Key Points
Melphalan, in combination with bortezomib, should be maintained as one of the standards of care for the treatment of elderly MM patients.
Complete response and particularly flow complete response should be an important goal in the treatment of elderly myeloma patients.
Abstract
Melphalan (M), in combination with prednisone (MP), has been the backbone of new combinations, including bortezomib plus MP (VMP). However, new alkylator-free schemes, such as lenalidomide plus low-dose dexamethasone, are challenging the role of alkylators in myeloma treatment of elderly patients. Here we have updated, after a long follow-up (median 6 years), the results of the GEM2005 study that addressed this question by comparing VMP with bortezomib plus thalidomide and prednisone (VTP) as induction. Between April 2005 and October 2008, 260 patients were randomized to receive 6 cycles of VMP or VTP as induction. The median progression-free survival was 32 months for the VMP and 23 months for the VTP arms (P = .09). VMP significantly prolonged the overall survival (OS) compared with VTP (median of 63 and 43 months, respectively; hazard ratio [HR]: 0.67, P = .01). Achieving immunophenotypic complete response was associated with a significantly longer OS, especially in the VMP arm (66% remain alive after 8 years). Melphalan, plus bortezomib, should be maintained as standard care for the treatment of elderly multiple myeloma patients. This trial was registered at www.clinicaltrials.gov as #NCT00443235.
Introduction
Melphalan plus prednisone (MP) has been the standard treatment of elderly patients with newly diagnosed multiple myeloma (MM) for >30 years. Moreover, in the era of novel agents, it has been the backbone of combination regimens containing novel drugs,1 such as those with thalidomide (MPT), lenalidomide (MPR), and bortezomib (VMP).
In the VISTA trial, the 3-drug combination of bortezomib plus MP (VMP) resulted in a 30% complete response rate, and in a 13.3-month longer overall survival (OS) than with MP alone. It is currently one of the standard treatments for transplant-ineligible MM patients.2 However, VMP had significant side effects, in particular peripheral neuropathy (13% of cases grade 3 or worse), gastrointestinal symptoms (19% grade 3 or worse), and a high rate of early discontinuation.3 The VMP scheme in the VISTA trial, in which patients most commonly received bortezomib twice weekly, was subsequently optimized through the use of a soft induction based on weekly administration of bortezomib, followed by maintenance.4,5 This approach resulted in a favorable toxicity profile and high efficacy. The subcutaneous administration of bortezomib is an additional step toward its optimized use because it is less toxic and of similar efficacy.6 New therapeutic options, based on alkylator-free regimens, are also emerging for patients who are not eligible to transplant. Lenalidomide plus low-dose dexamethasone (Rd) as continuous treatment or for 18 cycles has been compared in a large randomized trial, the FIRST trial, with MPT, an alkylator-based combination accepted as a standard of care for transplant-ineligible patients. Continuous treatment with Rd resulted in a significant improvement over MPT with respect to progression-free survival (PFS) (25.5 vs 21.2 months) and OS (59% vs 51% at 4 years).7
Currently, one of the burning questions in the treatment of transplant-ineligible newly diagnosed MM patients is whether alkylators should be maintained as part of their regimen. The phase 3 GEM2005MAS65 Spanish trial addressed this specific question prospectively by comparing VMP vs bortezomib plus thalidomide and prednisone (VTP) as induction in newly diagnosed elderly MM patients. Initial results showed that patients receiving VMP had slightly longer PFS and OS than did those receiving VTP, and this, together with the reduction in the frequency of side effects, suggested that melphalan was preferable to thalidomide for bortezomib-based combinations. However, this conclusion was based on a relatively short median follow-up of 32 months.4 We now provide an update of this phase 3 randomized trial after a median follow-up of 6 years, confirming the role of the alkylator as part of bortezomib-based combinations for transplant-ineligible MM patients. In addition, these updated results demonstrate that the better the quality of the response the longer are the PFS and OS, reinforcing the value of CR in transplant-ineligible MM patients.
Patients and methods
Patient population
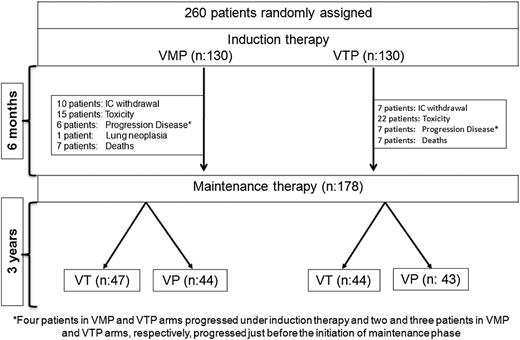
The study was originally conducted in 63 Spanish centers between March 2006 and October 2008. The details of this phase 3 randomized trial have already been reported4 and are updated here after a median follow-up of 72 months for survivors. Briefly, the Spanish GEM05MAS65 trial included 260 patients aged 65 years or older with newly diagnosed, untreated, symptomatic, and measurable MM. Equal numbers of patients were randomly assigned to receive either VMP or VTP as induction therapy, and those who completed the induction therapy were subsequently randomly assigned in approximately equal numbers to maintenance therapy with either bortezomib plus prednisone (VP) or bortezomib plus thalidomide (VT) (Figure 1).
Trial profile. Four patients in each of the VMP and VTP arms progressed under induction therapy. Two patients in the VMP group and 3 in the VTP group progressed just before starting the maintenance phase. M, melphalan; P, prednisone; T, thalidomide; V, bortezomib.
Trial profile. Four patients in each of the VMP and VTP arms progressed under induction therapy. Two patients in the VMP group and 3 in the VTP group progressed just before starting the maintenance phase. M, melphalan; P, prednisone; T, thalidomide; V, bortezomib.
Study design and treatment groups
VMP induction therapy consisted of 6 cycles: 1 cycle of IV bortezomib given twice per week for 6 weeks (1.3 mg/m2 on days 1, 4, 8, 11, 22, 25, 29, and 32), plus oral melphalan 9 mg/m2 and prednisone 60 mg/m2 on days 1 to 4, followed by 5 cycles of bortezomib once per week for 5 weeks (1.3 mg/m2 on days 1, 8, 15, and 22) plus the same doses of MP. VTP induction therapy consisted of the same schedule of bortezomib and prednisone plus oral, continuous thalidomide at a dose of 100 mg per day instead of melphalan. Patients from each arm who completed the 6 induction cycles were then randomly assigned to maintenance therapy with either VT or VP. Maintenance consisted of 1 conventional cycle of bortezomib (1.3 mg/m2 on days 1, 4, 8, and 11) every 3 months, plus either oral prednisone 50 mg every other day or oral thalidomide 50 mg per day, for up to 3 years (Figure 1).
The induction phase had a dual purpose: to evaluate whether weekly administration of bortezomib during induction was feasible and to investigate whether the best combination drug for bortezomib was melphalan (VMP) or thalidomide (VTP).
The institutional review board and/or independent ethics committee of each participating center approved the study. All patients provided written informed consent before screening in accordance with the Declaration of Helsinki. Data were monitored by an external contract research organization and centrally assessed.
Efficacy assessments
Disease response was assessed according to the European Group for Blood and Marrow Transplantation criteria, including standard CR (negative immunofixation) and near CR (positive immunofixation). Patients were considered to be in CR if they had a bone marrow aspirate containing <5% plasma cells. Disease response was assessed at the beginning of each induction cycle and at the end of induction. During maintenance therapy, assessments were made every month during the first year and every 2 months thereafter. After the end-of-treatment visit, all patients were followed every 3 months for response and outcome. Subanalysis of minimal residual disease (MRD) monitoring was planned after 6 induction cycles as part of the initial protocol design. MRD was analyzed by 4-color multiparametric flow cytometry, as previously described.4
Statistical analyses
Updated analyses were performed using data collected up to January 31, 2014. All results were evaluated in an intention-to-treat analysis. For univariate analyses, the OS and PFS curves were estimated by the Kaplan-Meier method and compared by the 2-sided log-rank test. The Cox proportional hazards model was used to estimate the hazard ratio (HR) values and the 95% confidence intervals (CIs). All statistical analyses were done with SPSS (version 15.0; SPSS Inc.).
Results
Two hundred sixty patients were included in the trial and randomly assigned to receive VMP (n = 130) or VTP (n = 130) as induction. One hundred seventy-eight patients completed the 6 induction cycles and were randomly assigned to maintenance therapy with VP (n = 87) or VT (n = 91). Induction and maintenance groups were balanced according to baseline characteristics, as has been previously reported.4 Of note, 41 patients (32%) and 47 patients (36%) were older than 75 years in VMP and VTP, respectively.
The VMP and VTP regimens yielded similar complete response rates of 20% and 28%, respectively. VMP produced more hematologic toxic effects than did VTP, particularly grade 3 or worse neutropenia and thrombocytopenia. However, patients older than 75 years of age did not develop a higher frequency of hematologic adverse events. The frequency of infections was higher with VMP (7%) than with VTP (1%) but they were balanced in the group of patients between 65 and 75 years (45%) and older than 75 years (55%). However, VTP treatment was associated with severe cardiac complications in 8% of patients (most of them [8 of 11] occurring in patients older than 75 years), compared with no cardiac complications in those patients who received VMP. Seven percent and 9% of patients in the VMP and VTP groups, respectively, had grade 3 or 4 peripheral neuropathy and were well balanced in the population older than 75 years (55% and 65% in VMP and VTP, respectively). Three (2%) and 2 patients (1.5%) in the VMP and VTP, respectively, developed second primary tumors whereas no hematologic malignancies have so far been reported (Table 1).
Toxicity profile (grade 3 or worse) during induction therapy in the whole series and in patients older than 75 y
| Adverse event . | VMP . | VTP . | ||
|---|---|---|---|---|
| Overall series, N = 130, n (%) . | ≥75 y, N = 41, n (%) . | Overall series, N = 130, n (%) . | ≥75 y, N = 47, n (%) . | |
| Hematological toxicity | ||||
| Anemia | 15 (11) | 4 (10) | 10 (8) | 3 (6) |
| Neutropenia | 51 (39) | 18 (44) | 29 (22) | 11 (23) |
| Thrombocytopenia | 35 (27) | 13 (31) | 16 (12) | 4 (8) |
| Nonhematological toxicity | ||||
| Gastrointestinal toxicity | 9 (7) | 5 (12) | 2 (2) | 1 (2) |
| Infections | 9 (7) | 4 (10) | 1 (<1) | — |
| Peripheral neuropathy | 9 (5) | 5 (12) | 12 (9) | 8 (17) |
| DVT/thromboembolism | 1 (<1) | — | 3 (2) | 1 (2) |
| Cardiologic events | — | 11 (8) | 8 (17) | |
| Solid tumors | 3 (2) | 2 (1.5) | ||
| Adverse event . | VMP . | VTP . | ||
|---|---|---|---|---|
| Overall series, N = 130, n (%) . | ≥75 y, N = 41, n (%) . | Overall series, N = 130, n (%) . | ≥75 y, N = 47, n (%) . | |
| Hematological toxicity | ||||
| Anemia | 15 (11) | 4 (10) | 10 (8) | 3 (6) |
| Neutropenia | 51 (39) | 18 (44) | 29 (22) | 11 (23) |
| Thrombocytopenia | 35 (27) | 13 (31) | 16 (12) | 4 (8) |
| Nonhematological toxicity | ||||
| Gastrointestinal toxicity | 9 (7) | 5 (12) | 2 (2) | 1 (2) |
| Infections | 9 (7) | 4 (10) | 1 (<1) | — |
| Peripheral neuropathy | 9 (5) | 5 (12) | 12 (9) | 8 (17) |
| DVT/thromboembolism | 1 (<1) | — | 3 (2) | 1 (2) |
| Cardiologic events | — | 11 (8) | 8 (17) | |
| Solid tumors | 3 (2) | 2 (1.5) | ||
DVT, deep venous thrombosis; y, years.
The median cumulative dose of the different drugs was significantly lower in patients aged older than 75 as compared with patients younger than 75 (Table 2). However, the proportion of the planned bortezomib dose that was actually delivered in patients older than 75 years was higher in the VMP arm (79%) as compared with VTP arm (66%). This was due to the lower number of bortezomib discontinuations and dose reductions required during VMP treatment.
Cumulative dose of drugs delivered in patients between 65 and 75 y and >75 y, overall as mean plus standard deviation
| Drug . | Total planned dose . | Cumulative dose of drug 65-75 y, mean (SD) . | Cumulative dose of drug ≥75 y, mean (SD) . | P . |
|---|---|---|---|---|
| Bortezomib, mg/m2 | 36.4 mg/m2 | 30 (10) | 24 (12) | <.0001 |
| Melphalan, mg/m2 | 216 mg/m2 | 194 (49) | 169 (64) | .02 |
| Thalidomide, mg | 17250 mg | 15359 (6960) | 10 710 (7994) | .001 |
| Prednisone, mg/m2 | 1440 mg/m2 | 1281 | 1043 | <.0001 |
| Drug . | Total planned dose . | Cumulative dose of drug 65-75 y, mean (SD) . | Cumulative dose of drug ≥75 y, mean (SD) . | P . |
|---|---|---|---|---|
| Bortezomib, mg/m2 | 36.4 mg/m2 | 30 (10) | 24 (12) | <.0001 |
| Melphalan, mg/m2 | 216 mg/m2 | 194 (49) | 169 (64) | .02 |
| Thalidomide, mg | 17250 mg | 15359 (6960) | 10 710 (7994) | .001 |
| Prednisone, mg/m2 | 1440 mg/m2 | 1281 | 1043 | <.0001 |
At the time of the first report, the median follow-up was 32 months from first randomization and although we had found no significant differences between the 2 arms, VMP was slightly superior to VTP in terms of PFS (HR: 1.2, 95% CI: 0.9-1.7; P = .1) and OS (HR: 1.2, 95% CI: 0.7-1.9; P = .3).
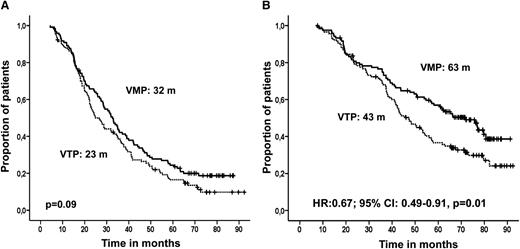
The median follow-up for surviving patients is currently 72 months. Progressive disease or death occurred in 105 patients (81%) in the VMP arm and in 113 (87%) of the patients who received VTP (P = .1). VMP delayed disease or death by 9 months compared with VTP. The median PFS was 32 months in the VMP group and 23 months in the VTP arm (P = .09) (Figure 2A). No differences in outcome were observed between patients receiving VT or VP as maintenance.
Survival in an intention-to-treat analysis by induction therapy received. (A) PFS and (B) OS.
Survival in an intention-to-treat analysis by induction therapy received. (A) PFS and (B) OS.
Deaths occurred in 73 patients treated with VMP (56%) and 91 patients (70%) in the VTP arm. OS was significantly prolonged in VMP compared with VTP (median of 63 and 43 months, respectively; HR: 0.67, 95% CI: 0.49-0.91; P = .01) (Figure 2B). Again, no statistically significant differences were observed for maintenance with VT or VP in either of the induction arms. The same analysis of the 178 patients who received both induction and maintenance also showed significantly longer OS for VMP than for VTP (77 and 54 months, respectively; P = .05).
The superiority of VMP over VTP in terms of PFS was observed in both the group of patients between 65 and 75 years (31 vs 27 months), and it was more evident in the group of patients older than 75 years (median PFS of 32 months vs 18 months for VMP and VTP arms, respectively; HR: 1.6, 95% CI: 1.1-2.5; P = .04). As far as OS is concerned, median OS for VMP patients between 65 and 75 years was 66 months vs 50 months for VTP patients. In patients older than 75 years, the median OS for VMP arm was 51 months vs 34 months for VTP (HR: 1.7, 95% CI: 1.1-2.9; P = .02).
The median PFS of patients with high-risk cytogenetic abnormalities was not significantly different for VMP (18 months) or VTP (20 months). By contrast, OS was shorter in patients treated with VTP (29 months) as compared with VMP (51 months).
Subsequent drugs used in first relapse were balanced in the VMP and VTP arms and included lenalidomide-based combinations in 40 patients (43.4%), bortezomib-based therapy in 19 patients (20.5%), other combinations in 25 patients (27.1%), and bendamustine-prednisone in 2 patients (2.1%). Six patients (6.5%) received supportive care.
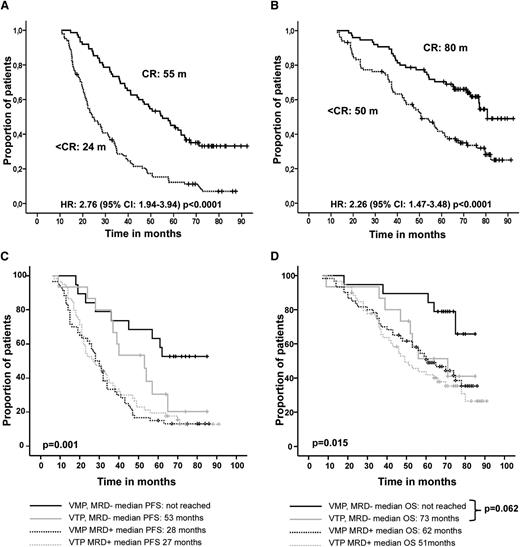
With the long follow-up we wanted to evaluate the impact of the quality of response. Patients who achieved conventional CR had significantly longer PFS (median of 55 months) than patients with less than CR (median of 24 months; HR: 2.76, 95% CI: 1.94-3.94; P < .0001) (Figure 3A). This benefit was more evident in patients who received VMP, resulting in a median PFS benefit of 10 more months than CR patients in VTP arm (60 vs 50 months; P > .05).
Survival in an intention-to-treat analysis by response category. (A) PFS and (B) OS. (C) PFS and (D) OS with respect to achievement of flow CR and induction therapy (VMP or VTP) received. CR, hematological CR; < CR, less than hematological CR; Flow-CR, immunophenotypic CR; < Flow-CR, less than immunophenotypic CR; MRD−, MRD negative, immunophenotypic CR; MRD+, MRD positive.
Survival in an intention-to-treat analysis by response category. (A) PFS and (B) OS. (C) PFS and (D) OS with respect to achievement of flow CR and induction therapy (VMP or VTP) received. CR, hematological CR; < CR, less than hematological CR; Flow-CR, immunophenotypic CR; < Flow-CR, less than immunophenotypic CR; MRD−, MRD negative, immunophenotypic CR; MRD+, MRD positive.
Achieving CR also translated into significantly prolonged OS, with a median of 80 months compared with 50 months for patients in less than CR (HR: 2.26, 95% CI: 1.47-3.48; P < .0001) (Figure 3B). Similar to what occurred with PFS, among patients in CR, those receiving VMP had a significant OS advantage over those receiving VTP (median of 81 and 76 months for patients in CR receiving VMP and VTP, respectively; HR: 0.66, 95% CI: 0.44-0.98; P = .045). Comparing maintenance with VT or VP revealed no differences in outcome (PFS and OS) associated with depth of response in either of the induction arms.
The prognostic value of MRD monitoring by multiparametric flow cytometry was assessed in the cohort of 153 patients in which this subanalysis was performed. Thirty-four patients (22%) achieved flow CR, and this translated into longer PFS (median of 59 vs 29 months; HR: 2.50, 95% CI: 1.53-4.05; P < .001) and OS (median not reached, 55% at 8 years vs 57 months; HR: 2.07, 95% CI: 1.14-3.74; P = .013). It should be noted that those patients in flow CR after VMP had a remarkable outcome, neither attaining median PFS nor OS (53% and 66% at 8 years, respectively; Figure 3C-D); these results are significantly better than those obtained in flow-CR patients after VTP. We then used MRD monitoring to compare the quality of conventional CR achieved after VMP or VTP; interestingly, although 70% of patients in CR after VMP were also in flow CR, only 45% of patients in CR after VTP attained flow CR (P = .09).
In the multivariate analysis, the covariates independently predicting longer PFS were CR achievement (HR: 2.82, 95% CI: 1.94-4.09; P < .0001) and International Stage System 1,2 (HR: 1.64, 95% CI: 1.08-2.49; P = .019). The analysis identified VMP as induction (HR: 1.68, 95% CI: 1.09-2.5; P = .01), standard risk cytogenetic abnormalities (HR: 1.92, 95% CI: 1.14-3.23; P = .014), and attainment of CR (HR = 2.25, 95% CI: 1.44-3.53; P < .0001) as factors independently associated with significantly longer OS. Flow CR was not included in the multivariate analysis because data for this were available from only a subset of patients after induction.
Discussion
After a median follow-up of 6 years, this randomized phase 3 trial shows that melphalan and prednisone in combination with bortezomib should be maintained as one of the standards of care for the treatment of elderly MM patients. When the results of this trial were first reported at the beginning of 2010, we concluded that, considering its efficacy and toxicity, melphalan was probably preferable to thalidomide for bortezomib-based combinations in the setting of elderly MM patients4 ; 4 years later, the treatment with VMP as induction has been confirmed as an independent variable associated with longer OS than with VTP (HR: 1.68, 95% CI: 1.09-2.5; P = .01).
Our study has 2 major findings. First, melphalan remains an important and cost-effective drug for the treatment of newly diagnosed elderly myeloma patients, and second, the value of prolonged treatment and depth of response in elderly patients is confirmed.
This study is the first specifically designed to address whether the alkylator should be maintained as part of the induction in elderly MM patients. Ludwig et al compared thalidomide plus high-dose dexamethasone (TD) with MP in a cohort of elderly MM patients and the OS was significantly shorter with TD (41.5 vs 49.4 months; P = .024); this result was due to the higher frequency of disease-unrelated deaths observed in the TD group and led to the conclusion that TD in elderly patients is not a good option unless patients receive reduced doses of both drugs.8 The phase 3b UPFRONT trial evaluated the efficacy and safety of 3 full doses of bortezomib-based induction regimens, 2 nonalkylator-based combinations, bortezomib with dexamethasone (VD) and bortezomib with thalidomide and dexamethasone (VTD), and 1 containing melphalan (VMP). The rate of partial response or better was slightly higher for VTD (80% vs 73% for VD, and 69% for VMP), but as in our trial, this was also associated with more frequent side effects, especially peripheral neuropathy (24% of grade 3 or higher vs 19% for VD and VMP). Although no significant differences in time-to-event data have so far been reported between the 3 arms, the bortezomib-based combination including melphalan, VMP, shows a trend to longer PFS and OS compared with VTD.9
The schemes used as rescue therapies in our trial were well balanced in the 2 arms, but survival from relapse was significantly shorter for patients who received VTP as induction rather than VMP (data not shown). We can therefore speculate that the longer OS we have observed with VMP could be the result of selection of more resistant clones by the VTP combination.
One limitation of our conclusion is that the results cannot be extrapolated to lenalidomide, which has a better safety profile and, probably, greater efficacy than thalidomide. The FIRST trial (Intergroupe Francophone du Myélome 07-01, MM-020) has just reported results of the comparison of Rd until disease progression vs Rd up to 18 cycles vs MPT up to 12 cycles, whereby PFS was 5 months longer for continuous Rd than with MPT. Though the comparison between alkylator upfront or not was not a planned objective of this trial, the use of MPT will probably decline in this patient population according to the results and Rd will become a new standard of care for this patient population. However, the conclusion does not apply to melphalan as a single drug but to the MPT combination. Of note, other new combinations are emerging considering Rd as backbone and although the information is still preliminary, based on phase 1/2 studies and including newly diagnosed patients (not all of them nontransplant candidates), the results are encouraging. Carfilzomib plus Rd resulted in a 100% overall response rate (ORR) with 65% stringent CR, and bortezomib plus Rd also in a 100% ORR with a 52% CR rate.10,11
In line with this, if we consider the results reported in the MM-015 trial in which the addition of lenalidomide to MP gave a significantly better PFS but not a longer OS, and in which there was a high frequency of adverse effects, especially in patients older than 75 years,12 we may conclude that melphalan is not the best partner for combining with immunomodulatory drugs, but is nevertheless an excellent drug in combination with bortezomib, as our analysis suggests. Furthermore, the combination of second-generation proteasome inhibitors with MP is yielding promising results. Carfilzomib plus MP is being evaluated in a phase 1/2 trial with an ORR of 90%.13 In an attempt to optimize the management of elderly patients, in 2010 the Spanish Myeloma Group activated a phase 2 randomized trial in which a total therapy approach was planned, including not only VMP but Rd, in a sequential or alternating approach. Although the median follow-up is short (20 months) preliminary results are encouraging, with estimated 3-year PFS and OS of 70% and 87%, respectively.14
In the elderly population, frail patients and those older than 75 years require particular attention. VMP was also shown to be superior in the group of patients aged over 75 years. The evaluation of the toxicity profile confirms that patients older than 75 years represent a frail patients’ population, with higher probability for developing nonhematological toxicities. In our study, this trend was seen in all patients aged over 75 years, but it was particularly evident for the cardiac toxicity in the VTP arm. The higher frequency of toxic effects translated into lower cumulative dose drugs delivered in patients older than 75 years and this effect was more significant for bortezomib in the VTP arm as compared with VMP. The results in terms of PFS and OS in this group of patients aged older than 75 years also support the benefit of VMP over VTP. However, the advanced age continues to be a feature associated with poor prognosis and although this trial confirms that VMP with bortezomib twice per week in the first cycle, followed by a less intensive weekly bortezomib dosing, is well tolerated, it could probably be optimized in patients over the age of 75 years using weekly since the first cycle and subcutaneous bortezomib administration.
A similar approach including weekly administration of bortezomib has been evaluated by an Italian group with similar conclusion in both studies.15
Finally, the role of CR has been investigated in elderly patients, and in a retrospective analysis of pooled data from 1175 patients with newly diagnosed MM, treated with novel agents and MP, CR achievement was associated with improved PFS and OS.16 In our prospective, phase 3 trial, the achievement of CR during the treatment was an independent prognostic factor in the multivariate analysis for both PFS (HR: 2.66) and OS (HR: 2.44), with an impressive median PFS and OS of 55 and 80 months, respectively, for patients in CR. This benefit was even more pronounced in patients receiving VMP as induction and proved to be an independent factor predicting longer OS. These results are comparable to those obtained in young patients eligible for transplantation. Moreover, our data confirm that immunophenotypic response is also relevant in the elderly population, with 55% of patients in flow CR free of progression and 66% alive after 8 years. These results are better than those obtained with conventional CR, and the group of patients who received VMP as induction gained the greatest benefit, suggesting that the DNA damage induced by the alkylator used in the VMP combination may help induce a bigger reduction of the tumor clone, resulting in a better outcome. In fact, 70% of patients in CR after VMP were also in flow CR, whereas only 45% of patients in CR after VTP were also in flow CR. These results also suggest that undetectable levels of MRD among patients receiving VTP were most likely responsible for late relapses, and that ongoing clinical trials should benefit from high-sensitivity, semiautomated multidimensional flow cytometry immunophenotyping.
In conclusion, although new combinations, some of them alkylator-free are emerging with encouraging efficacy and safety results, long-term follow-up is needed. At the present time melphalan, as part of the bortezomib-based combinations, should continue to be a cost-effective standard in most countries and VMP should remain one of the standards of care for the treatment of newly diagnosed transplant-ineligible MM patients. Although the maintenance approach is not approved yet, our study confirms that reduced-intensity induction with VMP followed by maintenance with VT or VP produces significantly better OS than achieved with VTP. In addition, CR, and particularly flow CR, should be an important goal in the treatment of this patient population.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Arturo Touchard and Lucía López-Anglada for providing data management support.
This work was funded and sponsored by Pethema (Spanish Program for the Treatment of Hematologic Diseases), Madrid, Spain, and was coordinated by the Spanish Myeloma Group (PETHEMA/Gem). Multiparametric flow cytometry studies were supported by a grant from FIS (Fondo de Investigación Sanitaria) 06/1354. This work was partially supported by the FIS Intrasalud Project (PS2009/01897) and by grants from RTICC (Red Temática Cooperativa en Cáncer) (RD12/0036/0046/0036/0058).
Authorship
Contribution: M.-V.M., J.-J.L., J. Bladé, and J.-F.S.M., the principal investigators, were involved in the conception and design of the study; M.-V.M. and J.F.S.-M. wrote the protocol and report, and analyzed and interpreted the data; M.-V.M., A.O., J.M.-L., A.-I.T., E.B., R.M., F.d.A., L.P., J.M.H., J. Bargay, F.-J.P., and M.-L.M.-M. contributed with the inclusion of patients; B.P. and M.-A.M. did the flow cytometry analysis; A.L.d.l.G., J.L., M.P., Y.G., M.G., and J.-L.B contributed with inclusion of patients; and all the authors have read and approved the manuscript before its submission for publication.
Conflict-of-interest disclosure: M.-V.M. has received honoraria from Janssen and Celgene. B.P. has received honoraria from Janssen, Millennium, Celgene, and Binding Site. J. Bladé has received honoraria for lectures and advisory boards from Janssen and Celgene and grant support from Janssen. J.-J.L. has received honoraria from Janssen and Celgene. J.F.S.-M. has received honoraria from Janssen, Millennium, and Celgene. The remaining authors declare no competing financial interests.
Correspondence: María-Victoria Mateos, University Hospital of Salamanca/IBSAL, Paseo San Vicente, 58-182, 37007 Salamanca, Spain; e-mail: mvmateos@usal.es.