Abstract
Human neutrophil antigen-3a (HNA-3a) antibodies contained in donor plasma can result in severe, sometimes fatal transfusion-related acute lung injury (TRALI). Recent developments in TRALI secondary to antibodies to HNA-3a antigen span diagnosis, pathophysiology, treatment, and prevention resulting in improved understanding, potential treatments, and mitigation strategies. First, on the molecular level, characterization of HNA-3 antigen has allowed for genotyping methods that clarify population prevalence. Related work has led to generation of multiple antibody detection assays. These assays aid in determining potential populations at risk and potential mitigation strategies. Second, the development of TRALI requires a hit from the patient and from the product. Anti-HNA-3a is one of the product-derived factors and appears to result in TRALI by binding directly to pulmonary endothelium as well as to neutrophils expressing the corresponding antigen. Finally, potential mitigation strategies include red blood cell product filtration to remove anti-HNA-3a as well as other antibodies.
Medscape Continuing Medical Education online
This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education through the joint providership of Medscape, LLC and the American Society of Hematology.
Medscape, LLC is accredited by the ACCME to provide continuing medical education for physicians.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 75% minimum passing score and complete the evaluation at http://www.medscape.org/journal/blood; and (4) view/print certificate. For CME questions, see page 2004.
Disclosures
The authors, Associate Editor Mario Cazzola, and CME questions author Laurie Barclay, freelance writer and reviewer, Medscape, LLC, declare no competing financial interests.
Learning objectives
Describe transfusion-related acute lung injury and the role of antibodies, based on a review.
Assess the role of anti-human neutrophil antigen-3a in transfusion-related acute lung injury.
Identify potential mitigation strategies for transfusion-related acute lung injury.
Release date: September 18, 2014; Expiration date: September 18, 2015
Introduction
Transfusion-related acute lung injury (TRALI) remains the leading cause of transfusion-related fatalities as indicated by both the US Food and Drug Administration (FDA) and international hemovigilance systems such as the United Kingdom Serious Hazards of Transfusion and the French Agency for the Safety of Health Products. TRALI accounted for 38% of transfusion-related fatalities reported to the FDA during the period 2008-2013.1 Decreases in the incidence of TRALI have occurred through mitigation strategies, particularly with male, never-pregnant female, or solvent detergent plasma. The use of male-only plasma decreased TRALI risk of all transfused units by 68% from 2006 to 2009,2 and solvent detergent plasma has reportedly eliminated risk.3 TRALI usually arises secondary to transfused donor white blood cell antibodies (HLA class I or class II, or human neutrophil antigen [HNA] antibodies), but patient factors also play a role. Antibodies to HNAs, in particular anti-HNA-3a, are associated with severe cases of TRALI, although why these antibodies cause more severe disease than HLA class I or class II antibodies remains unknown. Although antibodies to HNA-1a, HNA-1b, and HNA-2a are also implicated in TRALI, given the particular association of anti-HNA-3a with severe and fatal cases, this review will highlight HNA-3a and its corresponding antibodies, including their role in TRALI development, incidence, and potential testing and mitigation strategies. Please refer to other recent reviews that more broadly discuss the clinical entity, epidemiology, and pathogenesis of TRALI.4,5
TRALI
TRALI is a clinical syndrome defined by hypoxia and pulmonary edema, occurring within 6 hours of transfusion in the absence of alternate causes of pulmonary injury.6 HNA antibodies are implicated in a significant proportion of cases. A prospective case-controlled study of TRALI demonstrated the presence of HNA antibodies in 42% of TRALI cases, of which 12% were exclusively HNA.2 Two large case series of TRALI showed HNA antibodies in 33% and 23% of cases, respectively.7,8 Similarly, FDA data from 2007 to 2008 and United Kingdom hemovigilance data from 1996 to 2006 show HNA antibodies in 22% and 25% of cases with demonstrated donor antibodies, although not all cases were tested for recipient cognate antigens.9,10
TRALI develops in a 2-hit model, the first hit from the recipient and the second hit from the blood product. The product can result in TRALI either from an antibody-mediated or non-antibody-mediated mechanism. Then patient characteristics, such as higher interleukin-8 levels, together with the product determine if TRALI is none, mild, or severe.2,4 The antibody-mediated mechanism is that TRALI is caused by the transfusion of blood products containing HLA class I and II or HNA alloantibodies directed against the cognate antigen of the recipient. Non-antibody-mediated mechanisms involve infusion of biologically activated lipids or cytokines that stimulate neutrophil activation. Both mechanisms then result in lung damage through capillary leakage and subsequent pulmonary edema.
A recent large prospective case-controlled study of TRALI identified significant factors associated with TRALI.2 First, the amount of anti-HNA determined by granulocyte immunofluorescence test (GIFT) and unit volume was a product risk factor. Second, other product risk factors included volume of HLA class II antibody with normalized background ratio >27.5 and plasma or whole blood from female donors. Third, recipient risk factors were higher interleukin-8 levels, liver surgery, chronic alcohol abuse, shock, higher peak airway pressure during mechanical ventilation, current smoking, and positive fluid balance. Finally, older red blood cell units, noncognate or weak cognate class II antibody, and class I antibody were not found to be associated with TRALI occurrence. Overall, antibodies were only found in 52% of the 89 TRALI patients in this study.
Current mitigation strategies
Current mitigation strategies focus on reducing the risk from antibody-mediated TRALI because the majority of TRALI cases are attributable to large volume plasma-containing products associated with HLA and/or HNA antibodies. Accordingly, such measures target high plasma volume products, designated as whole blood, plasma products (fresh frozen plasma, plasma frozen within 24 hours, and cryoprecipitate reduced plasma), apheresis platelets (PLTs), and buffy coat PLTs resuspended in plasma; the efficacy of applied mitigation measures regarding TRALI risk from PLT products, however, is uncertain at this time. Mitigation interventions for plasma products have included using plasma from exclusively male donors or never-pregnant females, resuspending pooled buffy coat PLTs in male-only plasma, HLA antibody screening of female donors based on parity, and using solvent detergent plasma. National and international data (United Kingdom, The Netherlands, and Canada) show that all TRALI cases from plasma transfusions in the United States were reduced by 23 to 10 cases (57%) from 2006 to 2009,2 and by 32 to 7 cases (80%) from 2006 to 200711 ; in the United Kingdom, by 36 to 10 (72%) in 2003 vs 200610 in the Netherlands, by 36 to 8 (77%) from 2002 to 200912 ; and in Canada, by 57 to 34 cases (40%) from 2007 to 200813 since the adoption of strategies utilizing predominantly male plasma.2,10,12,13
HNA-3/CTL2
Recent advances have provided information on the structure of the HNA-3 antigen. Although HNA-3 was discovered 45 years ago, only recently has the antigen system been characterized molecularly.14 Two alleles designated HNA-3a and HNA-3b result from a single nucleotide polymorphism at position 154 in the first extracellular loop of choline transporter-like protein 2 (CTL2), with arginine in HNA-3a and glutamine in HNA-3b.14,15 The HNA-3 antigen is located on CTL2, which is a 70-95 kDa glycoprotein encoded by the SLC44A2 gene.14,16 In addition to neutrophils, HNA-3 is expressed on monocytes, lymphocytes, and PLTs, as well as pulmonary endothelium.17,18 CTL2 is a membrane-bound protein comprising 10 transmembrane domains and 5 extracellular loops.16,19 Two different isoforms exist, designated CTL2-P1 and CTL2-P2, both equally able to bind HNA-3a antibodies, but with choline transporter activity limited to CTL2-P2.16,18 It was recently demonstrated that neutrophils, mononuclear cells, and PLTs express only CTL2-P1, implying that choline transport is not instrumental for neutrophil activation. Both transcript variants, however, are expressed in lung, suggesting a potential role of choline transport in anti-HNA-3a binding directly to endothelium resulting in TRALI.17
HNA-3a antibodies and TRALI
HNA-3a antibodies are the most frequently detected of the HNA antibodies in TRALI cases, seen in up to 85% of cases7 ; can induce TRALI in the context of minimal residual plasma volume, such as from red cell transfusion; and account for a disproportionate share of TRALI fatalities compared with other mediators.7,20,21 The higher fatality association was demonstrated in a study by Reil et al, who reported 36 TRALI cases, 12 of which were attributable to HNA antibodies (Table 1).7 Of these, 10 were attributable to HNA-3a antibodies, and 6 of the 10 cases were fatal.7 In contrast, of the remaining 24 cases attributable to non-HNA antibodies, only 4 cases were fatal.
HNA antibodies in published TRALI reports
| Reference . | Cases (no.) . | Antibody . | Donors (no.) . | Donor gender . | Mean pregnancies per donor (range) . | Fatal cases . | Products transfused . | Type of study . |
|---|---|---|---|---|---|---|---|---|
| 22 | 15 | HNA-3a | 1 | Female | 3 | 0 | Plasma | Lookback |
| 20 | 2 | HNA-3a | 2 | Female | 2 | 2 | Platelets, plasma | Case review |
| 21 | 9 | HNA-2a | 1 | Male | NA | 0 | Platelets | Lookback |
| 7 | 12 | 1 HNA-1a, 1 HNA-2a, 10 HNA-3a | — | Female | (1-6) | 6 | Plasma, red blood cells | Interventional |
| 8 | 8 | 1 HNA-2a, 7 HNA-3a | 2 | Female | 2.5 | 2 | Plasma | Observational cohort |
| Reference . | Cases (no.) . | Antibody . | Donors (no.) . | Donor gender . | Mean pregnancies per donor (range) . | Fatal cases . | Products transfused . | Type of study . |
|---|---|---|---|---|---|---|---|---|
| 22 | 15 | HNA-3a | 1 | Female | 3 | 0 | Plasma | Lookback |
| 20 | 2 | HNA-3a | 2 | Female | 2 | 2 | Platelets, plasma | Case review |
| 21 | 9 | HNA-2a | 1 | Male | NA | 0 | Platelets | Lookback |
| 7 | 12 | 1 HNA-1a, 1 HNA-2a, 10 HNA-3a | — | Female | (1-6) | 6 | Plasma, red blood cells | Interventional |
| 8 | 8 | 1 HNA-2a, 7 HNA-3a | 2 | Female | 2.5 | 2 | Plasma | Observational cohort |
NA, not applicable.
Lookback studies have also provided important information about the severity of TRALI cases involving HNA-3a antibodies. Kopko et al evaluated 36 patients who had received transfusion from a donor with HNA-3a antibodies associated with a fatality and found 8 additional cases of severe reactions consistent with TRALI, only 2 of which had been reported.22 In contrast, Davoren et al20 did not find any instance of adverse reaction in 25 previous donations from an implicated donor with HNA-3a antibodies; however, this information was only obtained from communication with the involved blood banks and did not include recipient chart reviews.
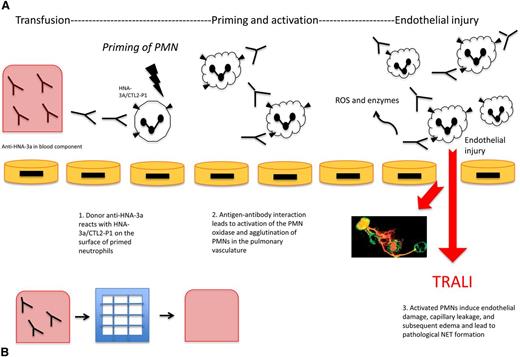
HNA-3a antibodies result in TRALI both through neutrophil activation and direct pulmonary endothelial damage. It has been suggested that HNA-3a antibodies serve as the second of a 2-hit pathogenesis, following the first hit of a clinical insult such as sepsis or shock. Silliman et al developed an analogous in vitro model showing that HNA-3a antibodies primed the N-formyl-methionyl-leucyl-phenylalanine respiratory burst, and that endotoxin-activated human microvascular endothelial cells were able to prime polymorphonuclear neutrophils (PMNs) through increased intercellular adhesion molecule 1 and chemokine synthesis. Then the subsequent addition of HNA-3a antibodies was able to activate the primed PMNs leading to release of reactive oxygen species (ROS) causing neutrophil-mediated destruction of pulmonary endothelial cells (Figure 1).23
Anti-HNA-3a induced TRALI and the effect of filtration on TRALI. (A) Anti-HNA-3a induced TRALI. Antibodies to HNA-3a are postulated to bind to primed neutrophils expressing HNA-3a, leading to activation, agglutination, and subsequent pulmonary injury resulting in TRALI. First, anti-HNA-3a in the blood component binds to human neutrophils expressing HNA-3a/CTL2-P1. Next, antibody-antigen interactions lead to agglutination of neutrophils and activation of the PMN oxidase. Then activated PMNs release ROS and enzymes that mediate endothelial damage, vascular compromise, and edema, subsequently leading to TRALI. NETs are postulated to form as a pathological bioproduct of neutrophil activation, leading to further endothelial injury. (B) Effect of filtration on TRALI. Modified and experimental Pall BPF4 leukoreduction filters deplete blood products of anti-HNA-3a among other antibodies. Reduction of TRALI-inducing substances in the blood component can result in decreased subsequent lung injury, as a potential preventative mechanism.
Anti-HNA-3a induced TRALI and the effect of filtration on TRALI. (A) Anti-HNA-3a induced TRALI. Antibodies to HNA-3a are postulated to bind to primed neutrophils expressing HNA-3a, leading to activation, agglutination, and subsequent pulmonary injury resulting in TRALI. First, anti-HNA-3a in the blood component binds to human neutrophils expressing HNA-3a/CTL2-P1. Next, antibody-antigen interactions lead to agglutination of neutrophils and activation of the PMN oxidase. Then activated PMNs release ROS and enzymes that mediate endothelial damage, vascular compromise, and edema, subsequently leading to TRALI. NETs are postulated to form as a pathological bioproduct of neutrophil activation, leading to further endothelial injury. (B) Effect of filtration on TRALI. Modified and experimental Pall BPF4 leukoreduction filters deplete blood products of anti-HNA-3a among other antibodies. Reduction of TRALI-inducing substances in the blood component can result in decreased subsequent lung injury, as a potential preventative mechanism.
The direct binding of HNA-3a antibodies to HNA-3a+ neutrophils results in neutrophil agglutination, priming and activation of the PMN oxidase, and subsequent neutrophil-mediated destruction of pulmonary endothelial cells.4 An early study using an ex vivo lung model showed that infusing explanted rabbit lungs with human anti-HNA-3a induced significant alterations in synthetic arachidonic acid metabolites, endothelial permeability, and lung edema after cotransfusion of HNA-3a–positive but not HNA-3a–negative human neutrophils.24,25
The 2-hit pathogenesis was also recently demonstrated in a mouse model showing the formation of neutrophil extracellular traps (NETs) in TRALI. NETs, defined as decondensed chromatin fibers decorated with neutrophil granular proteins, are released from activated neutrophils and trap extracellular proteins.26,27 NET formation, although potentially beneficial in capturing pathogens and killing bacteria, can also lead to pathological tissue injury, such as when excessive activation of neutrophils leads to TRALI.26,28,29 Thomas et al showed that NETs were produced in vitro by a 2-hit mechanism of neutrophils primed with tumor necrosis factor α and then exposed to HNA-3a antibodies, which were previously implicated in TRALI.26 No significant NET formation was seen from tumor necrosis factor α primed PMNs without HNA-3a antibody. Importantly, further experiments showed improved oxygenation from administration of intranasal DNase1, which destroys NETs, suggesting that NETs play a role in TRALI and that DNase1 is a potential treatment option.
In contrast to anti-HNA-3a mediating TRALI through PMN binding, recent work suggests that anti-HNA-3a mediates endothelial destruction by direct binding. Bayat et al developed an in vitro model showing that treatment of HNA-3a–positive endothelial cells with anti-HNA-3a can induce production of ROS by endothelial cells, although treatment of HNA-3a–positive neutrophils with anti-HNA-3a did not induce ROS production, consistent with previous work.17,23 Additional effects of anti-HNA-3a on endothelium included a decrease in endothelial resistance and increased endothelial permeability leading to impaired barrier integrity.17 Further, recent mouse models have demonstrated that HNA3 (choline transporter) antibodies can bind to the analogous mouse choline transporter epitope,30 and these antibodies can induce TRALI-like symptoms when injected into mice.17
Most recently, experimental filters have shown efficacy in mitigating TRALI attributable to HNA antibodies (Figure 1). Silliman et al31 describe a filtration system based on Pall BPF4 leukoreduction filters that depletes both antibodies and biological response modifiers from the red blood cell products. In a murine model of HNA-3a antibody–induced TRALI, these filters resulted in reduced neutrophil activation and prevention of lung injury.31 By showing that prevention of neutrophil priming with filtration reduces acute lung injury, the study provides further support to a PMN-dependent model.
HNA genotyping
Genotyping methods recently were developed based on the molecular characterization of HNA-332,33 that could allow for screening of donors at risk of developing HNA-3a antibodies (eg, previously pregnant women). Reil et al recently developed such a method using polymerase chain reaction sequence-specific primers to genotype HNA-3a and HNA-3b of 398 blood donors. They concurrently phenotyped 40 individuals by granulocyte agglutination test, GIFT, and lymphocyte immunofluorescence test and found the results concordant with the genotypes obtained by their polymerase chain reaction sequence-specific primers method.7 Genotyping methods now exist for HNA-1, HNA-3, HNA-4, and HNA-5, although a method is still unavailable for HNA-2a as it represents a defect in transcription of the HNA-2 gene.
Population studies demonstrate variation in the frequencies of the genes encoding the HNA alleles 3a and 3b. The study by Reil et al found 22 out of 398 (5.5%) donors in Germany homozygous for the HNA-3b allele and thus at risk for alloimmunization to HNA-3a.7 Other studies similarly show ∼5% to 6% of both the European and American white population homozygous for HNA-3b.14,32 In contrast, 16% of the Han Chinese population is homozygous for HNA-3b, and no African Americans were HNA-3b homozygous.32 These findings suggest a potential value in stratifying donors for screening based on race/ethnicity.
Antibody detection
Current testing for HNA-3a antibodies includes the granulocyte agglutination test and GIFT, as recommended by the ISBT Working Party on Granulocyte Immunobiology.34 These classical methods, however, are cumbersome because they require fresh granulocytes that must be prepared daily and used immediately. Consequently, screening for HNA-3a antibodies is not commonly performed. A recent national survey demonstrated that none of 47 participating US blood centers were screening for anti-HNA-3a.35 Although a combined Luminex test is available that detects HLA class I and class II and HNA-1a, HNA-1b, and HNA-1c antibodies (LABScreen MULTI, One Lambda Inc., Canoga Park, CA), it does not currently include HNA-3a antigens because until recently HNA-3 had not been molecularly defined.36
The recent characterization of HNA-3a could potentially allow for the development of solid phase assays for future high-throughput donor screening tests, with recombinant antigens targeting the first extracellular loop of CTL2; however, such efforts have proved difficult. For example, cyclic and linear synthetic peptides corresponding to HNA-3a do not consistently bind HNA-3a antibodies.37 Berthold et al attempted to characterize HNA-3a antibody binding by developing an enzyme immunoassay to screen blood donors for HNA-3a antibodies using a library of synthetic peptides covering the first extracellular loop of CTL2.38 However, they found that at most 9 out of 21 plasma samples containing anti-HNA-3a recognized CTL2 peptides corresponding to segments of the first extracellular loop containing the R154 peptide.38 Other studies have found similarly that approximately half of HNA-3a antibodies from blood donors implicated in TRALI recognize such peptides.36,37
Because of the inability of synthetic peptides to recognize all clinically relevant HNA-3a antibodies, efforts have been made to express the CTL2 protein in transfected HEK293 cells.36,39,40 Several studies found that the full-length CTL2 protein was recognized by all clinically relevant anti-HNA-3a sera as detected by flow cytometry.39 Despite the relative success of these studies, a cell-based assay is not ideal for large-scale screening of blood donors.
Future directions
In summary, recent advances in TRALI research focus on the role of HNA-3a antigens and antibodies. Typing of HNA-3a antigens allows for identification of donors at risk of developing HNA-3a antibodies, and progress in antibody detection will potentially lead to widespread screening of HNA-3a antibodies, both of which will enhance the safety of the blood supply. New understanding of the mechanism of HNA-3a antibodies through NET formation introduces the possibility of novel treatment options, such as intranasal DNase/pulmozyme, whereas PMN independent mechanisms pose remaining treatment challenges. Although important preventative measures have been proposed in the form of experimental filtration, work remains to determine if the concept will prove clinically relevant.
Authorship
Contribution: All authors contributed to the writing of this manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Beth Shaz, New York Blood Center, 310 East 67th St, New York, NY 10065; e-mail: bshaz@nybloodcenter.org.