Abstract
Src family kinases (SFKs) play a central role in mediating the rapid response of platelets to vascular injury. They transmit activation signals from a diverse repertoire of platelet surface receptors, including the integrin αIIbβ3, the immunoreceptor tyrosine–based activation motif–containing collagen receptor complex GPVI-FcR γ-chain, and the von Willebrand factor receptor complex GPIb-IX-V, which are essential for thrombus growth and stability. Ligand-mediated clustering of these receptors triggers an increase in SFK activity and downstream tyrosine phosphorylation of enzymes, adaptors, and cytoskeletal proteins that collectively propagate the signal and coordinate platelet activation. A growing body of evidence has established that SFKs also contribute to Gq- and Gi-coupled receptor signaling that synergizes with primary activation signals to maximally activate platelets and render them prothrombotic. Interestingly, SFKs concomitantly activate inhibitory pathways that limit platelet activation and thrombus size. In this review, we discuss past discoveries that laid the foundation for this fundamental area of platelet signal transduction, recent progress in our understanding of the distinct and overlapping functions of SFKs in platelets, and new avenues of research into mechanisms of SFK regulation. We also highlight the thrombotic and hemostatic consequences of targeting platelet SFKs.
Src family kinases: critical initiators of platelet activation
Platelets are highly reactive fragments of megakaryocytes that rapidly adhere to sites of vascular injury and nucleate formation of thrombi that prevent excessive blood loss. They have also been implicated in maintenance of vascular integrity, the inflammatory response, and blood-lymphatic vessel separation. Platelets can also have detrimental effects on health, forming life-threatening thrombi on ruptured atherosclerotic plaques that culminate in myocardial infarction and stroke. Conversely, reduced platelet counts and reactivity can predispose to bleeding. Although highly effective in preventing thrombosis, current antiplatelet therapies have their limitations, including increased risk of bleeding and resistance in some patients. Moreover, antiplatelet drugs are contraindicated in conditions such as hemorrhagic stroke, for which there are currently no effective therapies. Thus, understanding the molecular basis of platelet activation has important clinical implications.
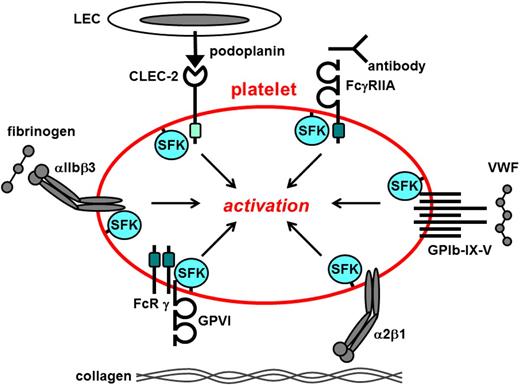
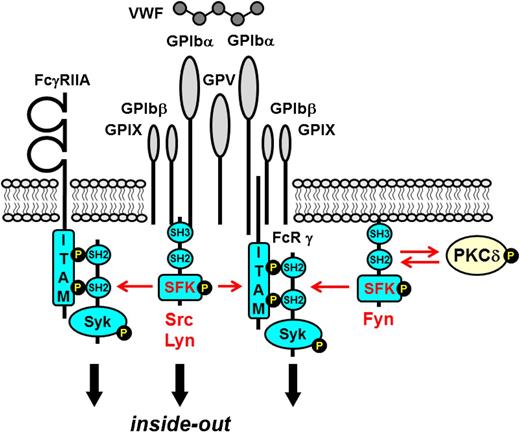
The rapid response of platelets to vascular injury is mediated by a diverse repertoire of tyrosine kinase–linked receptors, including the von Willibrand factor (VWF) receptor complex GPIb-IX-V that mediates tethering to sites of vascular injury1 ; the immunoreceptor tyrosine–based activation motif (ITAM)–containing collagen receptor complex GPVI-FcR γ-chain that mediates platelet activation2 ; the integrins α2β1 and αIIbβ3 that mediate firm adhesion and aggregation to exposed extracellular matrix3 ; the hemi-ITAM–containing podoplanin receptor CLEC-24 ; and the ITAM-containing low-affinity immunoglobulin receptor FcγRIIA (Figure 1).5 Ligand-mediated clustering of these receptors triggers transmission of primary activation signals through the phosphorylation of downstream tyrosine residues in proteins. None of these receptors have intrinsic kinase activity; instead, they rely on a family of protein-tyrosine kinases (PTKs) called Src family kinases (SFKs) that are either associated with or in close proximity to their cytoplasmic tails, to transmit signals (Figure 1). Downstream effectors of SFKs include adaptors, enzymes, and cytoskeletal proteins that collectively coordinate cytoskeletal remodeling, degranulation, membrane flipping, and integrin activation. A growing body of evidence has established that SFKs also contribute to signaling via G protein–coupled receptors (GPCRs), including the Gq-coupled thrombin protease-activated receptors 1 and 4 (PAR-1 and PAR-4)6-9 and the Gi-coupled adenosine 5′-diphosphate (ADP) receptor P2Y1210-12 that synergize with primary activation signals to maximally activate platelets. Some SFKs concomitantly initiate inhibitory pathways involving immunoreceptor tyrosine–based inhibition motif (ITIM)–containing receptors, lipid and protein-tyrosine phosphatases (PTPs) that attenuate platelet activation,13 thus limiting thrombus size.
Src family kinases initiate primary activation in platelets. Src family kinases (SFKs) phosphorylate adaptors, enzymes, and cytoskeletal proteins downstream of a variety of platelet surface receptors that collectively coordinate platelet activation. Dark green box, ITAM; light green box, hemi-ITAM. LEC, lymphatic endothelial cell.
Src family kinases initiate primary activation in platelets. Src family kinases (SFKs) phosphorylate adaptors, enzymes, and cytoskeletal proteins downstream of a variety of platelet surface receptors that collectively coordinate platelet activation. Dark green box, ITAM; light green box, hemi-ITAM. LEC, lymphatic endothelial cell.
SFKs have been intensively investigated in platelets for more than 2 decades, yet much remains to be learned about their functions and regulation. The primary aim of this review is to discuss our current understanding of the functional roles of SFKs in platelets by highlighting key discoveries that laid the foundation for this field and by introducing new concepts and future areas of research. We also discuss the thrombotic and hemostatic consequences of inhibiting platelet SFKs, which has broad clinical implications because PTK inhibitors are increasingly used in the treatment of cancer and inflammatory and autoimmune diseases.
Structure, function, and expression of SFKs
Cellular-Src (short for “sarcoma”) was the first proto-oncogene discovered and its protein product Src is the prototype of this family of PTKs. There are 8 members of the family, including Src, Yes, Fyn, and Fgr, which make up the SrcA subfamily, and Lyn, Hck, Lck, and Blk, which make up the SrcB subfamily (Table 1).14 The Src-related kinases Frk, Brk, and Srm are referred to as SFKs, but they lack structural features common to all SFKs. Src, Yes, and Fyn are widely expressed, whereas the remaining SFKs are primarily expressed in hematopoietic cells.
SFKs involved in regulating platelet activation
| Subfamily . | Src kinase . | Species . | Interacting proteins . | Substrates . | Receptor signaling regulated by SFKs . | Phenotypes of knockout mouse models . |
|---|---|---|---|---|---|---|
| SrcA | Src | Human26 | β3,31 GPIbα,71 SHIP-144 | β3,95 FcγRIIA,43 SHIP-144 | αIIbβ3,25,31,44 α2β1,96 α6β197 | Reduced αIlbβ3-mediated signaling and functional responses21,31 |
| Mouse21,31 | GPIb-IX-V,71 PAR-1, PAR-4,6,83 TP,98 α2A99 | |||||
| Yes | Human16,19 | β325 | Unknown | αIIbβ3,100 PAR-1, PAR-4101 | ||
| Fyn | Human18 | GPVI,50 β3,62 PECAM-160 | FcR γ-chain,50,51 PKCδ73 | αIIbβ3,62 GPVI-FcR γ-chain,50,51 GPIb-IX-V,75 PAR-1, PAR-4,7,12 P2Y127 | Reduced unknown αIIbβ3-mediated signaling and functional responses21,62 | |
| Mouse51 | Reduced GPVI-FcR γ-chain–mediated signaling and aggregation/secretion21,51 | |||||
| Potentiation of PAR-4–mediated aggregation12 | ||||||
| Mild bleeding diathesis62 | ||||||
| Fgr | Human20 | Unknown | Unknown | αIIbβ3,21 GPVI-FcR γ-chain21 | Marginal reduction in GPVI-mediated aggregation/secretion21 | |
| Mouse21 | ||||||
| SrcB | Lyn | Human17 | GPVI,50 β3,25,45 GPIbα,71 PECAM-1,60 SHIP-145 | FcR γ-chain,50,51 SHIP-145 | αIIbβ3,25,31,45 GPVI-FcR γ-chain,50,51 PECAM-1,13,60 GPIb-IX-V,102 | Increased αIIbβ3-mediated spreading21,45 |
| Mouse51 | PAR-1, PAR-4,7,103 P2Y12,7 Mpl57 | Delayed onset of GPVI-mediated signaling and aggregation/secretion21,51 | ||||
| Recovery of GPVI-mediated signaling and enhanced aggregation/secretion at later time points21,51 | ||||||
| Reduced GPIb-IX-V–mediated spreading102 | ||||||
| Increased megakaryopoiesis and Mpl signaling57 | ||||||
| Cholesterol sensor in megakaryocyte progenitors58 | ||||||
| Hck | Human60 | Unknown | ||||
| Lck | Human19,20 | |||||
| Blk | Not detected21 | |||||
| Src-related kinases | Frk | Unknown | ||||
| Brk | Unknown | |||||
| Srm | Unknown | |||||
| Subfamily . | Src kinase . | Species . | Interacting proteins . | Substrates . | Receptor signaling regulated by SFKs . | Phenotypes of knockout mouse models . |
|---|---|---|---|---|---|---|
| SrcA | Src | Human26 | β3,31 GPIbα,71 SHIP-144 | β3,95 FcγRIIA,43 SHIP-144 | αIIbβ3,25,31,44 α2β1,96 α6β197 | Reduced αIlbβ3-mediated signaling and functional responses21,31 |
| Mouse21,31 | GPIb-IX-V,71 PAR-1, PAR-4,6,83 TP,98 α2A99 | |||||
| Yes | Human16,19 | β325 | Unknown | αIIbβ3,100 PAR-1, PAR-4101 | ||
| Fyn | Human18 | GPVI,50 β3,62 PECAM-160 | FcR γ-chain,50,51 PKCδ73 | αIIbβ3,62 GPVI-FcR γ-chain,50,51 GPIb-IX-V,75 PAR-1, PAR-4,7,12 P2Y127 | Reduced unknown αIIbβ3-mediated signaling and functional responses21,62 | |
| Mouse51 | Reduced GPVI-FcR γ-chain–mediated signaling and aggregation/secretion21,51 | |||||
| Potentiation of PAR-4–mediated aggregation12 | ||||||
| Mild bleeding diathesis62 | ||||||
| Fgr | Human20 | Unknown | Unknown | αIIbβ3,21 GPVI-FcR γ-chain21 | Marginal reduction in GPVI-mediated aggregation/secretion21 | |
| Mouse21 | ||||||
| SrcB | Lyn | Human17 | GPVI,50 β3,25,45 GPIbα,71 PECAM-1,60 SHIP-145 | FcR γ-chain,50,51 SHIP-145 | αIIbβ3,25,31,45 GPVI-FcR γ-chain,50,51 PECAM-1,13,60 GPIb-IX-V,102 | Increased αIIbβ3-mediated spreading21,45 |
| Mouse51 | PAR-1, PAR-4,7,103 P2Y12,7 Mpl57 | Delayed onset of GPVI-mediated signaling and aggregation/secretion21,51 | ||||
| Recovery of GPVI-mediated signaling and enhanced aggregation/secretion at later time points21,51 | ||||||
| Reduced GPIb-IX-V–mediated spreading102 | ||||||
| Increased megakaryopoiesis and Mpl signaling57 | ||||||
| Cholesterol sensor in megakaryocyte progenitors58 | ||||||
| Hck | Human60 | Unknown | ||||
| Lck | Human19,20 | |||||
| Blk | Not detected21 | |||||
| Src-related kinases | Frk | Unknown | ||||
| Brk | Unknown | |||||
| Srm | Unknown | |||||
SFKs are involved in a broad range of cellular processes, including proliferation, differentiation, survival, adhesion, and motility. For a time, platelets were the primary cell of choice for studying SFK function because they express high levels of SFKs and are easy to obtain.15 Seven SFKs (Src, Yes, Lyn, Hck, Fyn, Fgr, and Lck) have been reported in human platelets and four (Src, Lyn, Fyn, and Fgr) have been reported in mouse platelets (Table 1).16-21 A number of structurally distinct broad-spectrum SFK inhibitors (PP1, PP2, SU6656, PD0173952) and knockout mouse models have been used to elucidate the contributions of these SFKs to platelet signal transduction and function. The most well-established functions are summarized in Table 1.
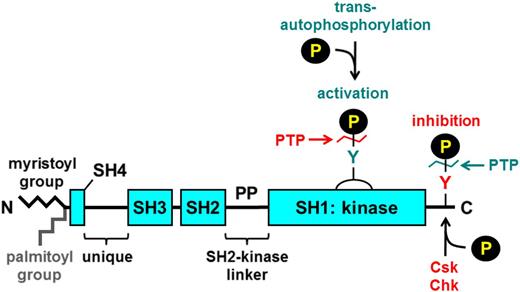
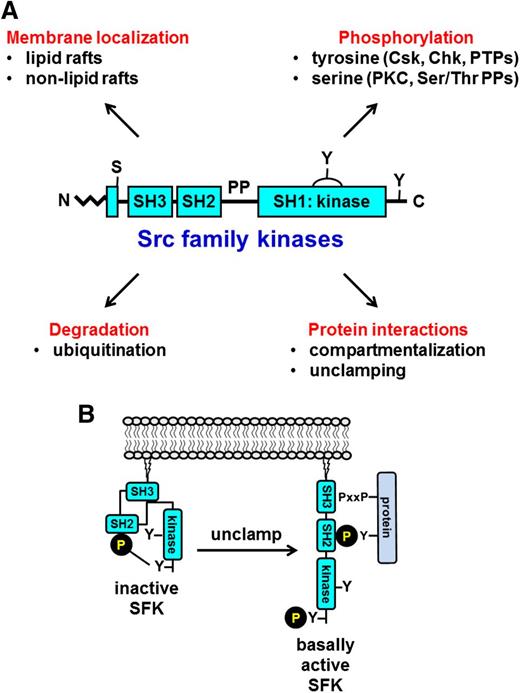
SFKs consist of an N-terminal myristoyl group attached to an Src homology 4 (SH4) domain, followed by a unique region, an SH3 domain, an SH2 domain, a proline-rich SH2-kinase linker region, and an SH1 or kinase domain (Figure 2).14 Myristoylation of the N-terminus facilitates binding to the inner leaflet of the plasma membrane, which is required for function. Yes, Fyn, Fgr, Lyn, Hck, and Lck also contain a cysteine residue within their myristoylation peptide that supports palmitoylation and localization to specialized regions of the plasma membrane referred to as lipid rafts that contain high concentrations of glycosphingolipids and cholesterol and serve as organizing centers of signaling proteins and receptors. Src and Blk are not palmitoylated, and hence they are excluded from lipid rafts. The SH3 and SH2 domains of SFKs mediate intra- and intermolecular interactions with proline-rich regions and phospho-tyrosine residues, respectively. In addition, all SFKs contain 2 highly conserved tyrosine residues, one in their C-terminal tail and the other in their activation loop that are critical for regulating SFK activity (Figure 2).22 Phosphorylation of the C-terminal tyrosine residue by C-terminal Src kinase (Csk) or its family member Csk homologous kinase inhibits SFK activity,23 whereas trans-autophosphorylation of the activation loop tyrosine residue maximally activates the SFK (Figure 2).22 Conversely, dephosphorylation of the C-terminal inhibitory and activation loop tyrosine residues by PTPs increases and decreases SFK activity, respectively (Figure 2).24
General structure of SFKs. All SFKs share a common structure consisting of an N-terminal myristoyl group attached to an SH4 domain, a unique region, an SH3 domain, an SH2 domain, an SH2-kinase proline-rich linker region, and an SH1 or kinase domain. Yes, Fyn, Fgr, Lyn, Hck, and Lck contain a cysteine residue within the myristoylation peptide sequence that gets palmitoylated and mediates localization to lipid rafts. There is a conserved tyrosine residue in the activation loop and one in the C-terminal tail. Phosphorylation of the activation loop tyrosine by trans-autophosphorylation increases SFK activity, whereas phosphorylation of the C-terminal tyrosine by C-terminal Src kinase (Csk) or the structurally related Csk homologous kinase (Chk) inhibits SFK activity. Dephosphorylation of the activation loop and C-terminal inhibitory tyrosine residues by PTPs attenuates and increases SFK activity, respectively. Green denotes activation, and red denotes inhibition of SFK activity.
General structure of SFKs. All SFKs share a common structure consisting of an N-terminal myristoyl group attached to an SH4 domain, a unique region, an SH3 domain, an SH2 domain, an SH2-kinase proline-rich linker region, and an SH1 or kinase domain. Yes, Fyn, Fgr, Lyn, Hck, and Lck contain a cysteine residue within the myristoylation peptide sequence that gets palmitoylated and mediates localization to lipid rafts. There is a conserved tyrosine residue in the activation loop and one in the C-terminal tail. Phosphorylation of the activation loop tyrosine by trans-autophosphorylation increases SFK activity, whereas phosphorylation of the C-terminal tyrosine by C-terminal Src kinase (Csk) or the structurally related Csk homologous kinase (Chk) inhibits SFK activity. Dephosphorylation of the activation loop and C-terminal inhibitory tyrosine residues by PTPs attenuates and increases SFK activity, respectively. Green denotes activation, and red denotes inhibition of SFK activity.
For the remainder of this review, we focus specifically on the 3 most highly expressed and studied SFKs in platelets, namely Src, Lyn, and Fyn, all of which have been implicated in integrin, ITAM-containing, and GPIb-IX-V receptor signaling. Src and Fyn are primarily regarded as positive regulators, whereas Lyn plays a dual role as a positive and a negative regulator of platelet activation. All 3 SFKs also contribute to Gq- and Gi-coupled receptor signaling. Fgr and Yes are expressed at much lower levels than Src, Lyn, and Fyn in platelets and have been implicated in ITAM-containing and integrin receptor signaling, respectively.21,25 Below, we discuss our current understanding of how Src, Lyn, and Fyn work in conjunction to regulate an optimal level of platelet activation.
Src: positive regulator of αIIbβ3 signaling
αIIbβ3 is the most abundant platelet surface receptor and is essential for adhesion, aggregation, and clot retraction. Inside-out signals emanating from various activation receptors trigger αIIbβ3 to adopt a high-affinity conformation (Figure 3A). Subsequent ligand-mediated clustering initiates outside-in signaling that facilities platelet activation, spreading, secretion, and clot retraction. Src is the most abundant SFK in human platelets26 and is essential for initiating and propagating signals from αIIbβ3.27
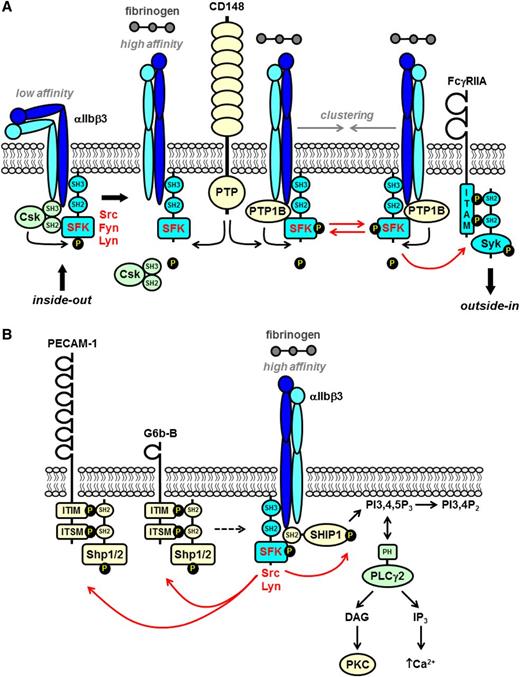
SFKs in integrin αIIbβ3 proximal signaling. (A) The integrin αIIbβ3 is in a low-affinity conformation on the surface of resting platelets. The SFKs Src and Fyn constitutively associate with the cytoplasmic tail of the β3 subunit via their SH3 domains. Src is maintained in an inactive conformation by Csk, which forms a complex with β3 and Src. Inside-out signaling induces the integrin to adopt a high-affinity conformation and fibrinogen binding. Csk subsequently dissociates from the complex and is replaced by the nontransmembrane PTP-1B that dephosphorylates the C-terminal inhibitory tyrosine residue of Src and activates it. The receptor-like PTP CD148 plays a major role in maintaining a pool of activated SFKs at the plasma membrane that contribute to αIIbβ3 signaling. Fibrinogen-mediated clustering of the integrin induces trans-autophosphorylation of the activation loop tyrosine residue of SFKs and maximal activation. (B) The ITIM/immunoreceptor tyrosine-based switch motif (ITSM)–containing inhibitory receptors PECAM-1 and G6b-B are phosphorylated by SFKs downstream of αIIbβ3. The SH2 domain–containing nontransmembrane PTPs Shp1 and Shp2 bind to the tandem phosphorylated ITIM/ITSM. The exact contributions of PECAM-1 and G6b-B to αIIbβ3 signaling remain ambiguous and are denoted by the dashed arrow. SFKs also phosphorylate and activate SH2 domain-containing SHIP-1, which forms a complex with SFKs and the β3 tail. SHIP-1 attenuates integrin signaling by dephosphorylating PI3,4,5P3 to PI3,4P2 and disrupting membrane localization of (PLCγ2), which binds to PI3,4,5P3 via its pleckstrin homology (PH) domain. PLCγ2 must associate with the plasma membrane in order to hydrolyze PI4,5P2 to the second messenger’s DAG and IP3 that activate serine/threonine PKC and facilitate Ca2+ mobilization, respectively.
SFKs in integrin αIIbβ3 proximal signaling. (A) The integrin αIIbβ3 is in a low-affinity conformation on the surface of resting platelets. The SFKs Src and Fyn constitutively associate with the cytoplasmic tail of the β3 subunit via their SH3 domains. Src is maintained in an inactive conformation by Csk, which forms a complex with β3 and Src. Inside-out signaling induces the integrin to adopt a high-affinity conformation and fibrinogen binding. Csk subsequently dissociates from the complex and is replaced by the nontransmembrane PTP-1B that dephosphorylates the C-terminal inhibitory tyrosine residue of Src and activates it. The receptor-like PTP CD148 plays a major role in maintaining a pool of activated SFKs at the plasma membrane that contribute to αIIbβ3 signaling. Fibrinogen-mediated clustering of the integrin induces trans-autophosphorylation of the activation loop tyrosine residue of SFKs and maximal activation. (B) The ITIM/immunoreceptor tyrosine-based switch motif (ITSM)–containing inhibitory receptors PECAM-1 and G6b-B are phosphorylated by SFKs downstream of αIIbβ3. The SH2 domain–containing nontransmembrane PTPs Shp1 and Shp2 bind to the tandem phosphorylated ITIM/ITSM. The exact contributions of PECAM-1 and G6b-B to αIIbβ3 signaling remain ambiguous and are denoted by the dashed arrow. SFKs also phosphorylate and activate SH2 domain-containing SHIP-1, which forms a complex with SFKs and the β3 tail. SHIP-1 attenuates integrin signaling by dephosphorylating PI3,4,5P3 to PI3,4P2 and disrupting membrane localization of (PLCγ2), which binds to PI3,4,5P3 via its pleckstrin homology (PH) domain. PLCγ2 must associate with the plasma membrane in order to hydrolyze PI4,5P2 to the second messenger’s DAG and IP3 that activate serine/threonine PKC and facilitate Ca2+ mobilization, respectively.
A proportion of Src associates with the cytoskeleton upon platelet activation allowing it to phosphorylate key substrates, including cortactin and WASp, that regulate cytoskeletal remodeling.28,29 A pool of Src is also constitutively associated with the β3 subunit of αIIbβ3.25 This interaction, mediated by the SH3 domain of Src and the RGT amino acid sequence in the extreme C-terminal tail of β3, disrupts the intramolecular SH3-proline-rich linker interaction and primes Src.25 β3-associated Src is maintained in an inactive conformation by Csk, which forms a complex with β3 and Src in resting platelets and phosphorylates the C-terminal inhibitory tyrosine of Src (human Tyr529; mouse Tyr534) (Figure 3A).25,30,31 Csk gets replaced by the nontransmembrane PTP-1B, released from the cytosolic surface of the endoplasmic reticulum upon fibrinogen binding, which dephosphorylates Tyr529 and activates Src.30,32 The receptor-like PTP CD148 contributes to αIIbβ3 outside-in signaling by maintaining a pool of active SFKs at the plasma membrane and also by dephosphorylating the C-terminal inhibitory tyrosine residue of all SFKs (Figure 3A).33 Subsequent ligand-mediated clustering of the integrin induces trans-autophosphorylation of human Tyr418 (mouse Tyr423) in the activation loop and maximal activation of Src.30,33 Downstream Src-dependent phosphorylation events include Tyr773 and Tyr785 in human β3 (mouse Tyr747 and Tyr759) that provide docking sites for myosin light chain and the adaptor Shc,34 adaptor and cytoskeletal proteins (paxillin, vinculin, and actinin) that provide docking sites for assembly of signaling complexes,35-37 tyrosine kinases (FAK, Pyk, and Syk),38 small guanosine triphosphatase (Rap1B), guanine nucleotide exchange factors (Vav1 and Vav3),39,40 and the lipid kinase phosphoinositide 3 kinase (PI3K) that propagate the signal.38,41 Src-dependent activation of phospholipase Cγ2 (PLCγ2) results in the hydrolysis of phosphatidylinositol 4,5-bisphosphate (PI4,5P2) to the second messengers diacylglycerol (DAG) and inositol triphosphate (IP3) that activate the serine/threonine protein kinase C (PKC) family and facilitate Ca2+ mobilization, respectively.42 Tyrosine phosphorylation of the ITAM-containing FcγRIIA receptor by SFKs provides a high-affinity docking site for the tandem SH2 domain-containing tyrosine kinase Syk, which facilitates spreading on fibrinogen (Figure 3A).43
Src and Lyn concomitantly activate the SH2 domain–containing inositol 5-phosphatase 1 (SHIP-1) that attenuates αIIbβ3 signaling. SHIP-1 associates with Src, Lyn, and β3 and is phosphorylated by Src and Lyn (Figure 3B).44,45 Activated SHIP-1 subsequently dephosphorylates phosphatidylinositol 3,4,5-trisphosphate (PI3,4,5P3) to PI3,4P2, which downregulates membrane localization of pleckstrin homology domain–containing PLCγ2, IP3, and DAG generation and Ca2+ mobilization.44,45 Enhanced spreading of Lyn-deficient mouse platelets on fibrinogen is thus explained by loss of SHIP-1 activation.45
The ITIM-containing inhibitory receptors PECAM-1 and G6b-B also get phosphorylated downstream of αIIbβ3, but their exact contributions to αIIbβ3 signaling remain ambiguous. Both associate with Shp1 and Shp2 following SFK-dependent phosphorylation of tyrosine residues within tandem ITIM/immunoreceptor tyrosine-based switch motifs.46,47 Paradoxically, mouse platelets lacking PECAM-1 exhibit reduced spreading on fibrinogen and clot retraction, and G6b-B–deficient platelets exhibit reduced lamellipodia formation on fibrinogen in the presence of thrombin (Figure 3B),46 suggesting that both receptors facilitate αIIbβ3-mediated responses.47
Mouse platelets lacking Src exhibit severely impaired tyrosine phosphorylation and spreading on fibrinogen.21,31 Similarly, disruption of the Src-β3 interaction, either by deleting or mutating the β3 RGT sequence reduces outside-in signaling and platelet spreading on fibrinogen and clot retraction, which culminates in reduced thrombus formation and a mild bleeding diathesis.48 However, defects are not as severe as those seen in the presence of SFK inhibitors,31,49 suggesting that there is functional redundancy between SFKs. Indeed, deletion of Lyn or Fyn in combination with Src exacerbates integrin-mediated functional defects.21
Dual roles of Lyn in ITAM-containing and integrin receptor signaling
Lyn is highly expressed in human platelets19 and is the most abundant SFK in mouse platelets.21 Unlike Src, it is localized to lipid and nonlipid rafts and performs dual functions as positive and negative regulator of ITAM-containing receptor and integrin signaling. A pool of active Lyn is constitutively associated with the cytoplasmic tail of GPVI, allowing for rapid signal transduction (Figure 4A).50-53 This interaction, mediated by the SH3 domain of Lyn and the proline-rich juxtamembrane region of GPVI, activates and maintains Lyn in close proximity to the FcR γ-chain. Lyn-mediated phosphorylation of the tandem tyrosine residues in the FcR γ-chain ITAM provides a high-affinity docking site for Syk (Figure 4A). Lyn subsequently phosphorylates and activates Syk, and together they propagate the signal. The phosphorylation status of GPVI-associated Lyn suggests that it is maximally active, because it is phosphorylated on its activation loop tyrosine (human and mouse Tyr396) and not on its C-terminal inhibitory tyrosine residue (human and mouse Tyr507).53 It is hypothesized that this allows GPVI to signal more rapidly than a typical ITAM-containing immune receptor lacking associated SFKs. How the FcR γ-chain remains only marginally phosphorylated under resting conditions is not known.54 One hypothesis is that the FcR γ-chain is embedded in the plasma membrane and is inaccessible to Lyn.55 Another is that the FcR γ-chain gets dephosphorylated at a faster rate than it gets phosphorylated. Perhaps another inhibitory mechanism is at play. This is an intriguing question that warrants investigation.
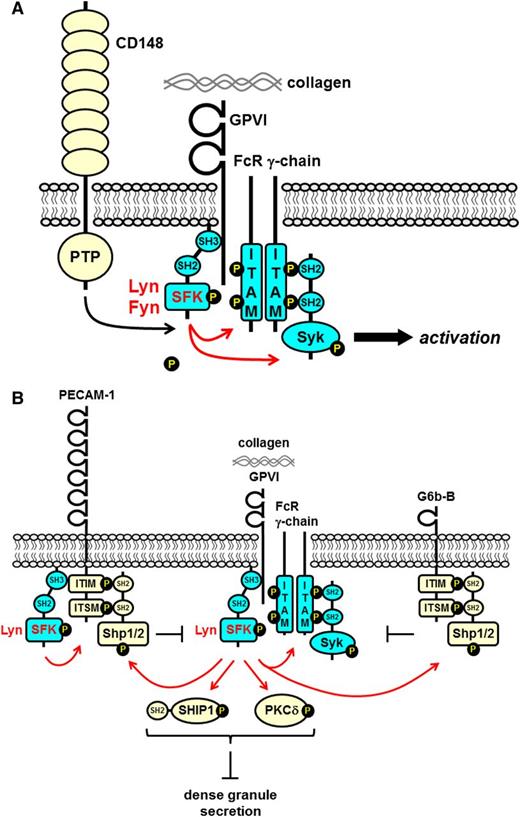
SFKs in GPVI-FcR γ-chain proximal signaling. (A) The SFKs Lyn and Fyn constitutively associate with the proline-rich juxtamembrane region of GPVI via their SH3 domains. This interaction unclamps and activates the SFKs. The receptor-like PTP CD148 maintains the SFKs in an activated state by dephosphorylating their C-terminal inhibitory tyrosine residues. Collagen-mediated clustering of the receptor induces trans-autophosphorylation of the activation loop tyrosine residue and maximal SFK activation. SFKs phosphorylate tandem tyrosine residues in the ITAM-containing FcR γ-chain, which provides a high-affinity docking site for the tandem SH2 domain–containing protein-tyrosine kinase Syk. SFKs also phosphorylate and active Syk. SFKs and Syk phosphorylate downstream effectors and propagate the signal. (B) The ITIM/ITSM-containing inhibitory receptors PECAM-1 and G6b-B inhibit GPVI-FcR γ-chain signaling. Lyn phosphorylates tandem tyrosine residues in the ITIM/ITSM in the cytoplasmic tails of PECAM-1 and G6b-B that provides a high-affinity binding site for the SH2 domain–containing nontransmembrane PTPs Shp1 and Shp2. Lyn associated with the cytoplasmic tail of PECAM-1 facilitates phosphorylation. Lyn also phosphorylates and activates SH2 domain–containing SHIP-1 and PKCδ that form a complex and inhibit dense granule secretion.
SFKs in GPVI-FcR γ-chain proximal signaling. (A) The SFKs Lyn and Fyn constitutively associate with the proline-rich juxtamembrane region of GPVI via their SH3 domains. This interaction unclamps and activates the SFKs. The receptor-like PTP CD148 maintains the SFKs in an activated state by dephosphorylating their C-terminal inhibitory tyrosine residues. Collagen-mediated clustering of the receptor induces trans-autophosphorylation of the activation loop tyrosine residue and maximal SFK activation. SFKs phosphorylate tandem tyrosine residues in the ITAM-containing FcR γ-chain, which provides a high-affinity docking site for the tandem SH2 domain–containing protein-tyrosine kinase Syk. SFKs also phosphorylate and active Syk. SFKs and Syk phosphorylate downstream effectors and propagate the signal. (B) The ITIM/ITSM-containing inhibitory receptors PECAM-1 and G6b-B inhibit GPVI-FcR γ-chain signaling. Lyn phosphorylates tandem tyrosine residues in the ITIM/ITSM in the cytoplasmic tails of PECAM-1 and G6b-B that provides a high-affinity binding site for the SH2 domain–containing nontransmembrane PTPs Shp1 and Shp2. Lyn associated with the cytoplasmic tail of PECAM-1 facilitates phosphorylation. Lyn also phosphorylates and activates SH2 domain–containing SHIP-1 and PKCδ that form a complex and inhibit dense granule secretion.
Mice lacking Lyn exhibit a complex platelet phenotype, including mild thrombocytopenia after 10 weeks of age, secondary to enhanced inflammation, increased numbers of megakaryocyte progenitors and mature megakaryocytes in the bone marrow, and aberrant platelet function.56,57 The phenotype can be partially explained by increased Mpl-mediated Erk1, Erk2, and Akt activation in Lyn-deficient megakaryocytes. SHIP-1 phosphorylation is concomitantly decreased, suggesting that Lyn negatively regulates the Ras/MAPK and PI3K/Akt pathways in a SHIP-1–dependent manner.57 More recently, Lyn was implicated as a membrane cholesterol sensor in megakaryocyte progenitors, linking platelet production with membrane cholesterol levels and atherogenesis.58 Increased membrane cholesterol accumulation in mouse megakaryocyte progenitors led to increased Mpl expression and signaling, platelet overproduction, arterial thrombosis, and atherogenesis in a hypercholesterolemicmouse model.58 Mechanistically, Lyn was proposed to be the dominant SFK mediating downregulation of Mpl via the E3-ubiquitin ligase Cbl.58 Excessive membrane cholesterol accumulation led to decreased Lyn kinase activity and reduced Cbl-mediated downregulation of Mpl by Tpo.58,59
Platelets from Lyn-deficient mice exhibit a delay in the onset of GPVI-induced tyrosine phosphorylation and functional responses, followed by a recovery of tyrosine phosphorylation and enhanced responses at later time points.51 These findings suggest that Lyn activates negative feedback pathways concomitantly with initiating GPVI signaling. The delay in GPVI signaling in the absence of Lyn is likely the result of less efficient phosphorylation of the FcR γ-chain by other SFKs, whereas the enhanced responses at later stages may be the result of the absence of Lyn-dependent negative feedback signals, most likely mediated by PECAM-1, G6b-B, and SHIP-1 (Figure 4B). Lyn associates with the cytoplasmic tail of PECAM-1, facilitating phosphorylation of the tandem ITIM/immunoreceptor tyrosine-based switch motifs and binding of Shp2.60 Deletion of PECAM-1 and Lyn does not have an additive effect, which supports the notion that they act via the same inhibitory pathway.13 Whether Lyn also contributes to tyrosine phosphorylation of G6b-B remains to be determined.47 Lyn also associates with and phosphorylates SHIP-1 and PKCδ downstream of GPVI, thus negatively regulating cytosolic Ca2+ concentration and dense granule secretion (Figure 4B).45,61
Mouse platelets lacking Src, Fyn, or Fgr along with Lyn respond less well to GPVI-specific agonist collagen-related peptide (CRP) and fibrinogen compared with platelets lacking any of these SFKs on their own, suggesting a degree of functional redundancy.21 The one unique aspect of the Lyn knockout phenotype is the hyperreactivity to CRP and fibrinogen, which suggests that only Lyn mediates these inhibitory functions in mouse and presumably human platelets.21
Fyn: positive regulator of ITAM-containing and integrin receptor signaling
Fyn is highly expressed in human and mouse platelets.18,21 Like Lyn, Fyn is palmitoylated and constitutively associated with the proline-rich region in the cytoplasmic tail of GPVI via its SH3 domain.50,51 In vitro interaction studies demonstrate that the Lyn SH3 domain preferentially binds to this site.53 Despite these similarities, Fyn- and Lyn-deficient platelets exhibit unique GPVI-mediated defects.51 Aggregation and secretion of Fyn-deficient platelets are reduced in response to low and intermediate concentrations of CRP.21,51 Tyrosine phosphorylation of the FcR γ-chain, Syk, and the downstream adaptors LAT and SLP-76 are also reduced in Fyn-deficient platelets in response to a low concentration of CRP.51 However, Fyn-deficient platelets aggregate normally in response to high concentrations of thrombin, collagen, and ADP, which suggests that other SFKs compensate in the absence of Fyn.51 Indeed, additive effects are seen if Lyn or Src are deleted in addition to Fyn.21 The Src/Fyn double-knockout result is particularly intriguing, because Src is not detectable in lipid rafts.21
A proportion of Fyn is also constitutively associated with the cytoplasmic tail of the β3 subunit of αIIbβ3. Unlike Src, which binds to the extreme C-terminus of β3, Fyn associates with amino acid residues 721 to 725 (IHDRK) in the juxtamembrane region and remains associated with β3 following thrombin-induced platelet aggregation.62 Loss of the Src binding site is thought to be calpain-mediated.63 Fyn-deficient mouse platelets exhibit a less severe spreading defect on fibrinogen compared with Src-deficient platelets, demonstrating the dominant role of Src downstream of αIIbβ3.21,62 Mouse platelets lacking Src and Fyn exhibit a comparable spreading defect to that of Src-deficient platelets.21
Distinct roles of SFKs in ITAM- and hemi-ITAM–containing receptor signaling
SFKs play a central role in initiating signaling via the ITAM-containing receptors GPVI-FcR γ-chain and FcγRIIA53,64 ; however, their role downstream of the hemi-ITAM–containing podoplanin receptor CLEC-2 is incompletely defined. CLEC-2 has garnered much attention recently for its involvement in blood-lymphatic vessel separation.65 Unlike the FcR γ-chain and FcγRIIA that contain tandem tyrosine residues in their ITAMs, CLEC-2 contains a single tyrosine residue in its hemi-ITAM that binds a single Syk SH2 domain with low affinity when phosphorylated.66 Tandem phosphorylated CLEC-2 receptors are thus required to provide a high-affinity docking site for a single Syk molecule.66 Rhodocytin-mediated tyrosine phosphorylation of CLEC-2 and downstream signaling proteins is inhibited in human platelets treated with PP2, which suggests an important role of SFKs in initiating CLEC-2 signaling.67 However, phosphorylation of the CLEC-2 is normal in rhodocytin-stimulated Src-, Lyn- and Fyn-deficient mouse platelets.68 Surprisingly, rhodocytin-mediated CLEC-2 phosphorylation is normal, and downstream phosphorylation of PLCγ2 and platelet activation are abolished in mouse platelets treated with an SFK inhibitor.68 On the basis of these findings, SFKs are thought to play an auxiliary role in initiating CLEC-2 signaling, activating Syk, and amplifying proximal signaling events, whereas Syk plays the main role in phosphorylating CLEC-2.66
SFKs initiate GPIb-IX-V signaling
VWF engagement of GPIb-IX-V induces inside-out signaling to αIIbβ3, which mediates firm adhesion to sites of vascular injury and thrombus formation.1 Src, Lyn, and Fyn have all been implicated in the early stages of GPIb-IX-V signaling. Lipid raft localization and filamin-dependent cytoskeletal association are also required for optimal GPIb-IX-V signaling.69,70 Src and Lyn associate with the cytoplasmic tail of GPIbα and the cytoskeleton following VWF engagement (Figure 5).71 PI3K, FAK, SHIP-1, and PTP-1B subsequently associate with the plasma membrane in an SFK-dependent manner.72 Concomitantly, Fyn interacts with PKCδ, inducing transphosphorylation and reciprocal activation.73 Studies using Lyn-deficient mouse platelets revealed that Lyn is essential for initiating GPIb-IX-V signaling, whereas Src enhances it.74 The FcR γ-chain is also phosphorylated downstream of GPIb-IX-V and associates with the GPIbα subunit (Figure 5).75 Syk binds to the phosphorylated FcR γ-chain and transmits signals in a manner similar to that of an ITAM-containing receptor; however, GPVI-FcR γ-chain transmits a stronger activation signal relative to GPIb-IX-V. Downstream targets of SFKs include the adaptor proteins LAT and ADAP, PLCγ2, the tyrosine kinase Btk, and the mitogen-activated protein kinases Erk1 and Erk2.1 FcγRIIA is also reported to be phosphorylated downstream of GPIb-IX-V and is required for signaling (Figure 5).76 However, subsequent studies demonstrated that GPIb-IX-V signals in an ITAM-independent manner, culminating in PLCγ2 activation and Ca2+ oscillations.77,78 Inhibition of PI3K and Ca2+ chelation blocked GPIb-IX-V–mediated integrin activation but not tyrosine phosphorylation, whereas inhibition of SFKs blocked all GPIb-IX-V–mediated responses,78 highlighting the central role played by SFKs in this signaling pathway.
SFKs in GPIb-IX-V proximal signaling. The SFKs Src and Lyn associate with the GPIbα subunit and initiate inside-out signaling. Binding of VWF to the extracellular region of GPIbα induces SFK activation and phosphorylation of downstream substrates, including the ITAM-containing FcR γ-chain (FcR γ) and FcγRIIA, both of which act as high-affinity docking sites for the tandem SH2 domain–containing protein-tyrosine kinase Syk. The FcR γ-chain is reported to associate with the GPIbα subunit. However, GPIb-IX-V can signal in an ITAM-independent manner. Fyn and PKCδ associate and reciprocally activate one another, propagating the signal.
SFKs in GPIb-IX-V proximal signaling. The SFKs Src and Lyn associate with the GPIbα subunit and initiate inside-out signaling. Binding of VWF to the extracellular region of GPIbα induces SFK activation and phosphorylation of downstream substrates, including the ITAM-containing FcR γ-chain (FcR γ) and FcγRIIA, both of which act as high-affinity docking sites for the tandem SH2 domain–containing protein-tyrosine kinase Syk. The FcR γ-chain is reported to associate with the GPIbα subunit. However, GPIb-IX-V can signal in an ITAM-independent manner. Fyn and PKCδ associate and reciprocally activate one another, propagating the signal.
SFKs in GPCR signaling
GPCRs signal via heterotrimeric guanine nucleotide-binding proteins (Gαβγ proteins). However, a growing body of evidence has established that SFKs associate with GPCRs and contribute to downstream signaling.79 Platelets express a variety of activating GPCRs that are broadly divided into Gq- and Gi-coupled receptors. Gq-coupled receptors for thrombin (PAR-1 and PAR-4), ADP (P2Y1), thromboxane A2 (TxA2, TP), and serotonin (5-HT2A) signal by activating PLCβ and inducing Ca2+ mobilization (Figure 6Ai). In contrast, Gi-coupled receptors for ADP (P2Y12) and adrenalin (α2A) signal by inhibiting adenylate cyclase and activating PI3K (Figure 6B).80 Signals transmitted by these receptors synergize with primary activation signals from tyrosine kinase–linked receptors to maximally activate platelets.
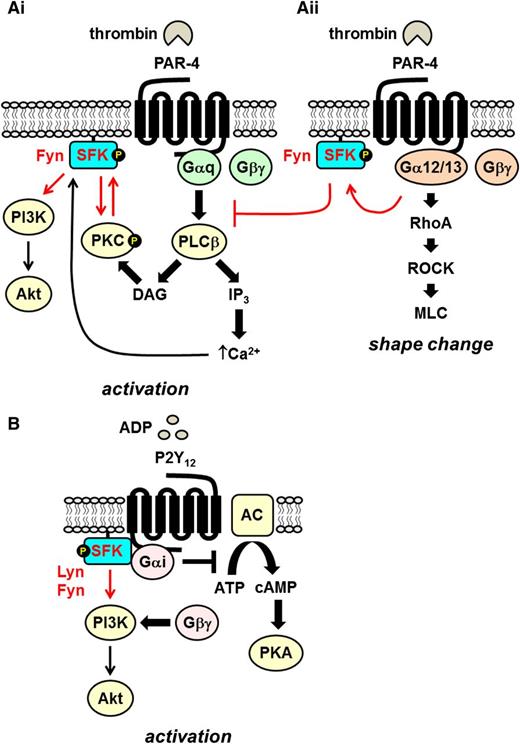
Src family kinases in Gq- and Gi-coupled receptor signaling. (Ai) The Gq-coupled PAR-4 signals via the activation of phospholipase β (PLCβ), which hydrolyses PI4,5P2 to the second messengers DAG and IP3, which in turn activate serine/threonine PKC and facilitate Ca2+ mobilization, respectively. The SFK Fyn associates with PKCδ downstream of PLCβ, and they reciprocally activate one another. Increased Ca2+ concentration contributes to SFK activation. SFKs lie upstream of the PI3K/Akt pathway. (Aii) PAR-4 coupled to G12/13 mediates platelet shape change via the RhoA/ROCK/MLC pathway. Fyn activated downstream of G12/13 inhibits signaling via Gq-coupled PAR-4. (B) The Gi-coupled ADP receptor P2Y12 signals by inhibiting adenylate cyclase/cAMP/protein kinase A (PKA) and Gβγ-mediated activation of PI3K/Akt. The SFKs Lyn and Fyn bind directly to the Gα subunit and play a critical role in initiating signaling via the PI3K/Akt pathway.
Src family kinases in Gq- and Gi-coupled receptor signaling. (Ai) The Gq-coupled PAR-4 signals via the activation of phospholipase β (PLCβ), which hydrolyses PI4,5P2 to the second messengers DAG and IP3, which in turn activate serine/threonine PKC and facilitate Ca2+ mobilization, respectively. The SFK Fyn associates with PKCδ downstream of PLCβ, and they reciprocally activate one another. Increased Ca2+ concentration contributes to SFK activation. SFKs lie upstream of the PI3K/Akt pathway. (Aii) PAR-4 coupled to G12/13 mediates platelet shape change via the RhoA/ROCK/MLC pathway. Fyn activated downstream of G12/13 inhibits signaling via Gq-coupled PAR-4. (B) The Gi-coupled ADP receptor P2Y12 signals by inhibiting adenylate cyclase/cAMP/protein kinase A (PKA) and Gβγ-mediated activation of PI3K/Akt. The SFKs Lyn and Fyn bind directly to the Gα subunit and play a critical role in initiating signaling via the PI3K/Akt pathway.
Unlike integrin and ITAM-containing receptors, SFKs play an auxiliary role in Gq-coupled receptor signaling, lying downstream of PLCβ and Ca2+ mobilization (Figure 6Ai).7 General SFK inhibitors do not completely inhibit platelet activation, but rather they block or attenuate specific aspects of platelet function, such as TxA2 production, platelet aggregation, and adenosine triphosphate secretion.7 PAR-4–mediated SFK activation is inhibited by Ca2+ chelation, which supports the notion that SFKs are activated downstream of Ca2+ mobilization.7 SFKs have been reported to lie upstream of PI3K9 and work in conjunction with PKC to propagate PAR-4 signaling.81 PAR-4–mediated platelet activation is only partially inhibited in the presence of a PKC inhibitor, Ca2+ chelator, or SFK inhibitor, but it is abolished by a PKC inhibitor in combination with either a Ca2+ chelator or SFK inhibitor.6,7,81,82 Further evidence of the interplay between SFKs and PKC is that PKCδ interacts directly with Fyn and is tyrosine phosphorylated at positions Tyr311 and Tyr565 in an SFK-dependent manner that potentiates PKC activity in response to thrombin.81 This is consistent with the earlier finding that phosphorylation of Ser12 in the membrane-binding domain of Src by PKC induces cytoskeletal association and an increase in substrate affinity.83
Fyn has also been implicated as a negative regulator of PAR-4 signaling (Figure 6Aii).12 This is supported by findings that PAR-4–induced platelet aggregation and PKC activation are potentiated in Fyn-deficient platelets but not Lyn-deficient platelets.12 It is hypothesized that Fyn activated downstream of G12/13-coupled PAR-4, which signals via the RhoA/ROCK/MLC pathway and regulates shape change, attenuates Gq-coupled platelet responses by inhibiting PKC activation and intracellular Ca2+ mobilization (Figure 6Ai-ii).12 The mechanism of activation of Fyn downstream of G12/13 and how it inhibits Gq signaling remains unknown.
SFKs play a central role in initiating signaling via the Gi-coupled P2Y12 and α2A receptors. ADP- and adrenaline-induced primary wave aggregation is inhibited in platelet-rich plasma in the presence of SFK inhibitors.84 The involvement of SFKs in P2Y12 signaling is supported by findings that ADP-induced SFK trans-autophosphorylation is abolished by a P2Y12 antagonist, but not by a P2Y1 antagonist, and in mouse platelets lacking P2Y12 but not in Gq-deficient platelets.7,85 Consistent with these findings, Gi signaling is sufficient to activate SFKs, and Lyn and Fyn interact with Gi in platelets, suggesting that SFKs are direct effectors of Gi (Figure 6B). SFKs have also been proposed to lie upstream of the PI3K/Akt pathway activated by the Gβγ subunit of Gi (Figure 6B).7 Akt phosphorylation is inhibited by PP2 in ADP-stimulated Gq-deficient platelets or platelets treated with a P2Y1 receptor antagonist. However, SFKs are not involved in regulating adenylate cyclase and cAMP levels.7 SFKs have also been implicated in regulating cross-talk between P2Y1 and P2Y12.11 According to this model, SFKs activated downstream of P2Y1 negatively regulate Ca2+ mobilization mediated by P2Y12 and PI3K,11 providing further evidence of the inhibitory roles of SFKs downstream of GPCRs and mechanistic insights into how SFKs regulate cross-talk between these receptors.
Hemostatic consequences of inhibiting platelet SFKs
PTK inhibitors are increasingly used in the treatment of cancer and inflammatory and autoimmune diseases, examples of which are the Bcr-Abl inhibitors dasatinib, bosutinib, and saracatinib used as second-line treatments of chronic myelogenous leukemia.86 However, these compounds have off-target effects on other PTKs, including SFKs. Not surprisingly, they have severe adverse bleeding effects.86 Recent studies have revealed reduced platelet reactivity and mild thrombocytopenia in humans and mice treated with dasatinib, which underlie these adverse effects.87,88 Reduced platelet counts and normal half-life in dasatinib-treated mice suggest a block in platelet production. This is supported by delayed platelet recovery following immune-induced thrombocytopenia and increased numbers of megakaryocytes in the bone marrow of these mice.88 Megakaryocyte migration toward an SDF-1α gradient and proplatelet formation were inhibited by dasatinib ex vivo, supporting the hypothesis that dasatinib inhibits platelet production.88 Thus, caution must be taken when administering PTK inhibitors with known off-target effects on SFKs, especially when given in combination with platelet inhibitors, such as aspirin and P2Y12 antagonists. Such profound hemostatic effects of inhibiting SFKs raise important questions about the antithrombotic potential of selective and reversible SFKs inhibitors.
New frontiers and targets
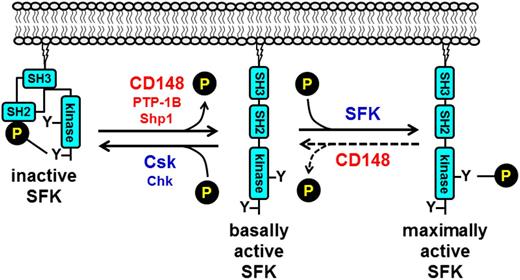
A key question that remains unresolved is how SFKs are regulated in platelets. Addressing this question will lead to a better understanding of how the threshold of platelet reactivity is set and the identification of novel antithrombotic drug targets. Only recently have we started to make some headway into this area of research. Findings from our group have established CD148 as a global activator of SFKs in platelets (Figure 7).33 Without it, mouse platelets are less reactive to collagen, fibrinogen, and to a lesser extent thrombin. Consequently, CD148-deficient mice exhibit reduced thrombus formation and a mild bleeding diathesis.33 Consistent with these findings, polymorphisms identified in the extracellular region of CD148 (Q276R and R326Q) have a protective effect in the development of heparin-induced thrombocytopenia through the downregulation of SFK activity and platelet reactivity,89 raising the possibility of targeting CD148 in this and thrombotic conditions. Subsequent findings from our group revealed that CD148 can also attenuate SFK activity by dephosphorylating the activation loop tyrosine residue of SFKs (Figure 7).90 This suggests that CD148 acts as a rheostat that optimizes SFK activity in resting and activated platelets; however, the conditions under which CD148 negatively regulates SFKs have yet to be determined.
Tyrosine phosphorylation–mediated regulation of SFK activity. SFK activity is regulated through the phosphorylation of conserved tyrosine residues in the C-terminal tail and activation loop. Phosphorylation of the C-terminal tyrosine residue inhibits SFK activity by mediating formation of an intramolecular interaction with the SH2 domain that blocks the active site. A second intramolecular interaction between the SH3 domain and the proline-rich SH2-kinase linker region maintains the SFK in an inactive conformation. Dephosphorylation of the C-terminal tyrosine residue by the protein-tyrosine phosphatases CD148, PTP-1B, and possibly Shp1, releases the intramolecular interactions and actives the SFK. Maximal activation is achieved through trans-autophosphorylation of the activation loop tyrosine residue. Phosphorylation of the C-terminal inhibitory tyrosine residue by Csk or Csk homologous kinase (Chk) re-establishes the SH2 C-terminal inhibitory phosphotyrosine interaction and returns the SFK to an inactive conformation. Dephosphorylation of the activation loop tyrosine returns the SFK to a basally active state. CD148 may be responsible for dephosphorylating this site in platelets.
Tyrosine phosphorylation–mediated regulation of SFK activity. SFK activity is regulated through the phosphorylation of conserved tyrosine residues in the C-terminal tail and activation loop. Phosphorylation of the C-terminal tyrosine residue inhibits SFK activity by mediating formation of an intramolecular interaction with the SH2 domain that blocks the active site. A second intramolecular interaction between the SH3 domain and the proline-rich SH2-kinase linker region maintains the SFK in an inactive conformation. Dephosphorylation of the C-terminal tyrosine residue by the protein-tyrosine phosphatases CD148, PTP-1B, and possibly Shp1, releases the intramolecular interactions and actives the SFK. Maximal activation is achieved through trans-autophosphorylation of the activation loop tyrosine residue. Phosphorylation of the C-terminal inhibitory tyrosine residue by Csk or Csk homologous kinase (Chk) re-establishes the SH2 C-terminal inhibitory phosphotyrosine interaction and returns the SFK to an inactive conformation. Dephosphorylation of the activation loop tyrosine returns the SFK to a basally active state. CD148 may be responsible for dephosphorylating this site in platelets.
The structurally distinct nontransmembrane PTPs PTP-1B and Shp1 have also been implicated as positive regulators of SFKs in platelets by a similar mechanism. PTP-1B has been shown to activate Src specifically downstream of αIIbβ3 (Figures 3A and 7),30,91 whereas Shp1 appears to act as a positive regulator of SFKs downstream of GPVI and αIIbβ3 (Figure 7).92 In contrast, SFK activity was not altered in platelets lacking the structurally related PTP Shp2,92 demonstrating the specificity of PTPs. Thus, a picture is emerging of the central role played by PTPs in regulating SFKs in platelets.24
Surprisingly little is known about how SFKs are inhibited in platelets. This also applies to megakaryocytes, which presumably also contain high levels of SFKs and are continuously exposed to agonists in the bone marrow that signal via SFKs. There is the question of why megakaryocytes do not become transformed under these conditions. Src is, after all, the product of a proto-oncogene. The prevailing hypothesis is that this is achieved by Csk, a well-established inhibitor of SFKs in other cell types (Figure 7).93 Preliminary findings from Csk conditional knockout mice have revealed that Csk is indeed a major inhibitor of SFKs in platelets (J.M. and Y.A.S., unpublished data). Platelets also express Chk that may work in conjunction with Csk to inhibit subsets of SFKs under specific conditions.94 Alternative inhibitory mechanisms cannot be excluded, particularly because platelets contain such high levels of SFKs.
Other modes of regulation that warrant investigation include serine phosphorylation, unclamping, and degradation (Figure 8A). Neither the PKC isoform that phosphorylates Ser12 of Src nor the Ser/Thr protein phosphatase that dephosphorylates this residue and regulates the compartmentalization and substrate affinity of Src have been identified. Protein-protein interactions can also activate SFKs by unclamping the intramolecular interactions that maintain SFKs in inactive conformations (Figure 8B), in which case dephosphorylation of the C-terminal inhibitory tyrosine residue is not necessary to active the SFK. Ubiquitin-dependent degradation is yet another mechanism of downregulation that has yet to be explored in platelets.
Alternative mechanisms of regulation. (A) Established and novel mechanisms of regulating SFK activity in platelets. (B) Unclamping and activation of SFKs by disruption of the intramolecular interactions by a proline-rich (PxxP)/phosphotyrosine-containing protein. Disruption of either the SH3-proline-rich linker or the SH2 C-terminal phosphotyrosine intramolecular interactions facilitates SFK activation, irrespective of whether the C-terminal inhibitory tyrosine residue is phosphorylated. Ser/Thr PPs, protein-phosphatases.
Alternative mechanisms of regulation. (A) Established and novel mechanisms of regulating SFK activity in platelets. (B) Unclamping and activation of SFKs by disruption of the intramolecular interactions by a proline-rich (PxxP)/phosphotyrosine-containing protein. Disruption of either the SH3-proline-rich linker or the SH2 C-terminal phosphotyrosine intramolecular interactions facilitates SFK activation, irrespective of whether the C-terminal inhibitory tyrosine residue is phosphorylated. Ser/Thr PPs, protein-phosphatases.
Physiological substrates and interacting partners of SFKs also remain incompletely defined in platelets. Although seemingly of little clinical value, answers to these questions could yield novel, indirect strategies of inhibiting SFKs and their biological effects. Potential targets could include activators, inhibitors, docking sites, and downstream effectors of SFKs. The rationale is that more specialized inhibitors blocking specific aspects of platelet function required for thrombus growth and stability will likely have fewer adverse bleeding effects compared with broad-spectrum SFK inhibitors.
In conclusion, findings made over the past 2 decades have firmly established SFKs as critical regulators of platelet signaling and activation. They not only transmit primary activation signals that initiate and stabilize thrombus formation, they also contribute to signaling via GPCRs that amplify activation signals and mediate cross-talk between receptors and negative feedback pathways that attenuate platelet activation. The distinct and complimentary functions of SFKs are essential for optimizing the platelet response to all conditions. Despite the vast amount of knowledge that has accumulated about SFKs in platelets, many important questions remain unresolved, first and foremost the question of how they are regulated, and second, their involvement in setting the threshold of platelet reactivity. Answers to these questions could lead to novel, more specialized SFK inhibitors with antithrombotic potential. We are optimistic that the next decade will provide important new insights into the regulation and roles of SFKs in platelets. The search is far from over.
Acknowledgments
The authors thank Steve Watson, Michael Tomlinson, Silke Heising, and Louise Tee for insightful suggestions and the British Heart Foundation for generous support.
Authorship
Contribution: Y.A.S. wrote and revised the paper; A.M. contributed intellectually and proofread and revised the paper; and J.M. contributed intellectually and proofread and revised the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Yotis A. Senis, Centre for Cardiovascular Sciences, Institute of Biomedical Research, School of Clinical and Experimental Medicine, College of Medical and Dental Sciences, University of Birmingham, Birmingham, B15 2TT, United Kingdom; e-mail: y.senis@bham.ac.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal