In this issue of Blood, Vrecenak et al report a significant improvement in the level of donor chimerism and immune tolerance in the dog model of major histocompatibility complex (MHC)–mismatched in utero hematopoietic cell transplantation (IUHCT).1
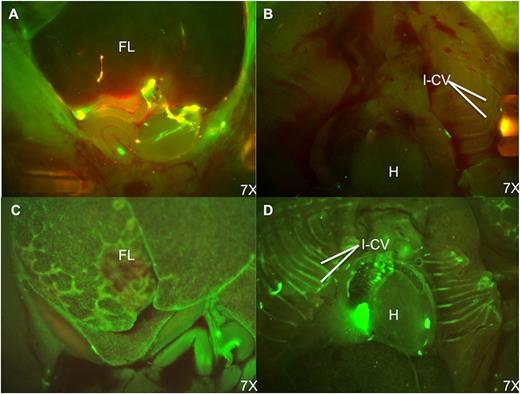
Fluorescence stereomicroscopic comparison of donor cell distribution at 48 hours postinjection of PKH-67–labeled bone marrow cells. (A) Fetal abdomen after IP injection; (B) fetal thorax after IP injection; (C) fetal abdomen after IC injection; and (D) fetal thorax after IC injection. Note the persistence of donor cells in the peritoneal cavity and paucity of engrafted donor cells in the fetal liver after IP injection. In contrast, the fetal liver and intercostal vascular bundles have large numbers of engrafted and circulating PKH-positive cells following IC injection. H, heart; FL, fetal liver; I-Cv, intercostal vessels. See Figure 4 in the article by Vrecenak et al that begins on page 1987.
Fluorescence stereomicroscopic comparison of donor cell distribution at 48 hours postinjection of PKH-67–labeled bone marrow cells. (A) Fetal abdomen after IP injection; (B) fetal thorax after IP injection; (C) fetal abdomen after IC injection; and (D) fetal thorax after IC injection. Note the persistence of donor cells in the peritoneal cavity and paucity of engrafted donor cells in the fetal liver after IP injection. In contrast, the fetal liver and intercostal vascular bundles have large numbers of engrafted and circulating PKH-positive cells following IC injection. H, heart; FL, fetal liver; I-Cv, intercostal vessels. See Figure 4 in the article by Vrecenak et al that begins on page 1987.
IUHCT is a potentially curative treatment for many congenital hematologic disorders, but enthusiasm for its clinical use has waned because of the general lack of sustained donor cell engraftment.2 There have been only a few successful cases of IUHCT with sustained donor T-cell chimerism in patients with X-linked severe combined immunodeficiency.3,4 To make progress in this field, it is essential to develop relevant large-animal models of IUHCT. However, for many years and in various animal models including the dog, donor cell engraftment after IUHCT has been either at very low levels or transient. Factors that limit engraftment include receptivity of the fetal host to donor cells, competition from host hematopoietic cells, and immunologic barriers to engraftment.2
Now, in a significant step forward, Drs Vrecenak and Pearson, along with their colleagues led by Dr Alan Flake at the Center for Fetal Research at the Children’s Hospital of Philadelphia, report the successful development of a dog model of IUHCT.1 They performed intravascular injection of maternal bone marrow cells into the fetus at gestation day 40 (term of gestation for dogs is 63 days) that reliably achieved long-term stable mixed chimerism with a relatively high level of donor cell engraftment without any form of conditioning. Importantly, they demonstrated donor-specific tolerance in IUHCT recipients 1 to 2 years later with acceptance of kidney grafts from the respective bone marrow donor without conditioning or immunosuppression.
The dog model is a particularly good model for IUHCT because individual placentas are immunologically separate (the placentas prevent mixing of fetal blood) and the issues of long-term hematopoiesis and immunologic barriers of graft-versus-host disease (GVHD) and solid organ transplantation have been well defined and closely resemble those of humans. Although sheep have previously been extensively used for IUHCT, their immunologic permissiveness and the relative ease of reaching high levels of donor-cell engraftment actually makes sheep inadequate as a predictive model for clinical outcome of human IUHCT.2
The authors demonstrate that the previously used method of intraperitoneal (IP) injection of donor bone marrow is very inefficient and results in donor chimerism levels consistently less than 3%. In contrast, intracardiac (IC) injection of IUHCT results in increased levels of sustained donor chimerism, on average greater than 10%.1 Figure 4 in the article by Vrecenak et al1 in this issue of Blood (reprinted here) shows the location of green fluorescent dye (PKH67)–labeled donor hematopoietic cells at 48 hours after either IP or IC injection in the fetus at day 40. As confirmed by the long-term follow-up chimerism studies, the IC injection of donor cells is significantly more efficient with homing to the fetal liver and intercostal vascular bundles, whereas IP injection results in most of the cells remaining in the abdomen, and apparently being unable to migrate to the sites of hematopoiesis in the fetal liver.
The timing of the IUHCT is also important. Consistent with findings by Blakemore and colleagues,5 it appears that donor cells infused too early or too late in embryonic development fail to adequately engraft. At day 39 of gestation, double-positive (CD4+CD8+) T cells begin to appear in the thymus and have completed significant expansion by day 46. The window of opportunity for donor-cell engraftment seems to be in the early phase of the rapid expansion of double-positive cells in the thymus, prior to onset of the transition of hematopoiesis to the fetal bone marrow.
Among recipient dogs with greater than 10% donor chimerism, kidney grafts from the original bone marrow donor were accepted without histologic evidence of rejection. Using their optimized protocol, the authors achieved 88% frequency of engraftment in the absence of any form of conditioning or immunosuppression. The levels of donor chimerism achieved may actually be sufficient to treat many target disorders, including hemoglobinopathies and immunodeficiencies.
Although none of the recipients developed GVHD, concern persists about the potential for development of GVHD after IUHCT. In this study, the investigators partially T-cell–depleted the maternal bone marrow grafts, so that T cells contributed ∼1% of the infused cell dose. The add-back of a portion of the CD34-depleted marrow cells to the CD34+ selected cells from the maternal bone marrow appeared to increase the level of donor chimerism. Future studies will need to determine the optimal T-cell dose and the degree of MHC mismatch acceptable for tolerance and stable mixed hematopoietic chimerism.
The significance of achieving high levels of donor chimerism and immune tolerance from an MHC-haploidentical graft is that a real barrier in IUHCT has finally been broken. For human patients, the authors are planning ultrasound-guided fetoscopic intravascular injection into the umbilical vein at the placental plate. They are initiating studies in nonhuman primates to technically mimic the clinical plan prior to beginning the studies in humans. If their approach is successful, it appears that in the not-too-distant future, IUHCT for hemoglobinopathies and immunodeficiencies that can be diagnosed by the end of the first in utero trimester may finally enter into the realm of clinical therapeutic reality.
Conflict-of-interest disclosure: The author declares no competing financial interests.