Key Points
AMCs home to tumor sites in MM.
CXCR7 inhibition delayed tumor progression in MM through specific regulation of AMC trafficking but not through a direct tumor effect.
Abstract
The CXCR4/stromal cell-derived factor-1 (SDF-1) axis is essential for cell trafficking and has been shown to regulate tumor progression and metastasis in many tumors including multiple myeloma (MM). A second chemokine receptor for SDF-1, CXCR7 was discovered recently and found on activated endothelial cells. We examined the role of CXCR7 in angiogenic mononuclear cells (AMCs) trafficking in MM. Our data demonstrate that AMCs are circulating in patients with MM and in vivo studies show that they specifically home to areas of MM tumor growth. CXCR7 expression is important for regulating trafficking and homing of AMCs into areas of MM tumor growth and neoangiogenesis. We demonstrate that the CXCR7 inhibitor, POL6926, abrogated trafficking of AMCs to areas of MM tumor progression leading to a significant inhibition of tumor progression. These effects were through regulation of endothelial cells and not through a direct tumor effect, indicating that targeting a bone marrow microenvironmental cell can lead to a delay in MM tumor progression. In conclusion, our studies demonstrate that CXCR7 may play an important role in the regulation of tumor progression in MM through an indirect effect on the recruitment of AMCs to areas of MM tumor growth in the bone marrow niche.
Introduction
Multiple myeloma (MM) is a plasma cell malignancy that depends on interactions with the bone marrow (BM) microenvironment for growth and survival.1 In turn, adhesion of MM cells to the BM microenvironment provides a mechanism of resistance to standard chemotherapeutic agents.2-4 Angiogenesis is a hallmark of progression in MM, and many studies have shown that angiogenesis should be considered as a therapeutic target in MM.5 Angiogenic mononuclear cells (AMCs) have been shown in solid tumors to play an essential role in tumor progression by secretion of proangiogenic growth factors,6 and by direct luminal incorporation into sprouting vessels.7 These cells migrate from the BM to the tumor site through a highly regulated process involving chemotaxis, adhesion, and invasion.8 The BM microenvironment in MM is characterized by an increased microvessel density and increased secretion of angiogenic factors.
The CXCR4/CXCL12 (stromal cell-derived factor-1 [SDF-1]) axis is essential for cell trafficking and has been shown to regulate tumor progression and metastasis in many tumors including MM.9 It has been previously shown that MM cells are more sensitive to chemotherapy after disrupting their adhesion using a selective CXCR4 antagonist.10 A second chemokine receptor for SDF-1, CXCR7, was discovered recently.11,12 This receptor was previously classified as the orphan G-protein coupled receptor, RDC1.13,14 It was shown that CXCR7 has two chemokine ligands, SDF-1 and CXCL11, and that CXCR7 binds SDF-1 10- to 20-fold greater than CXCL11.12 In landmark studies, CXCR7 surface expression was found on a number of transformed human and mouse cell lines, in addition to activated endothelial cells and embryonic fetal liver cells. Importantly, CXCR7 surface expression was not seen on normal nontransformed tissues despite the presence of CXCR7 messenger RNA.12 CXCR7 was found to form functional heterodimers with CXCR4 and enhanced CXCL12-induced signaling. The data also strongly suggested a specialized role for CXCR7 in endothelial biology.15 There is mounting evidence that CXCR7 itself plays a vital role in cell adhesion, survival, and tumor growth, as validated by recent in vitro and in vivo studies. Miao et al16 showed that CXCR7 overexpression, independent of CXCR4, promoted tumor growth in breast and lung cancer mouse models. These effects were abrogated by CXCR7 knockdown.16 Taken together, these findings provide a strong rationale for studying the role of CXCR7 in MM. CXCR7 was recently shown to play a key role in AMC trafficking17 and angiogenesis.18
In this study, we show for the first time that AMCs circulate in patients with MM, and specifically, home to areas of MM tumor growth. We also demonstrate that CXCR7 expression on AMCs is important for regulating trafficking and homing of AMCs into areas of MM tumor growth and neoangiogenesis. Inhibition of CXCR7 delays tumor progression through specific regulation of AMC trafficking and angiogenesis, and not through a direct tumor effect.
Methods
Cells
MM cell lines (MM1.S, U266, RPMI, OPM-1, and OPM-2) were used in this study. The MM1.S cell line was purchased from ATCC (Manassas, VA), while the OPM-1 and H929 cell lines were the kind gift from Prof Jesús F. San Miguel (Salamanca, Spain). All cell lines were cultured in RPMI-1640 containing 10% fetal bovine serum (Sigma-Aldrich, St. Louis, MO), 2 mM l-glutamine, 100 U/mL penicillin, and 100 µg/mL streptomycin (GIBCO, Grand Island, NY). The human umbilical vein endothelial cells (HUVECs) (Lonza, Walkersville, MD) were cultured in EGM-2 media (Lonza) and reconstituted according to the manufacturer’s instructions. MM patient samples were obtained after approval from the Dana-Farber Cancer Institute’s Institutional Review Board. Informed consent was obtained from all patients in accordance with the Declaration of Helsinki. Mononuclear cells (MNCs) from the BM and peripheral blood (PB) of MM patients and healthy subjects were obtained by Ficoll (Sigma-Aldrich, St. Louis, MO) gradient centrifugation, as previously described.10 Primary MM cells were obtained using CD138+ micro-bead selection (Miltenyi Biotec, Auburn, CA).
Reagents
The CXCR7 inhibitor, POL6926, a potent and selective protein epitope mimetic was obtained from Polyphor (Basel, Switzerland). It was synthesized with standard fluorenylmethoxycarbonyl solid-phase strategy on chlorotrityl chloride resin. The peptide, as final form in acetate salt, was purified by preparative reverse phase high-performance liquid chromatography and was recovered by lyophilisation.19 Monoclonal antibodies for flow cytometry were obtained from BD Biosciences (San Jose, CA). Calcein-AM cell labeling dye was obtained from Invitrogen (Carlsbad, CA).
Detection of presence of AMCs in BM and PB, and expression of CXCR7
MNCs from PB of MM patients and normal subjects (n = 20 and 5, respectively), MNCs from BM of MM patients and normal subjects (n = 5 and 5, respectively); MNCs from BM and PB (n = 5 and 5, respectively) of severe combined immunodeficiency (SCID) mice (which were injected with 2 × 106 MM1.S cells or vehicle, and tumor growth was allowed for 4 weeks, n = 5 for each group), and HUVECs were stained with fluorescein isothiocyanate-anti-CD31 (BD Biosciences), PE-Cy7-anti-CD34 (BD Biosciences), and anti-CXCR7 (ab72100; Abcam, Cambridge, MA) antibodies for 1 hour, followed by a secondary Alexa Fluor 647–conjugated ab (a21244; Invitrogen, Grand Island, NY) for 30 minutes on ice, and analyzed by flow cytometry for presence of AMCs and the expression of CXCR7 on a FACSCanto II flow cytometry system (BD Biosciences).
Moreover, MM cell lines were tested for expression of surface and intracellular CXCR7 expression. For surface expression, MM cells were stained with anti-CXCR7 antibodies for 1 hour, followed by a secondary Alexa Fluor 647–conjugated ab (a21244; Invitrogen) for 30 minutes on ice and analyzed by flow cytometry for expression of CXCR7. For intracellular expression, MM cells were fixed and permeabilized using Cytofix/Cytoperm Kit (BD Biosciences), according to the manufacturer’s instructions, and cells were then stained with anti-CXCR7 ab for 1 hour, followed by a secondary Alexa Fluor 647–conjugated ab (a21244; Invitrogen) for 30 minutes on ice and analyzed by flow cytometry.
Migration in vitro
Migration was performed in transwell migration assay, as previously described.20 Briefly, HUVECs (5 × 106/mL) were placed in the upper chamber, while the lower chamber had BM supernatant from MM patients or normal subjects; RPMI media or conditioned media (CM) from cultures of MM1.S, OPM-1, and OPM-2 cells lines and migrating cells in the lower chamber were counted by flow cytometry. In some cases, HUVECs were pretreated with POL6926 for 3 hours before the migration assay. Moreover, after the completion of the migration assay, the membrane of the upper chamber was isolated, washed, and fixed, and the lower side was imaged by a fluorescent microscope.
Proliferation assay
MM1.S, OPM-2, and HUVECs were treated with increasing concentrations (0-500 nM) of POL6926 for 24 hours, and analyzed by BrdU assay, as previously described.21 In some cases, MM cells were cocultured with endothelial cells in the presence or absence of POL6926 50 nM.
Tube formation assay
HUVECs were cultured in the presence or absence of MM1.S CM, MM1.S cells, and POL6926 on Matrigel for 8 hours, and tube formation was quantified by light microscopy, as previously described.22
Specific homing of HUVECs to MM tumors in vivo
MM1.S-GFP-Luc (0.5 × 106) cells were injected to the right tibia, while vehicle was injected to the left tibia of 6 SCID mice, as previously described.21 RFP-HUVECs (0.2 × 106) were injected intravenously to each of 3 mice at days 7 and 14; the next day, mice were euthanized and their tibias were collected, and then BM was analyzed by flow cytometry for the presence of MM cells (GFP+) and HUVECs (RFP+). The Dana-Farber Cancer Institute’s and the Massachusetts General Hospital’s Institutional Animal Care and Use Committees approved the animal experiments.
Effect of POL6926 on homing of HUVECs to MM tumors in vivo
MM1.S-GFP-Luc (2 × 106) cells were injected intravenously to 6 SCID mice and allowed to grow for 4 weeks. RFP-HUVECs were treated ex vivo in the presence or absence of 50 nM POL6926 for 3 hours and cells (0.2 × 106/mouse) were injected to 3 mice in each group. The mice were euthanized 24-hours after injection, their femurs collected, and their BM analyzed by flow cytometry for the presence of MM cells (GFP+) and HUVECs (RFP+).
Effect of POL6926 on number of AMCs in circulation
BALB/c mice were implanted with ALZET-pumps 1002 (each loaded with 3.036 mg and replaced after 2 weeks), 2002 (each loaded with 3.036 mg and replaced after 2 weeks), and 2004 (each loaded with 6.072 mg). Each type was implanted into 12 mice. Three mice were euthanized and blood was drawn at 0, 7, 14, 21, and 28 days, and circulating AMCs were analyzed by flow cytometry.
Effect of POL6926 on MM tumor growth
SCID mice were injected intravenously with MM1.S-GFP-Luc cells. Bioluminescence imaging (BLI) was done twice weekly for 21 days. Mice with similar tumor burden were divided into 2 groups and implanted with ALZET-pumps 2004 loaded with 0 or 6.072 mg of POL6926. Tumor growth was monitored weekly for 3 weeks by BLI.
β-Arrestin assay
A total of 3 × 103 CHO-CXCR7-βgal1:β-arrestin2-βgal2 (CHO-CXCR7) (DiscoveRX 93-0248P2) in 20 μl were seeded into 384 well plates and cultured overnight. The next day, 5 μl of the compounds at varying concentrations of ligands was added to the wells, and the plates were incubated in 5% CO2 at 37°C. After 90 minutes, 15 μl of β-gal substrate (DiscoveRX) was added to the wells and the plates were incubated at room temperature. After 90 minutes, light emission was analyzed in a Victor 2 V plate reader (Wallac/PerkinElmer).
Pharmacokinetic analysis of POL6926
Samples (50 µL) from all experiments with administration of POL6926 in vivo were spiked with an internal standard, extracted with acetonitrile-2% formic acid, supernatants were evaporated and reconstituted in ACN/DMSO/H2O-50/45/5+2% formic acid, and POL6926 analyzed using high-performance liquid chromatography (ACQUITY UPLC BEH C18, 100 × 2.1 mm, 1.7 µm; Waters) coupled to mass spectrometry detection (4000 QTRAP mass spectrometer; AB Sciex).
Immunoblotting
HUVECs were cultured for 24 hours before the experiment in 6-well plates at 5 × 104 cells/well, washed with phosphate-buffered saline and treated with POL6926 (0 or 50 nM) for 6 hours, and MM.1S cells were then applied for 1 hour. Nonactivated HUVECs (monoculture) were used as a negative control. After coculture, MM cells were separated from HUVECs using gentle pipetting, and HUVECs were washed with ice-cold phosphate-buffered saline, lysed, and protein concentration was normalized. Proteins were blotted using 8% to 12% acrylamide gels and transferred to a nitrocellulose membrane; membranes were blocked with 5% nonfat dry milk in tris-buffered saline/T-buffer, and incubated with primary antibodies for p-FAK, p-p130, p-Akt, pPI3K-85, phosphorylated extracellular signal-regulated kinase (pERK), and/or α-tubulin overnight at 4°C. The membranes were then washed, incubated with appropriate horseradish peroxidase-conjugated secondary antibody, washed, and developed using luminol-base assay. Luminescence was measured using radiograph films.
Results
MM-derived AMCs present with higher expression of CXCR7 in MM compared with healthy individuals
We first examined the number of circulating AMCs and CXCR7 expression on AMCs obtained from both MM patients and MM-harboring mice. Figure 1A shows a representative analysis of AMCs by flow cytometry, where AMCs were defined as CD31+/CD34+ as previously described.23-26 We found that the presence of AMCs was significantly higher in the BM and PB of MM patients compared with healthy subjects (Figure 1B), The number of AMCs in MM were three and sixfold higher than controls in BM and PB, respectively. Similar results were obtained in the BM and PB of mice engrafted with MM cells compared with control mice that were not engrafted with human MM cells. Here, the number of AMCs in MM mice was eight and sevenfold higher than control mice in BM and PB, respectively (Figure 1C). We further tested the expression of CXCR7 on the surface of AMCs and compared it to the expression levels on MNCs in BM and PB of MM patients and healthy subjects. We found that AMCs had a high expression of CXCR7 compared with the rest of the MNCs in both BM and PB. We also found that the surface expression of CXCR7 in AMCs in the BM was higher than the expression in the PB, whereas there was no difference in CXCR7 expression between AMCs isolated from MM patients or from healthy subjects (Figure 1D) suggesting that disease status does not change CXCR7 surface expression on AMCs.
AMCs are more prominent in MM BM and PB and express high CXCR7. (A) Representative images for gating of AMCs (CD31+/CD34+) and MNCs (CD31−/CD34−) populations (left), and diagram of expression of CXCR7 on each of these populations (right). (B) Presence of AMCs expressed as a percentage of total MNCs in the BM and PB of MM patients and normal subjects. (C) Presence of AMCs expressed as a percentage of total MNCs in the BM and PB of mice with and without MM1.S xenografts. (D) The expression of CXCR7 on AMCs from BM and PB isolated from MM patients and healthy subjects, normalized to mean fluorescence intensity of MNCs in each sample.
AMCs are more prominent in MM BM and PB and express high CXCR7. (A) Representative images for gating of AMCs (CD31+/CD34+) and MNCs (CD31−/CD34−) populations (left), and diagram of expression of CXCR7 on each of these populations (right). (B) Presence of AMCs expressed as a percentage of total MNCs in the BM and PB of MM patients and normal subjects. (C) Presence of AMCs expressed as a percentage of total MNCs in the BM and PB of mice with and without MM1.S xenografts. (D) The expression of CXCR7 on AMCs from BM and PB isolated from MM patients and healthy subjects, normalized to mean fluorescence intensity of MNCs in each sample.
AMCs home to MM-enriched BM niches
Because of their angiogenic cell-like features, we used HUVECs as representative cells for angiogenic cells in our models. We found that HUVECs have high surface expression of both CD31+ and CD34+, as well as high expression of CXCR7 (Figure 2A). These cells were found to migrate to BM-supernatant from MM patients with a threefold higher rate of migration compared with BM-supernatant from normal subjects (Figure 2B). These results were confirmed in vitro, in which HUVECs migrated to MM cell-line (MM1.S, OPM-1, and OPM-2) CM about twofold higher than non-CM (Figure 2C). To test the specificity of the homing of AMCs to MM tumors in vivo, we injected MM cells in the right tibia and vehicle control in the left tibia, and MM cells were allowed to grow for 2 weeks. Then, HUVECs were injected into the mice at 1 and 2 weeks after the injection of MM cells, and the homing of HUVECs to the right and left tibias, as well as the MM-cell percentage in the BM were analyzed 24-hours after injection of the HUVECs (see Figure 2D for a schematic description of the procedure). The growth of MM cells was higher in the right tibia at 1 and 2 weeks compared with the left tibia, indicating specific tumor growth, while some MM growth was observed in the left tibia at 2 weeks but to a lower extent compared with the right tibia (Figure 2E). HUVECs homed to the right tibia (that had more MM cells) about 2.5-fold higher compared with homing to the left tibia (Figure 2F), suggesting that AMCs home specifically to areas of MM tumor growth.
AMCs home specifically to MM-enriched BM niches. (A) Expression of CD31, CD34, and CXCR7 on HUVECs, as a model for AMCs. (B) Migration of HUVECs to BM supernatant from normal subjects and MM patients normalized to the average of migration of normal subjects. (C) Migration of HUVEC cells to conditioned culture media from MM cell lines that were incubated in the media for 24 hours before the migration test, normalized to migration to nonconditioned culture media. (D) A schematic description of the procedure. (E) MM tumor progression in the right and left tibias after intratibial injection of MM1.S cells into the right tibia. (F) Differential homing of HUVECs to tibias with higher involvement of MM after IV injection.
AMCs home specifically to MM-enriched BM niches. (A) Expression of CD31, CD34, and CXCR7 on HUVECs, as a model for AMCs. (B) Migration of HUVECs to BM supernatant from normal subjects and MM patients normalized to the average of migration of normal subjects. (C) Migration of HUVEC cells to conditioned culture media from MM cell lines that were incubated in the media for 24 hours before the migration test, normalized to migration to nonconditioned culture media. (D) A schematic description of the procedure. (E) MM tumor progression in the right and left tibias after intratibial injection of MM1.S cells into the right tibia. (F) Differential homing of HUVECs to tibias with higher involvement of MM after IV injection.
CXCR7 inhibition decreases the number of AMCs in circulation
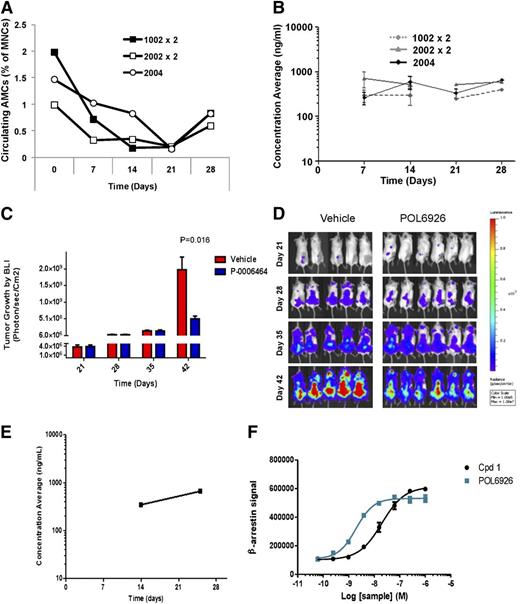
To study the role of CXCR7 in regulating endothelial cell trafficking, we examined the effect of the specific CXCR7 inhibitor POL6926, a protein epitope mimetic, on the circulating number of AMCs in healthy SCID-bg mice, and found that a continuous inhibition of CXCR7 delivery of POL6926 with osmotic pumps over 4 weeks (4 mg/kg per day [subcutaneous]) showed a significant decrease in the number of circulating AMCs in mice over 4 weeks. The 3 different types of pumps (1002, 2002, and 2004) showed similar patterns of reduction of the AMC numbers (Figure 3A). Pharmacokinetic analysis showed that, as expected, the concentration of POL6926 corresponded to a plateau around 300 nM for the 3 different types of pumps over the course of treatment (4 weeks) (Figure 3B). These studies indicate that continuous CXCR7 inhibition inhibits cell trafficking of AMCs.
Inhibition of CXCR7 prevents the AMC-mediated MM tumor growth. (A) Number of circulating AMCs at 0, 7, 14, 21, and 28 days after subcutaneous implantation of ALZET osmotic pumps 1002 (replaced at day 14), 2002 (replaced at day 14), and 2004 loaded with POL9626 in healthy SCID-bg mice. (B) Pharmacokinetic analysis of the levels of POL9626 in plasma over 4 weeks after implantation of the different pumps. The effect of POL9626 (delivered by 2004 osmotic pump) on MM tumor growth by BLI as detected at 28, 35, and 42 days after injection of MM cells; as shown in quantification of BLI (C) and images (D). (E) Pharmacokinetic analysis of the levels of POL9626 in plasma of the treatment group. (F) Compound 1 and POL6926 triggered association of β-arrestin with CXCR7 in a concentration-dependent manner.
Inhibition of CXCR7 prevents the AMC-mediated MM tumor growth. (A) Number of circulating AMCs at 0, 7, 14, 21, and 28 days after subcutaneous implantation of ALZET osmotic pumps 1002 (replaced at day 14), 2002 (replaced at day 14), and 2004 loaded with POL9626 in healthy SCID-bg mice. (B) Pharmacokinetic analysis of the levels of POL9626 in plasma over 4 weeks after implantation of the different pumps. The effect of POL9626 (delivered by 2004 osmotic pump) on MM tumor growth by BLI as detected at 28, 35, and 42 days after injection of MM cells; as shown in quantification of BLI (C) and images (D). (E) Pharmacokinetic analysis of the levels of POL9626 in plasma of the treatment group. (F) Compound 1 and POL6926 triggered association of β-arrestin with CXCR7 in a concentration-dependent manner.
CXCR7 inhibition decreases AMC-enhanced MM tumor growth
To study the role of AMCs in enhancing MM tumor growth and the role of CXCR7 in this process, mice were injected intravenously with MM1.S-GFP-Luc cells (3 × 106) and randomized into 2 groups with the same mean BLI after 21 days. Then, mice were treated with POL6926 by subcutaneous implantation of osmotic pumps (2004) and each mouse was delivered 4 mg/kg per day for 21 days; vehicle loaded pumps were implanted in a different group as a control. Mice treated with the CXCR7 inhibitor demonstrated a significant delay in tumor progression compared with the vehicle control group, as shown in quantification of BLI (Figure 3C) and in representative images of the BLI (Figure 3D). Analysis of the blood concentration of POL6926 over the treatment period showed that blood levels were well-above the concentration needed for inhibition of CXCR7 (Figure 3E). POL6926 potentially targets CXCR7 by recruiting β-arrestin with an EC50 of 4.6 nM without significantly modulating the activity of CXCR4 up to 10 μM (Figure 3F).
CXCR7 blockade exerts indirect inhibition of MM
To investigate the detection of CXCR7 expression in MM, flow cytometry was performed on a panel of MM cell lines showing lack of CXCR7 surface expression, and low CXCR7 intracellular expression (Figure 4A). Moreover, the effect of CXCR7 inhibition on MM was tested with POL6929, which showed no effect on proliferation of MM cells in vitro (Figure 4B). Similarly, POL6929 did not modulate the proliferation rate of HUVECs (Figure 4B). In contrast, the compound inhibited the proliferation of MM cells cultured in presence of HUVECs, suggesting its ability to overcome HUVEC-dependent growth advantage on MM cells (Figure 4C). Given that POL6926 does not inhibit the proliferation of tumor cells or HUVECs, we hypothesized that this in vivo inhibition of proliferation effect observed in Figure 3D is due to a specific effect of this agent on cell trafficking of AMCs into areas of MM proliferation.
Inhibition of CXCR7 exerts indirect inhibition of MM. (A) The expression of CXCR7 on the surface (upper panel) and intracellular (lower panel) of MM cell lines (MM1.S, OPM-1, OPM-2, H929, RPMI, U266, MM1R, and MOLP8). (B) The effect of a range of concentrations (0-500 nM) of POL9626 on proliferation of MM1.S, OPM-2, and HUVECs when each was cultured alone. (C) The effect of POL9626 (50 nM) on MM tumor proliferation induced by coculture with HUVECs.
Inhibition of CXCR7 exerts indirect inhibition of MM. (A) The expression of CXCR7 on the surface (upper panel) and intracellular (lower panel) of MM cell lines (MM1.S, OPM-1, OPM-2, H929, RPMI, U266, MM1R, and MOLP8). (B) The effect of a range of concentrations (0-500 nM) of POL9626 on proliferation of MM1.S, OPM-2, and HUVECs when each was cultured alone. (C) The effect of POL9626 (50 nM) on MM tumor proliferation induced by coculture with HUVECs.
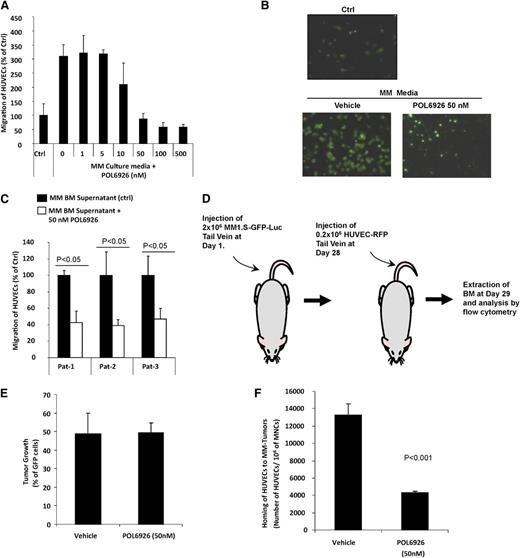
CXCR7 inhibition modulates homing of AMCs to MM-enriched BM niches
Prior studies have shown that trafficking of AMCs enhance tumor growth8 ; therefore, we sought to examine the role of AMCs in their specific homing into areas of MM tumor growth as a mechanism for the inhibition of tumor growth observed in our in vivo mouse model. Based on this hypothesis, we first examined the effect of POL6926 on the migration of HUVECs to CM from MM1.S cell line, and found that it inhibited the migration of HUVECs in a dose-dependent manner, with almost full abrogation of the migration at 50 nM (Figure 5A). These results were confirmed by fluorescent imaging of the lower side of the migration filter, which showed similar results of inhibition of the migration of HUVEC at 50 nM of POL6926 (Figure 5B). Similarly, POL6926 inhibited the migration of HUVECs to BM supernatant from 3 different MM patients to less than 40% of the nontreated cells (Figure 5C).
Inhibition of CXCR7 disrupts the homing of AMCs to MM-enriched BM niches. (A) The effect of increasing concentrations (0-500 nM) of the CXCR7 inhibitor POL6926 on migration of HUVECs to CM from MM1.S cells (24 hours incubation), normalized to the migration to non-CM as counted by flow cytometry. (B) Representative images of the effect of POL6926 (50 nM) on migration of AMCs to conditioned MM1.S media as detected by fluorescent microscopy. (C) The effect of POL6926 (50 nM) on migration of HUVECs to BM supernatant from 3 MM patients, counted by flow cytometry and normalized to migration of nontreated HUVECs in each. (D) A schematic description of the procedure. (E) The MM tumor burden of the 2 groups of MM-bearing mice. (F) The effect of CXCR7 on the homing of HUVECs, after IV injection to the BM of mice with established xenografts of MM1.S cells.
Inhibition of CXCR7 disrupts the homing of AMCs to MM-enriched BM niches. (A) The effect of increasing concentrations (0-500 nM) of the CXCR7 inhibitor POL6926 on migration of HUVECs to CM from MM1.S cells (24 hours incubation), normalized to the migration to non-CM as counted by flow cytometry. (B) Representative images of the effect of POL6926 (50 nM) on migration of AMCs to conditioned MM1.S media as detected by fluorescent microscopy. (C) The effect of POL6926 (50 nM) on migration of HUVECs to BM supernatant from 3 MM patients, counted by flow cytometry and normalized to migration of nontreated HUVECs in each. (D) A schematic description of the procedure. (E) The MM tumor burden of the 2 groups of MM-bearing mice. (F) The effect of CXCR7 on the homing of HUVECs, after IV injection to the BM of mice with established xenografts of MM1.S cells.
To further confirm the role of CXCR7 in modulating the homing of AMCs to MM-colonized BM niches, POL6926 was tested in vivo to examine its activity on delaying/preventing specific homing of endothelial cells to areas of MM tumor growth. First, MM cells were allowed to grow for 4 weeks in SCID-bg mice by intravenous injection of GFP-Luc+MM1S (2 × 106) and monitoring of tumor growth using BLI for 4 weeks. HUVECs were then treated in vitro in presence or absence of POL6926 (50 nM) for 3 hours, washed, and injected intravenously into the mice, and the homing of HUVECs to the BM was analyzed after 24 hours using flow cytometry. The schematic description of the procedure is shown in Figure 5D. The MM tumor burden in the BM of the 2 groups was found to be similar in the 2 groups of MM bearing animals (Figure 5E). Homing of POL6926-treated HUVECs to the MM tumor regions was significantly lower compared with the nontreated group (Figure 5F). These results indicate that CXCR7 plays an important role in the cell trafficking and homing of AMCs to MM tumors. Therefore, based on this data, we postulate that the mechanism by which the CXCR7 inhibitor decreased tumor growth in the MM tumor model is due to decreased cell trafficking and an inhibition of specific homing of AMCs to areas of MM tumor growth, leading to a delay in tumor progression.
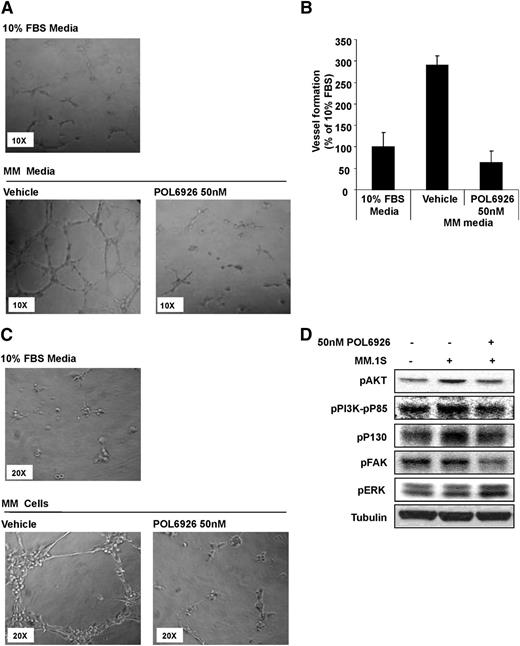
CXCR7 regulates endothelial vessel formation and angiogenesis
To further confirm that neoangiogenesis within the BM is the major mechanism by which CXCR7 inhibition regulates tumor growth, we examined the effect of CXCR7 inhibition on vessel formation of HUVECs. We tested the effect of MM cell-derived CM on tube formation of HUVECs, using Matrigel, and found that MM CM induced a significant increase in tube formation, whereas the effect was abolished by the treatment of HUVECs with the CXCR7 inhibitor, POL6929 (Figure 6A). Relative quantification of tube length formation is provided in Figure 6B. We next evaluated MM cells dependent ability to increase tube formation in presence or absence of POL6929, and found that the compound was able to inhibit MM-cell–induced tube formation of HUVECs (Figure 6C). Activation of HUVECs with MM cells increased the proliferative (pPI3K-P85 and pAKT) and cytoskeletal (pP130 and pFAK) pathways in HUVECs, whereas the inhibition of CXCR7 with POL6926 decreased this activation of these pathways. In addition, CXCR7-inhibition increased the phosphorylation of ERK (Figure 6D).
Inhibition of CXCR7 reverses tube formation induced by MM. (A) Representative images of the effect of POL9626 (50 nM) on the HUVEC tube formation induced by CM from MM1.S cells (non-CM was used a control). (B) Quantification of the length of tubes formed due to MM CM with or without the presence of POL9626 (50 nM), normalized to the length of tubes formed in non-CM. (C) Representative images of the effect of POL9626 (50 nM) on the tube formation of HUVECs induced by coculture with MM1.S cells, and the direct cell-adhesion of MM cells to the formed tubes. (D) Following activation of HUVECs with MM cells, the effect of CXCR7 inhibition on downstream signaling in HUVECells was shown by immunoblotting.
Inhibition of CXCR7 reverses tube formation induced by MM. (A) Representative images of the effect of POL9626 (50 nM) on the HUVEC tube formation induced by CM from MM1.S cells (non-CM was used a control). (B) Quantification of the length of tubes formed due to MM CM with or without the presence of POL9626 (50 nM), normalized to the length of tubes formed in non-CM. (C) Representative images of the effect of POL9626 (50 nM) on the tube formation of HUVECs induced by coculture with MM1.S cells, and the direct cell-adhesion of MM cells to the formed tubes. (D) Following activation of HUVECs with MM cells, the effect of CXCR7 inhibition on downstream signaling in HUVECells was shown by immunoblotting.
In summary, these studies demonstrate that AMCs play a critical role in tumor progression in MM and that CXCR7 expression on AMCs is important for regulating trafficking and homing of AMCs into areas of MM tumor growth and neoangiogenesis. This indicates that inhibition of CXCR7 delays tumor progression through the specific regulation of AMC trafficking and angiogenesis, and not through a direct tumor effect.
Discussion
Recent studies have shown that the scavenging receptor CXCR7 enhances metastasis complementary to CXCR4.27 Treatment with a small-molecule inhibitor of CXCR7 chemokine limited the growth of CXCR4+ breast cancer cells in tumors that also contained malignant CXCR7+ cells.28 In addition, upregulation of CXCR7 in gastric mucosa-associated lymphoid tissue lymphomas has been associated with progression to diffuse large B-cell lymphoma, indicating that this chemokine receptor is also critical in tumor progression in B-cell malignancies.29 However, its role in tumor progression in MM has not been previously examined. Our study demonstrates that CXCR7 has a role in tumor progression and cell dissemination in MM not through a direct activity of the CXCR7 receptor in MM cells, but rather through indirect recruitment and activity of AMCs and vasculogenesis in MM. This is consistent with plasma cell biology of CXCR7 null mice where there is little effect on B-cell composition.15
Prior studies have shown that angiogenesis is critical during tumor progression in MM with an increase in mean vessel density in the BM of patients with MM during tumor progression.30-32 Endothelial progenitor cells have been examined in 31 MM patients and were shown to increase in a number of patients with active disease compared with healthy control. Together, these studies indicate that angiogenesis and endothelial cells play an important role in tumor progression in MM. However, the exact mechanisms of regulation of these cells and whether targeting them with a therapeutic agent can have an effect in delaying tumor progression have not been previously examined.
The precise functional role of CXCR7 has remained elusive. While Burns et al12 confirmed that the SDF-1 ligand (CXCL12) binds to CXCR7 with high affinity, it was not found to induce calcium mobilization or cell migration as expected for a G-protein–coupled chemokine receptor. The role of CXCR7 as a partner (or interceptor) for CXCR4 is also being elucidated. Balabanian et al11 found that blocking both CXCR4 and CXCR7 resulted in an additive inhibitory effect on T-cell migration mediated by SDF-1. This suggested that the 2 chemokine receptors may act together. However, another possibility proposed by Sierro et al15 is that CXCR7 may not function alone but may modulate CXCR4 function via heterodimerization. In support of this, preformed dimers of CXCR4/7 were seen on the surface of HEK293-transfected cells by false-color merged image. In turn, Ca+ flux was enhanced with CXCR4/7 coexpressed cells. Coexpression also affected pERK signaling pattern, resulting in delayed but sustained pERK activation compared with singly expressing CXCR4 cells.15 Although it was shown that CXCR7 forms heterodimers with CXCR4 in vitro, heterodimerization in vivo remains to be demonstrated.33 Recently, Mazzinghi et al34 showed that CXCR4 and CXCR7 have differential roles in the homing of human renal progenitor cells. SDF-1–induced migration of renal progenitor cells was dependent only on CXCR4, while trans-endothelial migration required both CXCR4 and CXCR7.34 Interestingly, POL6926 and other recently published CXCR7 targeting agents (eg, “Compound 1”35 and CXCR7 modulators from ChemoCentryx, Inc.), act as a CXCR7 agonist for the β-arrestin recruitment36 and were described as functional antagonists35,37 by scavenging CXCL12 and negatively regulating CXCL12 functions, suggesting that CXCR7 agonists may have a therapeutic benefit in pathological conditions where CXCL12 is involved.
In this study, we found that the level of AMCs was elevated in the PB and BM of MM patients compared with normal subjects, a finding that was confirmed in a MM mouse model in which CXCR7 was highly expressed on AMCs. Injection of a specific CXCR7 antagonist for a prolonged period of time (4 weeks) decreased the numbers of AMCs in the PB. Moreover, in vitro and in vivo studies confirmed that the CXCR7 inhibitor POL6926, abrogated trafficking of AMCs to areas of MM tumor progression leading to a significant inhibition of tumor progression in MM. These results demonstrate that CXCR7 has a role in the cell-trafficking and recruitment of AMCs in MM. Our results also indicate that targeting a BM microenvironmental cell (and not direct inhibition of the tumor clone) can lead to a delay in MM tumor progression. Although prior studies have shown that antiangiogenic drugs had limited activity on tumor growth in MM,38,39 our studies suggest that targeting AMCs before their recruitment into areas of tumor progression may provide a window of opportunity for targeted antiangiogenic therapy, or AMC-targeted therapy that delays/prevents tumor progression. Further studies into the mechanisms of AMC-mediated tumor progression in MM and their specific role in inducing tumor progression are warranted.
The role of CXCR7 antagonists remains to be elucidated in patients with hematologic malignancies, specifically in MM. CXCR4 inhibitors have been used as stem-cell mobilizers or as chemosensitizers in blood cancers, including MM.40-42 Based on our preclinical studies, we believe that CXCR7 inhibitors are critical for regulating the interaction of endothelial cells with tumor cells and preventing ongoing angiogenic signals that lead to tumor progression and dissemination. Therefore, early interventions with CXCR7 inhibitors in patients with precursor conditions such as smoldering myeloma, or in minimal residual disease states posttransplant, are likely to be the most critical time points for therapeutic interventions with agents that disrupt the dependency of tumor cells on microenvironmental cells. Most importantly, we do not believe that agents such as CXCR7 inhibitors should be used as single agents in cases where the tumor cells are not dependent on the BM microenvironment, as in extramedullary involvement or plasma cell leukemia.
In conclusion, our studies demonstrate that CXCR7 is a crucial regulator of tumor progression in MM through an indirect effect on the recruitment of AMCs to areas of MM tumor growth in the BM niche.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported in part by the National Cancer Institute (R01CA154648 and R01CA125690), and the Leukemia and Lymphoma Society.
Authorship
Contribution: A.K.A. and I.S. performed research, designed research, analyzed data, and wrote the manuscript; F.A., M.M., Y.M., A.M.R., and N.B. performed research and analyzed data; K.P., B.R., J.Z., and E.C. provided the CXCR7 inhibitor, pharmacokinetic studies, analyzed data, and revised the manuscript; I.M.G. designed research, revised the manuscript, and supervised the study.
Conflict-of-interest disclosure: I.M.G. is on the advisory board for Onyx, Millennium, BMS, and Celgene, and receives research laboratory support from Genzyme/Takeda, BMS, and Noxxon. K.P., B.R., J.Z., and E.C. are employees of Polyphor, Ltd. The remaining authors declare no competing financial interests.
Correspondence: Irene M. Ghobrial, Department of Medical Oncology, Dana-Farber Cancer Institute, Harvard Medical School, 450 Brookline Ave Boston, MA 02115; e-mail: irene_ghobrial@dfci.harvard.edu.
References
Author notes
A.K.A. and I.S. contributed equally to this study.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal