In this issue of Blood, Sui and colleagues have identified novel and interesting roles for Tropomodulin (Tmod)-3, a protein involved with actin filament organization, in erythroid proliferation, survival, and enucleation.1
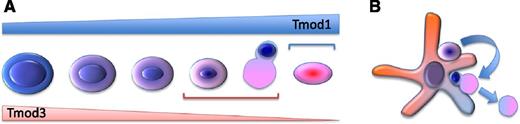
(A) Different stages of erythroid cell differentiation, enucleation, and erythrocyte formation. The triangular shapes indicate the relative level of expression of Tmod1 and Tmod3 during erythroid differentiation. The horizontal brackets indicate where Tmdo1 and Tmod3, respectively, might play a major role during erythropoiesis. (B) In erythroid islands, the potential role of macrophages and Tmod3 in support of the program that coordinates cell-cycle exit with enucleation in erythroid cells.
(A) Different stages of erythroid cell differentiation, enucleation, and erythrocyte formation. The triangular shapes indicate the relative level of expression of Tmod1 and Tmod3 during erythroid differentiation. The horizontal brackets indicate where Tmdo1 and Tmod3, respectively, might play a major role during erythropoiesis. (B) In erythroid islands, the potential role of macrophages and Tmod3 in support of the program that coordinates cell-cycle exit with enucleation in erythroid cells.
Actin filaments are one of the major components of the cytoskeleton, involved in many processes such as contraction, mobility, intracellular transport, cell division, and enucleation.2 Following a specific technique known as “decoration,” actin filaments can be visualized by electron microscopy as structures that look like arrows. The “pointed” end of the arrow corresponds to the slow growing end of the filament, while the “barbed” end is the site where actin monomers are preferentially incorporated during filament polymerization.3,4
Tmods are a family of proteins that “cap” or block association and dissociation of actin monomers at the pointed ends. In this way, Tmods control actin dynamics and turnover, stabilizing actin filament dimension and cytoskeletal architecture.2 In mammals, 4 Tmods have been described so far. In the interest of this commentary, Tmod1 is found predominantly in differentiating cells such as erythroid cells and erythrocytes, while Tmod3 is expressed ubiquitously.2 Tmod1 in erythroid cells interacts with tropomyosin and additional proteins to form the spectrin-actin network conferring stability and plasticity to the membrane skeleton.5 Pan deletion of Tmod1 is lethal, but Tmod1-null mice can be rescued by expression of a Tmod1 transgene under the control of the cardiac-specific α-myosin heavy chain (α-MHC) promoter.6 Interestingly, these rescued animals show a mild, compensated, sphero-elliptocytic anemia due to dysregulation of actin filament lengths and additional alterations in red blood cell shapes and plasticity.5 In these animals, expression of Tmod3 was observed in erythroid cells, suggesting that Tmod3 partially compensates for absence of Tmod1.6
In the present study by Sui et al,1 the group led by Dr Fowler further characterized the role of Tmod1 and Tmod3 in erythropoiesis using human erythroid cells and Tmod3-null animals. Although ablation of Tmod3 led to embryonic lethality, analysis of definitive erythropoiesis was possible by analysis of erythropoiesis at embryonic day 14.5. At this stage, these animals where characterized by anemia, limited progenitor erythroid populations, and a low percentage of enucleated cells.
Tmod1 and Tmod3 showed an interesting pattern of expression during erythroid differentiation: although both Tmod1 and Tmod3 were expressed similarly during the early stages of erythropoiesis, the level of Tmod1 increased while Tmod3 decreased as the erythroid cells differentiated (see figure, panel A). These observations, together with the phenotypes observed comparing Tmod1-null and Tmod3-null mice, suggest that Tmod3 plays a major role during erythroid progenitor proliferation and differentiation, while Tmod1 might be more relevant in correctly defining the final structure of erythrocyte membranes (see figure, panel A).
Analysis of Tmod3-null erythroid cells led to a variety of very interesting and novel observations in cell survival, proliferation, and erythroid-macrophage interaction. One of the most striking phenotypes was the association between abnormal actin filaments in enucleating erythroblasts and their decreased ability to exit the cell cycle during terminal differentiation. This observation suggests that Tmod3, either directly or through the assembly of the cytoskeleton during enucleation, communicates to the cells that it is time to coordinate the last steps of erythroid differentiation and nuclear extrusion, including halting DNA replication. In other words, Tmod3 might represent an important player in restraining some of the functions of erythroid cells during the essential steps leading to reticulocyte biogenesis.
Macrophages play an important role in regulating erythroid survival, proliferation, and maturation, providing a major contribution to the erythroid microenvironment and modulating the phenotype of erythropoietic disorders.7-10 Because Sui and colleagues also showed that Tmod3 is expressed in macrophages, the ability of Tmod3-null macrophages in forming erythroid islands was investigated. In fact, native erythroblastic island formation was impaired in Tmod3-null fetal livers. Furthermore, using reconstitution experiments, the authors also showed that erythroid island formation was impaired if either Tmod3-null erythroblasts or macrophages were used with their wild-type counterpart. This indicated that Tmod3 is required in both erythroblasts and macrophages to correctly form erythroid islands. It is undermined whether the function of Tmod3 in erythroid cells and macrophages is the same. However, we can speculate that Tmod3 in both macrophages and erythroid cells plays a major role in determining the correct repertoire of adhesion molecules required by these 2 cell types to interact with each other. In addition, these observations might also suggest a role of macrophages in reinforcing the program in erythroid cells that coordinates cell-cycle exit with enucleation (see figure, panel B).
Conflict-of-interest disclosure: S.R. is supported by the National Institutes of Health (NIH) National Institute of Diabetes and Digestive and Kidney Diseases (grants R01DK090554 and R01DK095112), and NIH National Heart, Lung, and Blood Institute (grant R01HL102449), the European Community (FP7), Isis Pharmaceuticals, Bayer Pharmaceuticals, and Merganser Biotech; is a consultant for Novartis, Biomarin, Bayer, Alexion, and Isis Pharmaceuticals; and is a stockholder and consultant for Merganser Biotech. The consulting work and intellectual property of S.R. did not affect in any way the preparation of this commentary.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal