finally, our system opened up the possibility to study AMG 330 exposure over more than 4 weeks, which is essential to capture the full therapeutic potential of the BiTE antibody. We observed high cytotoxicity and reduction of CD33+ blasts with all patient samples by AMG 330-mediated engagement of residual, autologous T cells that had not been prestimulated. Even AML blasts from a chemotherapy-refractory patient (PT6) and blasts with rather low CD33 expression (PT3) were completely eliminated.
Several reasons are conceivable why CD33+ AML blasts were not completely eliminated in all experiments. Where incomplete elimination of AML blasts after 20 days was seen, a prolongation of the culture period might have been required, as in another case (PT9) that required 28 days for complete blast elimination that had not yet been achieved at day 20. For patients 1, 2, and 5, AMG 330 failed to induce complete target cell lysis. These patients showed a trend toward lower CD33 expression level (MFI ratio of 5.3, 8.6, and 44.3, respectively), potentially contributing to decreased efficacy of AMG 330-mediated target cell lysis. However, taking into account the limited number of experiments and the differences in E:T ratio, it is not possible to deduce the persistence on CD33 expression level only. Nevertheless, CD33 expression levels might contribute to target cell escape. As observed in PT4 (Figure 4C), the lack of T-cell stimuli after elimination of CD33BRIGHT cells caused T cells to decline, which potentially promoted persistence of remaining CD33DIM cells and outgrowth of CD33BRIGHT cells.
This hypothesis is supported by our observation that repetitive addition of AML blasts to the cell culture prevented T-cell decline and led to a continuous expansion of T cells. The initial situation may be specific for a closed cell culture system but could be different in vivo, where new T cells become available from the periphery and normal CD33+ cells provide continuous T-cell stimulation through AMG 330. Consistent with AMG 330 activating T-cells by the invariant CD3 subunit, we saw an expansion of both memory CD4+ and CD8+ T cells, but not naïve T cells that are dependent on co-stimulatory signals.2 TEMRA cells, which were shown to substantially contribute to target cell lysis,34 are of low proliferative capacity23 and were therefore not expanded. Importantly, we saw no proliferation of regulatory T cells following AMG 330 treatment. The up-regulation of activation markers LAG3, PD-1, and TIM3 that we observed was reversible shortly after complete target cell elimination. It is still under investigation whether this is correlated with an increasing expression of inhibitory molecules on AML blasts or if it just indicates the status of T-cell activation.
In summary, our analysis validates CD33 as a suitable target antigen for BiTE therapy in AML and provides a rational basis for the correlation between CD33 expression and genotype in AML. An AML blast long-term culture system was established, which gave us the opportunity to closely simulate therapeutic administration cycles. Hence, strong cytotoxic activity of residual AMG 330-activated T cells against autologous AML blasts could be demonstrated at low E:T ratios. Our results support the further development of AMG 330 for treating AML patients in high medical need for alternative therapies.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Elke Habben, Karin Hecht, Sabine Reinkunz, and Ewelina Zientara (Laboratory for Leukemia Diagnostics, University Hospital Munich) for their excellent technical support and AMGEN Research (Munich) GmbH for providing AMG 330 and control antibody.
This work was supported by funding from Prof. Dr. med. Dres. Prof Dr med Dres h.c. Gert Riethmüller (Emeritus from the Institute for Immunology, Ludwig-Maximilians-University, Munich, Germany) to C.K., and from the Deutsche Forschungsgemeinschaft (SFB 684, project A12) to K.S.
Authorship
Contribution: C.K. was involved in research design and performed the experiments, collected, analyzed, and interpreted the data, and wrote the manuscript; J.B. collected data, analyzed target antigen expression, and performed the statistical analysis; T.K. performed the statistical expression analysis; G.R., P.K., R.K., and G.Z. were involved in research design and data interpretation; G.R., P.K., R.K., G.Z., and P.A.B. provided AMG 330 and the control antibody; F.S.L. was involved in data interpretation and contributed to the writing of the manuscript; W.H., S.S., K.H.M., K.S., and M.F. provided patient characteristics including molecular and cytogenetic data; and M.S. designed the research, interpreted the data, and supervised the project.
Conflict-of-interest disclosure: P.K., R.K., G.Z., and P.A.B. are employed by Amgen Research (Munich) GmbH. P.K., R.K., G.Z., P.A.B., and G.R. were involved in invention and development of the antibody and are shareholders of Amgen Research (Munich) GmbH. The remaining authors declare no competing financial interests.
Correspondence: Marion Subklewe, Department of Internal Medicine III, Klinikum der Universität München, Marchioninistraße 15, 81377 Munich, Germany; e-mail: marion.subklewe@med.uni-muenchen.de.
References
Author notes
C.K., P.K., and R.K. contributed equally to this study.

![Figure 1. Association of CD33 expression with cytogenetic and molecular markers. (A) CD33 expression of 621 AML patient samples, subgrouped into 3 terciles. (B) Two representative examples of CD33 expression on primary AML blasts (tercile 1 [T1], left; tercile 3 [T3], right). (C-D) CD33 expression intensity correlated to specific cytogenetic/molecular markers. *Including abn(3q), inv(3)(q21q26)/t(3;3)(q21;q26), add(5q), −5/5q-, −7, add(7q)/del(7q), t(v;11)(v;q23), t(9;22)(q34;q11), −17/abn(17p), but excluding t(3;5)(q21∼25;q31∼35), t(9;11)(p21-22;q23) and t(11;19)(q23;p13). †Including t(9;11)(p21-22;q23), t(11;19)(q23;p13) and all other abnormalities not otherwise classified.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/123/3/10.1182_blood-2013-08-523548/4/m_356f1.jpeg?Expires=1769146464&Signature=NXO21PvFKEXcpliq3dlWlEVEv5r5uWSJ94ZuFfTcjBiezMk0jRbnfFMfqKTcAS1xOUE0MmQOBxvSq1xbqP8IOvQJJPu6sQufZz6U~aXYuAaVL84-4ncsQJkJhknJxMlSu~UzwnxhK04Ftv0E3BrdqtOdzDjB2SJdVMTdCeuTgw0o7AeUamm2EMGeKGBRlk8qgQih5FULftuilyKsWzEUVp5~i5TWdKHXsegxOWLs6UPvfvclF627protHS6jTL-6IGnc6qYzuVbDaj0-H5z8TuLgujTG5puKNu5ag0e1mMkd2KudrG4mFf7rWwd1siEzH2n9OOMaLOdAUF5d1677zQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)